
Medications/Drugs (Outpatient/Part B)
Page 1 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
UnitedHealthcare
®
Medicare Advantage
Coverage Summary
Medications/Drugs (Outpatient/Part B)
Policy Number: MCS057.32
Last Committee Approval Date: July 10, 2024
Effective Date: August 1, 2024
Instructions for Use
Table of Contents Page
Coverage Guidelines ............................................................. 1
• Outpatient Medications/Drugs ........................................ 1
• Unlabeled Use of a Part B Drug ..................................... 2
• Medications/Drugs Covered Under Part B ..................... 3
• Medications/Drugs Not Covered ..................................... 8
• Review at Launch ........................................................... 9
• Step Therapy Program ................................................. 10
• Maximum Dosage and Frequency ................................ 10
• Other Specific Medications ........................................... 10
Definitions ............................................................................ 11
Supporting Information ........................................................ 11
Policy History/Revision Information .................................... 30
Instructions for Use ............................................................. 31
Coverage Guidelines
Outpatient/Part B medications/drugs are covered when Medicare coverage criteria are met.
DME Face-to-Face Requirement: Section 6407 of the Affordable Care Act (ACA) established a face-to-face encounter
requirement for certain items of DME (including implantable infusion pumps; implantable programmable infusion pump;
external ambulatory infusion pump and nebulizers). For DME Face-to-Face Requirement information, refer to the
Coverage Summary titled
Durable Medical Equipment (DME), Prosthetics, Orthotics (Non-Foot Orthotics), Nutritional
Therapy, and Medical Supplies Grid.
Note: The guidelines in this Coverage Summary are for specific procedures/medications only. For procedures/
medications not addressed in this Coverage Summary, refer to the Medicare Coverage Database
to search for applicable
coverage policies (National Coverage Determinations, Local Coverage Determinations and Local Coverage Articles).
(Accessed July 1, 2024)
Outpatient Medications/Drugs
Part B Medications/Drugs
Outpatient (Part B) medications/drugs, in accordance with Medicare coverage criteria, are covered when furnished
“incident” to a physician service for drugs that are “not Usually Self-Administered By the Patient.” Refer to the definition of
Not Usually Self-Administered By the Patient
.
Coverage is Usually limited to drugs or biologicals Administered by infusion or injection. However, if the injection is
generally Self-Administered (e.g., Imitrex), it is not covered under Part B. Despite the general limitation on coverage for
outpatient drugs under Part B, some Self-Administered medications/drugs are also covered. For examples, refer to the
Medications/Drugs Covered Under Part B and Medications/Drugs Not Covered
sections.
For Medicare’s detailed coverage criteria for medications/drugs under Part B, refer to the
Medicare Benefit Policy Manual,
Chapter 15, §50 – Drugs and Biologicals. (Accessed July 1, 2024)
Related Medicare Advantage Policy Guidelines
• Halaven
®
(Eribulin Mesylate)
• Immune Globulin
• Testosterone Pellets (Testopel
®
)
• Xgeva
®
, Prolia
®
(Denosumab)

Medications/Drugs (Outpatient/Part B)
Page 2 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Part D Medications/Drugs
A Part D covered drug is available only by prescription, approved by the Food and Drug Administration (FDA), used and
sold in the United States, and used for a medically accepted indication.
A drug for which coverage is available under Part A or Part B, as it is being “prescribed and dispensed or Administered”
with respect to the individual, is excluded from the definition of a Part D drug and, therefore, cannot be included in Part D
basic coverage. CMS interprets this to mean that if payment could be available under Part A or Part B to the individual for
such drug, then it will not be covered under Part D.
Section 1860D-2(e)(4) of the Act defines “medically-accepted indication,” in part by reference to section 1927(k)(6) of the
Act, to any use of a covered Part D drug which is approved under the Federal Food, Drug, and Cosmetic Act, or the use
of which is supported by one or more citations included or approved for inclusion in any of the compendia described in
section 1927(g)(1)(B)(i) of the Act. The recognized compendia are:
American Hospital Formulary Service Drug Information, and
DRUGDEX
®
Information System.
Refer to the Medicare Prescription Drug Benefit Manual Chapter 6, §10.6 – Medically Accepted Indication
.
Note: Some members may have coverage for Part D drugs under UnitedHealthcare. Refer to the Member’s Pharmacy
Booklet or contact the Prescription Solutions Customer Service Department to determine coverage eligibility for Part D
prescription drug plan benefit.
For Medicare’s detailed coverage information for medications/drugs under Part D, refer to the
Medicare Prescription Drug
Benefit Manual, Chapter 6, §10 – Definition of Part D Drugs. (Accessed July 1, 2024)
Part B vs. Part D Medications/Drugs
For Part B vs. Part D medications/drugs guidelines, refer to the specific medications listed under the Medications/Drugs
Covered Under Part B section.
Unlabeled Use of a Part B Drug
Unlabeled use of a drug may be covered only if a UnitedHealthcare Medical Director or his/her designee determines the
use to be medically accepted, taking into consideration the major drug compendia, authoritative medical literature and/or
accepted standards of medical practice.
Refer to the Medicare Benefit Policy Manual, Chapter 15, §50.4.2 – Unlabeled Use of Drug
.
For the list of the major drug compendia for off-label use of drugs and biologicals in an anti-cancer chemotherapeutic
regimen, refer to the Medicare Benefit Policy Manual, Chapter 15, §50.4.5.B – Recent Revision to Compendia List
.
In the case of drugs used in anti-cancer chemotherapeutic regimen, refer to the
Medicare Benefit Policy Manual,
Chapter 15, §50.4.5 – Off-Label Use of Drugs and Biologicals in an Anti-Cancer Chemotherapeutic Regimen.
Notes:
The above information is for determining coverage for the unlabeled use of medication covered under Part B only, not
Part D. Refer to the Member’s Pharmacy Booklet or contact the Prescription Solutions Customer Service Department
for further information on Part D coverage, if any.
Definition of Compendium: CMS revised the definition of “compendium” to include this public transparency
requirement. In this revised definition, a compendium:
o Includes a summary of the pharmacologic characteristics of each drug or biological and may include information
on dosage, as well as recommended or endorsed uses in specific diseases; and
o Is indexed by drug or biological; and
o Has a publicly transparent process for evaluating therapies and for identifying potential conflicts of interests.
Refer to the Medicare Benefit Policy Manual, Chapter 15, §50 – Drugs and Biologicals §50.4.5.1.A
.
(Accessed July 1, 2024)

Medications/Drugs (Outpatient/Part B)
Page 3 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Medications/Drugs Covered Under Part B
Examples of medications/drugs that are covered under Part B include, but not limited to, the following medications/drugs.
Durable Medical Equipment (DME) Supply Drugs
Payment may be made for supplies that are necessary for the effective use of durable medical equipment. This includes
drugs and biologicals which must be put directly into the equipment in order to achieve the therapeutic benefit of the
durable medical equipment or to assure the proper functioning of the equipment. Refer to the
Medicare Benefit Policy
Manual, Chapter 15, §110.3 – Coverage of Supplies and Accessories.
Part B vs. Part D Guideline
Nebulizer Inhalation Drugs (e.g., Albuterol Sulfate, Ipratropium Bromide)
Certain inhalation drugs are generally covered under Part B when used with a nebulizer in the home. These drugs would
not be covered under Part D for use with a nebulizer. However, if these drugs were delivered with a metered dose inhaler
or other non-nebulized administration, they would be Part D drugs.
In the case of a member in a hospital, or a SNF bed, (1) who does not have Part A coverage, (2) whose Part A coverage
for the stay has run out or (3) whose stay is non-covered-infusible DME supply drugs are not covered under Part B
because the law limits coverage under Part B’s DME benefit to those items that are furnished for use in a patient’s home,
and specifies that a hospital or SNF cannot be considered the member’s “home” for this purpose. In this case, coverage
for the drugs would be available under Part D.
In addition to a hospital, a SNF or a distinct part SNF, the following facilities cannot be considered a home for purposes of
receiving the Medicare DME benefit:
A nursing home that is dually certified as both a Medicare SNF and a Medicaid nursing facility (NF); and
A Medicaid-only NF that primarily furnishes skilled care; and
A non-participating nursing home (i.e., neither Medicare or Medicaid) that provides primarily skilled care; and
An institution which has a distinct part SNF and which also primarily furnishes skilled care.
Refer to the
Medicare Prescription Drug Benefit Manual, Chapter 6, Appendix C – Medicare Part B versus Part D
Coverage Issues.
For the list of nebulizer drugs covered under Part B, refer to the DME MAC LCD for Nebulizers (L33370)
. Compliance with
these policies is required where applicable. (Accessed July 1, 2024)
Infusion Pump Medications (e.g., Some Chemotherapeutic Agents)
In general, the supplier would bill Part B if the drug was Administered using an infusion pump and bill the Part D plan for
infusion using other methods (e.g., IV push). While professional services and supplies related to the administration of the
infused drug are not payable under Part D, some coverage may be available under Part A or B home health benefits,
under Medicaid, or from secondary commercial health benefits.
As a rule, drugs infused using an implantable pump would be covered under Part B. Drugs infused in the home using an
external pump are covered under Part B if they are included under the local coverage policy of the applicable Medicare
DME MAC.
In the case of a member in a hospital, or a SNF bed, (1) who does not have Part A coverage, (2) whose Part A coverage
for the stay has run out or (3) whose stay is non-covered infusible DME supply drugs are not covered under Part B
because the law limits coverage under Part B’s DME benefit to those items that are furnished for use in a patient’s home,
and specifies that a hospital or SNF cannot be considered the member’s “home” for this purpose. In this case, coverage
for the drugs would be available under Part D.
In addition to a hospital, a SNF or a distinct part SNF, the following facilities cannot be considered a home for purposes of
receiving the Medicare DME benefit:
A nursing home that is dually certified as both a Medicare SNF and a Medicaid nursing facility (NF); and
A Medicaid-only NF that primarily furnishes skilled care; and
A non-participating nursing home (i.e., neither Medicare or Medicaid) that provides primarily skilled care; and
An institution which has a distinct part SNF and which also primarily furnishes skilled care.

Medications/Drugs (Outpatient/Part B)
Page 4 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Refer to the Medicare Prescription Drug Benefit Manual, Chapter 6, Appendix C – Medicare Part B versus Part D
Coverage Issues. (Accessed July 1, 2024)
Immunosuppressive Drugs
Immunosuppressive drug therapy following a Medicare covered organ transplant is covered.
Covered drugs include those immunosuppressive drugs that have been specifically labeled as such and approved for
marketing by the FDA. (This is an exception to the standing drug policy which permits coverage of FDA Approved Drugs
for non-labeled uses, where such uses are found to be reasonable and necessary in an individual case.)
Immunosuppressive drugs are substances that suppress or interfere with normal immune responses. They are used in
controlling autoimmune diseases and in enhancing the chances for survival of foreign-tissue grafts and transplants.
Examples of FDA-approved immunosuppressive drugs include, but are not limited to:
Sandimmune (cyclosporine), Sandoz Pharmaceutical.
Imuran (azathioprine), Burroughs Welcomes.
Agma (antithymocyte globulin), Upjohn.
Orthoclone OKT3 (Muromonab-CD3), Ortho Pharmaceutical.
Prograf (tacrolimus), Fujisawa USA, Inc.
Celicept (mycophenolate mofetil), Roche Laboratories.
Daclizumab (Zenapax).
Cyclophosphamide (Cytoxan).
Prednisone and Prednisolone.
Notes:
Prescription drugs, such as prednisone, used in conjunction with immunosuppressive drugs as part of a therapeutic
regimen are covered as reflected in FDA approved labeling for immunosuppressive drugs. Therapeutic regimen is a
combination of drugs which has been clinically recognized for the treatment of a specific type of disorder or to treat
toxicities or side effects of drugs which are used at different times following an approved transplant.
Immunosuppressive drugs for organ transplants are covered under Part B coverage except when furnished during an
inpatient stay or upon discharge from the hospital, then the drugs are covered as Part A.
CMS expects contractors to keep informed of FDA additions to the list of the immunosuppressive drugs.
Members may have additional coverage for immunosuppressive drugs under the Part D Prescription Drug Plan which
are not covered in this benefit interpretation policy. Refer to the Member’s Pharmacy Booklet or contact the
Prescription Solutions Customer Services Department to determine coverage eligibility for prescription drug plan
benefit.
Refer to the Medicare Benefit Policy Manual, Chapter 15, §50.5.1 – Immunosuppressive Drugs
. (Accessed July 1, 2024)
Part B vs. Part D Guideline
Part B would be billed if the individual had a Medicare-covered transplant; otherwise, the Part D plan would be billed.
Pharmacists would bill Part B or the individual’s Part D plan based on information received from the individual or the Part
D plan. Part B would be billed if the individual had a Medicare-covered transplant; otherwise, the Part D plan would be
billed. Part D plan eligibility systems could contain a marker for members who had a Medicare covered transplant. This
information could come from a question included on the Part D sponsor’s enrollment or coordination of benefit (COB)
survey form.
In determining whether to pay for an immunosuppressive drug under Part D, it would not be appropriate for a Part D
sponsor to institute a general policy of requiring a Part B claim rejection, as a substitute for maintaining information on
transplant status and paying claims based on that information. Such a policy would be disruptive to beneficiaries and
pharmacies and would unnecessarily increase Part B contractor costs. Instead, a prior authorization requirement would be
appropriate.
Refer to the
Medicare Prescription Drug Benefit Manual, Chapter 6, Appendix C – Medicare Part B versus Part D
Coverage Issues. (Accessed July 1, 2024)

Medications/Drugs (Outpatient/Part B)
Page 5 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Hemophilia Blood Clotting Factors
Part B vs. Part D Guideline
Hemophilia blood clotting factors would not be a Part D benefit because of the Part B coverage. Refer to the Medicare
Prescription Drug Benefit Manual, Chapter 6, Appendix C – Medicare Part B versus Part D Coverage Issues.
(Accessed July 1, 2024)
Oral Anti-Cancer Drugs and Oral Anti-Emetics
Oral anti-cancer drugs and oral anti-nausea (anti-emetic) drugs are covered when criteria are met.
For detailed coverage requirements, refer to the
Medicare Benefit Policy Manual, Chapter 15, §50.5.3 Oral Anti-Cancer
Drugs.
For claims payment and coding information, refer to the
Medicare Claims Processing Manual, Chapter 17, §80.1 Oral
Cancer Drugs.
Local Coverage Determinations (LCDs)/Local Coverage Articles (LCAs) exist and compliance with these policies is
required where applicable. These LCDs/LCAs are available at
https://www.cms.gov/medicare-coverage-database/new-
search/search.aspx.
Note: Members may have additional coverage for oral anti-cancer under the Part D. Prescription Drug Plan, which are not
covered in this coverage summary. Refer to the member’s pharmacy booklet or contact the Prescription Solutions
customer service department to determine coverage eligibility for prescription drug plan benefit. (Accessed July 1, 2024)
Part B vs. Part D Guideline
Certain oral chemotherapy agents used in cancer treatment for which there is an infusible version of the drug.
Pharmacists would need to determine the reason for treatment. If related to cancer treatment, Part B would be billed;
otherwise, the Part D plan should be billed.
To the extent that a Part B-covered oral anti-cancer drug has no other medically accepted indication besides cancer
treatment, Part D sponsors should not include these drugs on their formularies because of Part B coverage. For the
drugs that have other medically accepted indications, prior authorization programs or other mechanisms to obtain
diagnostic information could be used to ensure appropriate payment.
Oral anti-emetics used in cancer treatment as a full replacement for intravenous treatment.
Pharmacists would need to determine the reason for treatment. If both related to cancer treatment and a full
replacement for intravenous administration within 48 hours of cancer treatment, Part B would be billed; otherwise, the
Part D plan should be billed.
Note: In order to receive Part B payment, CMS currently requires that the prescribing physician indicate on the
prescription that the oral anti-emetic is being used “as a full therapeutic replacement for an intravenous anti-emetic
drug as part of a cancer chemotherapeutic regimen.”
If based on a prior authorization program or other mechanism to obtain diagnostic information, a Part D sponsor
determined that a) a Part B-covered oral anti-emetic was being billed, and b) the drug was being furnished in the
context of cancer treatment for use within 48 hours of cancer treatment, the Part D sponsor should deny payment.
Such drugs dispensed for use after the 48-hour period, or any oral anti-emetic prescribed for conditions other than the
effects of cancer treatment, would be Part D drugs.
Refer to the Medicare Prescription Drug Benefit Manual, Chapter 6, Appendix C – Medicare Part B versus Part D
Coverage Issues. (Accessed July 1, 2024)
Immunizations
Immunizations (e.g., pneumococcal vaccine, Hepatitis B vaccine, and influenza vaccine) are covered when criteria are
met. Refer to the Medicare Benefit Policy Manual, Chapter 15, §50.4.4.2 – Immunizations
for coverage criteria.
(Accessed July 1, 2024)
Part B vs. Part D Guideline
For Hepatitis B vaccine, physicians would need to determine the level of risk of the individual. If the individual is at high or
intermediate risk, Part B would be billed. For all other individuals, prior authorization programs could be used to ensure
appropriate level of risk.

Medications/Drugs (Outpatient/Part B)
Page 6 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Pneumococcal and influenza vaccines would not be covered under Part D because of Part B coverage. Refer to the
Medicare Prescription Drug Benefit Manual, Chapter 6, Appendix C – Medicare Part B versus Part D Coverage Issues
.
(Accessed July 1, 2024)
Antigens/Antihistamines
Antigens/antihistamines are covered when criteria are met. These are prepared by a physician (Usually an allergist) for a
specific patient. The physician or physician’s nurse generally administers them in the physician’s office. In some cases,
the physician prepares antigens and furnishes them to a patient who has been taught to self-administer them at home.
Refer to the Medicare Benefit Policy Manual, Chapter 15, §20.2 – Physician Expense for Allergy Treatment and
§50.2 –
Determining Self-Administration of Drug or Biological.
Also refer to the:
o Medicare Benefit Policy Manual, Chapter 15, §50.4.4.1 – Antigens.
o Medicare Claims Processing Manual, Chapter 12, §200 – Allergy Testing and Immunotherapy.
Local Coverage Determinations (LCDs)/Local Coverage Articles (LCAs) exist and compliance with these policies is
required where applicable. These LCDs/LCAs are available at
https://www.cms.gov/medicare-coverage-
database/search.aspx.
(Accessed July 1, 2024)
Part B vs. Part D Guideline
Antigens would not be a Part D benefit because of the Part B coverage. Refer to the Medicare Prescription Drug Benefit
Manual, Chapter 6, Appendix C – Medicare Part B versus Part D Coverage Issues. (Accessed July 1, 2024)
Parenteral Nutrition
Parenteral nutrition, including Intradialytic Parenteral Nutrition (IDPN), is covered under the prosthetic benefit when criteria
are met. Refer to the Coverage Summary titled
Durable Medical Equipment (DME), Prosthetics, Orthotics (Non-Foot
Orthotics), Nutritional Therapy, and Medical Supplies Grid for coverage criteria.
Part B vs. Part D Guideline
If the therapy was being provided because of a non-functioning digestive tract, Part B would be billed; if not, this would be
a Part D drug. Refer to the
Medicare Prescription Drug Benefit Manual, Chapter 6, Appendix C – Medicare Part B versus
Part D Coverage Issues. (Accessed July 1, 2024)
Intravenous Immune Globulin (IVIG)
Intravenous Immune Globulin (IVIG) in the Home
Intravenous immune globulin (IVIG) for the treatment of primary immune deficiency diseases is covered in the home
under Part B if all of the following criteria are met:
It is an approved pooled plasma derivative for the treatment of primary immune deficiency disease.
The patient has a diagnosis of primary immune deficiency disease.
Note: For specific ICD-10-CM codes that are covered, refer to the
Medicare Benefit Policy Manual, Chapter 15, §50.6
– Coverage of Intravenous Immune Globulin for Treatment of Primary Immune Deficiency Diseases in the Home. Also
refer to the applicable LCDs/LCAs.
The IVIG is Administered in the home.
The treating physician has determined that administration of the IVIG in the patient’s home is medically appropriate.
Refer to the
Medicare Benefit Policy Manual, Chapter 15, §50.6– Coverage of Intravenous Immune Globulin for Treatment
of Primary Immune Deficiency Diseases in the Home.
Local Coverage Determinations (LCDs)/Local Coverage Articles (LCAs) exist for IVIG and compliance with these policies
is required where applicable. For specific LCDs/LCAs, refer to the table for Intravenous Immune Globulin (IVIG)
.
(Accessed July 1, 2024)
Part B vs. Part D Guideline
Part B coverage for IVIG in the home is for individuals whose diagnosis is primary immune deficiency disease. Part D
would provide coverage for IVIG in the home for all other medically accepted indications. Prior authorization requirements
could be used to ensure appropriate payment in accordance with the Part D sponsor’s medical necessity criteria. It would
not be appropriate to routinely require a rejection of a claim under Part B before processing a Part D claim. Such a policy
would be disruptive to beneficiaries and pharmacies and would unnecessarily increase Part B contractor cost.

Medications/Drugs (Outpatient/Part B)
Page 7 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
The supplier would bill Part B if the diagnosis is primary immune deficiency disease. IVIG provided in the home for other
diagnoses would be a Part D benefit. As discussed above, it would not be appropriate, as a general rule, for Part D
sponsors to require a rejection of a claim under Part B before processing a Part D claim. Prior authorization programs
could be used to ensure medical necessity in accordance with the Part D sponsor’s policy.
Refer to the
Medicare Prescription Drug Benefit Manual, Chapter 6, Appendix C – Medicare Part B versus Part D
Coverage Issues. (Accessed July 1, 2024)
Treatment of Autoimmune Mucocutaneous Blistering Diseases
IVIg is covered for the treatment of biopsy-proven:
Pemphigus Vulgaris.
Pemphigus Foliaceus.
Bullous Pemphigoid.
Mucous Membrane Pemphigoid (a.k.a., Cicatricial Pemphigoid).
Epidermolysis Bullosa Acquisita.
For more specific coverage guidelines, refer to the
National Coverage Determination (NCD) for Intravenous Immune
Globulin for the Treatment of Autoimmune Mucocutaneous Blistering Diseases (250.3).
Local Coverage Determinations (LCDs)/Local Coverage Articles (LCAs) exist for IVIG and compliance with these policies
is required. For specific LCDs/LCAs, refer to the table for Intravenous Immune Globulin (IVIG)
. (Accessed July 1, 2024)
Other Indications
Medicare does not have an NCD for other indications other than the ones listed above. Local Coverage Determinations
(LCDs)/Local Coverage Article (LCAs) exist for all states/territories and compliance with these policies is required where
applicable. For specific LCDs/LCAs, refer to the table for Intravenous Immune Globulin (IVIG)
.
Injectable Drugs for the Treatment of Osteoporosis
Injectable drugs for the treatment of osteoporosis when provided by the home health agency and the following criteria are
met:
The member is unable to learn the skills needed to self-administer the drug, or is otherwise physically or mentally
incapable of administering the drug, and that her family or caregiver are unable or unwilling to administer the drug, as
documented by the home health agency, and
The member sustained a bone fracture that a physician certifies was related to (post-menopausal) osteoporosis; and
The member is Homebound.
Refer to the:
Medicare Benefit Policy Manual Chapter 7, §50.4.3 8– Covered Osteoporosis Drugs.
Coverage Summary titled Home Health Services, Home Health Visits, Respite Care, and Hospice Care.
(Accessed July 1, 2024)
Dermal Injections for the Treatment of Facial Lipodystrophy Syndrome (LDS)
(HCPCS Code Q2026)
Effective for claims with dates of service on and after March 23, 2010, dermal injections for LDS are only reasonable and
necessary using dermal fillers approved by the Food and Drug Administration (FDA) for this purpose, and then only in
HIV-infected beneficiaries when LDS caused by antiretroviral HIV treatment is a significant contributor to their depression.
Refer to the NCD for Dermal Injections for the Treatment of Facial Lipodystrophy Syndrome (LDS) (250.5)
.
(Accessed July 1, 2024)
Drugs for Chelation Therapy for the Treatment of Heavy Metal Toxicity and Non-
Overload Conditions
Medicare does not have a National Coverage Determination (NCD) for chelation therapy for lead poisoning. Local
Coverage Determinations (LCDs)/Local Coverage Articles (LCAs) do not exist at this time.
For coverage guidelines, refer to the UnitedHealthcare Commercial Medical Policy titled
Chelation Therapy for Non-
Overload Conditions.
Medications/Drugs (Outpatient/Part B)
Page 8 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Note: After searching the Medicare Coverage Database, if no LCD/LCA is found, then use the policy referenced above for
coverage guidelines. (Accessed July 1, 2024)
Drugs Treated as Hospital Outpatient Supplies
In certain circumstances, Medicare pays for drugs that may be considered Usually self-Administered By the Patient when
such drugs function as supplies. This is the case when the drugs provided are an integral component of a procedure or
are directly related to it, i.e., when they facilitate the performance of or recovery from a particular procedure. Except for
the applicable copayment, hospitals may not bill beneficiaries for these types of drugs because their costs, as supplies,
are packaged into the payment for the procedure with which they are used. Listed below are examples of when drugs are
treated as supplies and hospitals should bill Medicare for the drug as a supply and should not separately bill the member.
Sedatives Administered to a patient while he or she is in the preoperative area being prepared for a procedure.
Mydriatic drops instilled into the eye to dilate the pupils, anti-inflammatory drops, antibiotic drops/ointments, and
ocular hypotensives that are Administered to a patient immediately before, during, or immediately following an
ophthalmic procedure; this does not refer to the patient’s eye drops that the patient uses pre-and postoperatively.
Barium or low osmolar contrast media provided integral to a diagnostic imaging procedure.
Topical solution used with photodynamic therapy furnished at the hospital to treat non-hyperkeratotic actinic keratosis
lesions of the face or scalp.
Antibiotic ointments such as bacitracin, placed on a wound or surgical incision at the completion of a procedure.
The following are examples of when a drug is not directly related or integral to a procedure and does not facilitate the
performance of or recovery from a procedure. Therefore, the drug is not considered a packaged supply. In many of these
cases the drug itself is the treatment instead of being integral or directly related to the procedure or facilitating the
performance of or recovery from a particular procedure.
Drugs given to a patient for his or her continued use at home after leaving the hospital.
Oral pain medication given to an outpatient who develops a headache while receiving chemotherapy administration
treatment.
Daily routine insulin or hypertension medication given preoperatively to a patient.
A fentanyl patch or oral pain medication such as hydrocodone, given to an outpatient presenting with pain.
A laxative suppository for constipation while the patient waits to receive an unrelated X-ray.
These two lists of examples may serve to guide hospitals in deciding which drugs are supplies packaged as a part of a
procedure, and thus may be billed under Part B. Hospitals should follow CMS’ guidance for billing drugs that are
packaged and paid as supplies, reporting coded and uncoded drugs with their charges under the revenue code
associated with the cost center under which the hospital accumulates the costs for the drugs. Refer to the
Medicare
Benefit Policy Manual, Chapter 15, §50.2 – Determining Self-Administration of Drug or Biological, M-Drugs Treated as
Hospital Outpatient Supplies. (Accessed July 1, 2024)
Hereditary Angioedema (HAE) Treatment (HCPCS Codes J0596, J0597, J0598, and
J1290)
Medicare does not have a National Coverage Determination (NCD) for Hereditary Angioedema (HAE) treatment. Local
Coverage Determinations (LCDs)/Local Coverage Articles (LCAs) do not exist.
For coverage guidelines, refer to the UnitedHealthcare Commercial Medical Benefit Drug Policy titled
Hereditary
Angioedema (HAE), Treatment and Prophylaxis.
Note: After searching the Medicare Coverage Database
, if no LCD/LCA is found, then use the policy referenced above for
coverage guidelines. (Accessed July 1, 2024)
Medications/Drugs Not Covered
Examples of medications/drugs that are not covered are:
Vitamin B12 Injections
Vitamin B12 injections to strengthen tendons, ligaments, etc., of the foot are not covered under Medicare because:
There is no evidence that vitamin B12 injections are effective for the purpose of strengthening weakened tendons and
ligaments, and
This is non-surgical treatment under the subluxation exclusion.

Medications/Drugs (Outpatient/Part B)
Page 9 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Accordingly, Vitamin B12 injections are not considered reasonable and necessary. Refer to the NCD for Vitamin B12
Injections to Strengthen Tendons, Ligaments, etc., of the Foot (150.6). (Accessed July 1, 2024)
Investigational or Experimental Drugs
Investigational or experimental drugs are not covered. Refer to the Medical Benefit Policy Manual, Chapter 15, §50.4.3 –
Examples of Not Reasonable and Necessary. (Accessed July 1, 2024)
Placebos
Placebos are not covered.
Outpatient Prescription Drugs
Outpatient prescription drugs are not covered except those medications/drugs covered under the Member’s Part D
Prescription Drug Plan benefit.
Refer to the Member’s Pharmacy Program booklet or contact the Prescription Solutions Customer Services Department to
determine coverage eligibility for Part D Prescription Drug benefit.
Medications for the Treatment of Sexual Dysfunction
Medications for the treatment of sexual dysfunction including erectile dysfunction, impotence, anorgasmy, or
hypoorgasmy are not covered.
Erectile dysfunction (ED) drugs will meet the definition of a Part D drug when prescribed for medically accepted
indications approved by the FDA other than sexual or erectile dysfunction (such as pulmonary hypertension). However,
ED drugs will not meet the definition of a Part D drug when used off-label, even when the off label use is listed in one of
the compendia found in section 1927(g)(1)(B)(i) of the Act: American Hospital Formulary Service Drug Information, United
States Pharmacopeia-Drug Information (or its successor publications), and DRUGDEX
®
Information System.
Refer to the Medicare Prescription Drug Benefit Manual, Chapter 6, Section 20.1 – Excluded Categories
.
(Accessed July 1, 2024)
Medications for Elective Enhancement
Medications for elective enhancement, such as those used for weight loss, hair growth, sexual performance, athletic
performance, cosmetic purposes, anti-aging, and mental performance are not covered. Refer to the Coverage Summary
titled Cosmetic and Reconstructive Procedures
.
Drugs Included in the CMS Self-Administered Drug Exclusion List
Drugs included in the CMS Self-Administered Drug Exclusion List are not covered.
Notes:
• Self-Administered Drug (SAD) Exclusion List Report: Local Contractors have Self-Administered drugs exclusion
lists. Compliance with these lists is required where applicable. Refer to the Medicare Coverage Database
.
(Accessed July 1, 2024)
PCSK9 Inhibitors: PCSK9 Inhibitors, i.e., Praluent
™
(alirocumab) and Repatha
™
(evolocumab) are considered self-
Administered drugs and are not covered under the Part B medical benefit. Refer to the Member’s Pharmacy Program
booklet or contact the Prescription Solutions Customer Service Department to determine coverage eligibility for these
drugs under the Part D Prescription Drug benefit.
Off-Label/Unlabeled Drug Use
Off-Label/unlabeled drug use is not covered unless criteria are met. Refer to the Unlabeled Use of a Part B Drug section
for coverage criteria and guidelines.
Review at Launch (RAL)
A pre-service organization determination is highly recommended for certain Part B medications (as defined above):
That are new to the market; and
That have not yet undergone review by UnitedHealthcare; and
For which a utilization management strategy has not been established.

Medications/Drugs (Outpatient/Part B)
Page 10 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
These medications, referred to herein as RAL medications, are identified in the Other Examples of Specific
Drugs/Medications table. Upon receipt of a pre-service organization determination, RAL medications will be reviewed
against National Coverage Determinations (NCDs), Local Coverage Determinations (LCDs). In the absence of an NCD,
LCD or clear Medicare guidance, medical necessity reviews will be conducted using the following:
A UnitedHealthcare Pharmacy and Therapeutics approved medical drug policy; or
All of the following:
o Food and Drug Administration (FDA) approved labeling, including but not limited to indication, patient age
requirements, dosing recommendations, contraindications, and clinical trial inclusion criteria (ex. genetic testing,
comorbid conditions); and
o Compendia (if available); and
o Current standard of care, as per evidenced based literature (if available).
Providers are strongly encouraged to seek a pre-service organization determination for any RAL medication that has been
identified in the Other Examples of Specific Drugs/Medications
table. This will help to avoid gaps in coverage in the event
that a prior authorization program becomes effective at a later date. If a provider believes an item or service may not be
covered, or could only be covered under specific conditions, the appropriate process is to request a pre-service
organization determination.
Step Therapy Program
Certain classes of medical benefit injectables covered under Medicare Part B will include preferred and non-preferred
therapies. Non-preferred therapies will generally require history of use of a preferred therapy among other criteria. This
step therapy requirement will apply to some, but not all, Medicare Advantage Plans.
A medical injectable is subject to step therapy when it is listed in the Other Examples of Specific Drugs/Medications
table
and a notation to refer to the UnitedHealthcare Medicare Advantage Drug Policy titled: Medicare Part B Step Therapy
Programs is provided in the Step Therapy column.
Maximum Dosage and Frequency
Provides information about the maximum dosage per administration and dosing frequency for certain medications
Administered by a medical professional. Most medications have a maximum dosage and frequency based upon body
surface area or patient weight or a set maximal dosage and frequency independent of patient body size.
A medication is subject to maximum dosage and frequency when it is listed in the
Other Examples of Specific
Drugs/Medications table and a notation to refer to the UnitedHealthcare Commercial Medical Benefit Drug Policy titled
Maximum Dosage and Frequency is provided in the Maximum Dosage and Frequency column.
Note: Any LCD/LCA maximum dosage and frequency criteria would be applicable, if available.
Other Specific Medications (Not Listed Above)
For Oncology Medications
Check for available NCDs, LCDs or LCAs at https://www.cms.gov/medicare-coverage-database/new-
search/search.aspx. If there are no applicable NCDs, LCDs or LCAs found, refer to Supporting Information table
within this Coverage Summary.
Also refer to the Medicare Benefit Policy Manual, Chapter 15, §50.4 Reasonableness and Necessity.
o For any off label drug or biological with a NCCN Category 2B indication refer to the UnitedHealthcare Commercial
Medical Benefit Drug Policy titled Oncology Medication Clinical Coverage
.
For Non-Oncologic Medications
Check for available NCDs, LCDs or LCAs at https://www.cms.gov/medicare-coverage-database/new-
search/search.aspx. If there are no applicable NCDs, LCDs or LCAs found, refer to Supporting Information table
within this Coverage Summary.
For all other drugs or biologicals (non-oncologic) not listed in this Coverage Summary, for which there are no
applicable NCDs, LCDs or LCAs, refer to the relevant UnitedHealthcare Commercial Medical Benefit Drug Policy. If
there is no UnitedHealthcare Commercial Drug Policy, then use the compendia and evidence-based medical literature
for coverage guidance.
o For available UnitedHealthcare Commercial Medical Benefit Drug Policies, refer to
https://www.uhcprovider.com/en/policies-protocols/commercial-policies/commercial-medical-drug-policies.html
.
(Accessed July 1, 2024)

Medications/Drugs (Outpatient/Part B)
Page 11 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Definitions
FDA Approved Drug: A drug that has received final marketing approval by the Food and Drug Administration (FDA) and
as a part of its labeling contains its recommended uses and dosages as well as adverse reactions and recommended
precautions in using it. Medicare Benefit Policy Manual, Chapter 15, §50.4.1 – Approved Use of Drug
.
Homebound: An individual shall be considered “confined to the home” (Homebound) if the following two criteria are met:
The patient must either:
o Because of illness or injury, need the aid of supportive devices such as crutches, canes, wheelchairs, and
walkers; the use of special transportation; or the assistance of another person in order to leave their place of
residence, or
o Have a condition such that leaving his or her home is medically contraindicated.
If the patient meets one of the conditions above, then the patient must also meet two additional requirements defined
below.
o There must exist a normal inability to leave home, and
o Leaving home must require a considerable and taxing effort.
If the patient does in fact leave the home, the patient may nevertheless be considered Homebound if the absences from
the home are infrequent or for periods of relatively short duration or are attributable to the need to receive health care
treatment.
Any other absence of an individual from the home shall not so disqualify an individual if the absence is of infrequent or of
relatively short duration. For purposes of the preceding sentence, any absence for the purpose of attending a religious
service shall be deemed to be an absence of infrequent or short duration.
Medicare Benefit Policy Manual, Chapter 15,
§60.4.1 – Definition of Homebound Patient Under the Medicare Home Health (HH) Benefit.
Not Usually Self-Administered By the Patient (as defined by Medicare):
Administered: The term “Administered” refers only to the physical process by which the drug enters the patient's
body. It does not refer to whether the process is supervised by a medical professional (for example, to observe proper
technique or side-effects of the drug). Injectable drugs (including intravenous drugs) are typically eligible for inclusion
under the “incident to” benefit. With limited exclusions, other routes of administration including, but not limited to, oral
drugs, suppositories, topical medications are all considered to be Usually Self-Administered By the patient.
Usually: For the purposes of applying this exclusion, the term "Usually" means more than 50 percent of the time for
all Medicare beneficiaries who use the drug. Therefore, if a drug is Self-Administered by more than 50 percent of
Medicare beneficiaries, the drug is excluded from coverage and you may not make any Medicare payment for it.
By the Patient: The term "By the Patient" means Medicare beneficiaries as a collective whole. Include only the
patients themselves and not other individuals (which do not include spouses, friends, or other caregivers).
Medicare
Benefit Policy Manual, Chapter 15, §50.2 – Determining Self-Administration of Drug or Biological.
Unlabeled Use of Drug: A use that is not included as an indication of the drug’s label as approved by FDA.
Medicare
Benefit Policy Manual, Chapter 15, §50.4.2 – Unlabeled Use of Drug. (Accessed July 1, 2024)
Supporting Information
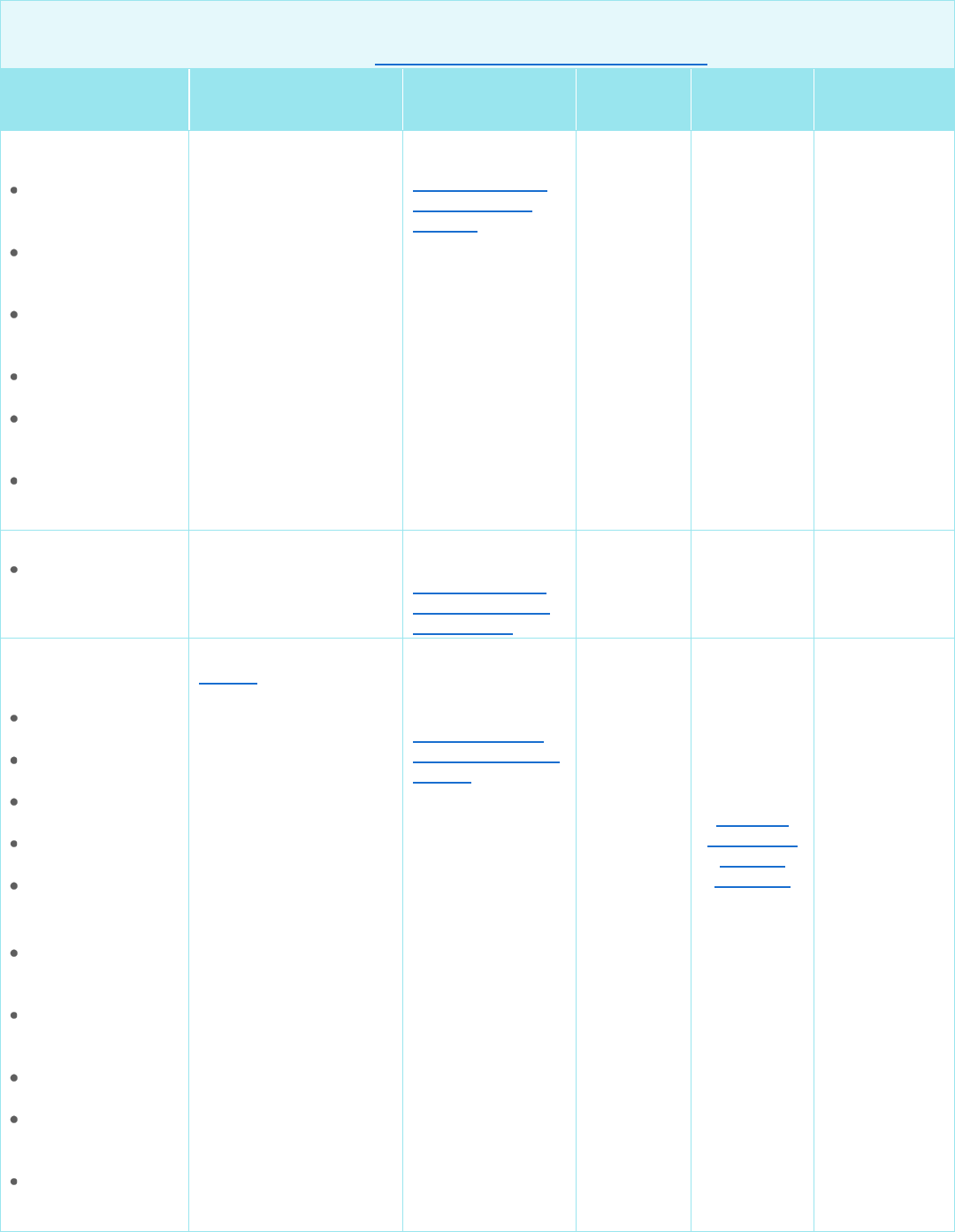
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Aduhelm
™
(aducanumab-avwa)
NCD for Monoclonal
Antibodies Directed
Against Amyloid for the
Treatment of
Alzheimer's Disease
(AD) 200.3
For payment rules for
NCDs requiring CED,
Not Applicable
(N/A)
No
No
No

Medications/Drugs (Outpatient/Part B)
Page 12 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Aduhelm
™
(aducanumab-avwa)
refer to the Medicare
Managed Care Manual,
Chapter 4, §10.7.3 –
Payment for Clinical
Studies Approved Under
Coverage with Evidence
Development (CED)
Not Applicable
(N/A)
No
No
No
Adzynma
(ADAMTS13,
recombinant-krhn)
None UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Adzynma
(ADAMTS13,
Recombinant-Krhn)
No No No
Amvuttra
™
(vutrisiran)
None UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
RNA-Targeted
Therapies
(Amvuttra
®
and
Onpattro
®
)
No No
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency
Antiemetics (oral) for
Oncology -
Neurokinin 1
Receptor Antagonist
(NK1 RA),
5-hydroxytrypta-mine
Receptor Antagonist
(5HT3 RA), NK1
RA/5HT3 RA
combination
Akynzeo
®
(netupitant and
palono-setron)
capsule
Emend
®
(aprepitant)
capsule
• Kytril
®
(granisetron)
tablets
• Varubi
®
(rolapitant) tablet
• Zuplenz, Zofran
ODT
®
, and
Zofran
®
(ondanset-ron)
tablets
Medicare Benefit Policy
Manual, Chapter 15,
§50.5.4 – Oral Anti-
Nausea (Anti Emetic)
Drugs
DME MAC L33827
N/A
No
No
No

Medications/Drugs (Outpatient/Part B)
Page 13 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Antiemetics
(injectable) for
Oncology -
Neurokinin 1
Receptor Antagonist
(NK1 RA),
5-hydroxytrypta-mine
Receptor Antagonist
(5HT3 RA), NK1
RA/5HT3 RA
combination
Akynzeo
®
(netupitant and
palonosetron)
injection
Aloxi
®
(palonosetron
hydrochlor-ide)
injection
Cinvanti
®
(aprepitant)
injectable
emulsion
Emend
®
(fosaprepitan)
injection
• Kytril
®
(granisetron)
injection
Sustol
®
(granisetron)
injection
• Zuplenz, Zofran
ODT
®
, and
Zofran
®
(ondansetron)
injection
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Antiemetics for
Oncology
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
No
Adakveo
®
(crizanlizumab-tmca)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Adakveo
®
(Crizanlizumab-
Tmca)
No No No
Beqvez
(fidanacogene
elaparvovec-dzkt)
None UnitedHealthcare
Commercial
Medical Drug
Policy titled
Gene
Therapies for
Hemophilia B
Yes
Refer to
Review at
Launch
(RAL)
No No

Medications/Drugs (Outpatient/Part B)
Page 14 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Bevacizumab
Alymsys
®
(bevacizumab-
maly)
Avastin
®
(bevacizu-mab)
Mvasi
®
(bevacizumab-
Awwb)
Vegzelma
®
(bevacizumab-
adcd)
Zirabev
®
(bevacizumab-
bvzr) – Oncology
Use Only
NGS
L33394 (A52370)
UnitedHealthcare
Commercial
Medical Drug
Policy titled
Oncology
Medication Clinical
Coverage
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
No
Botulinum toxin
Botox
®
(onabotulinum-
toxinA)
Daxxify
®
(daxibotulinum-
toxinA-lanm)
Dysport
®
(abobotulinum-
toxinA)
Myobloc
®
(rimabotulinum-
toxinB)
Xeomin
®
(incobotulinum-
toxinA)
CGS
L33949 (A56472)
First Coast
L33274
(A57715)
NGS
L33646
(A52848)
Noridian
L35170
(A57185)
L35172 (A57186)
Novitas
L38809
(A58423)
Palmetto
L33458
(A56646)
WPS*
L34635
(A57474)
Note: Local Coverage
Determinations
(LCDs)/Local Coverage
Articles (LCAs) exist and
compliance with these
policies is required
where applicable
All states/territories
have LCDs/LCAs
No
No
No
Briumvi
™
(ublituximab-xiiy)
None UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Briumvi
®
(Ublituximab-Xiiy)
No No No
Medications/Drugs (Outpatient/Part B)
Page 15 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
CAR-T Cellular
Therapy
Abecma
®
(idecaptagene
cicleucel)
Breyanzi
®
(lisocabtagene
maraluecel)
Carvykti
™
(ciltacabtagene
autoleucel)
Kymriah
®
(tisagenlecleucel)
Tecartus
®
(brexucabtagene
autoleucel)
Yescarta
®
(axicabtagene
ciloleucel)
None
Optum Clinical
Guidelines titled
Chimeric Antigen
Receptor T-cell
Therapy
No
No
No
Cellular Therapy
Amtagvi
™
(lifeucel)
None
Optum Clinical
Guidelines titled
Tumor-Infiltrating
Lymphocyte (TIL)
Cell Therapy
No
No
No
Colony stimulating
factors
Short acting
Granix
®
(tbo-
filgrastim)
Neupogen
®
(filgrastim)
Nivestym
®
(filgrastim-aafi)
Releuko
®
(filgrastim-ayow)
Zarxio
®
(filgrastim-sndz)
Long acting
Fulphila
®
(pegfilgrastim-
jmdb)
Fylnetra
®
(pegfilgrastim-
pbbk)
Neulasta
®
(pegfilgrastim)
Nyvepria
™
(pegfilgrastim-
apgf)
Rolvedon
™
(eflapegrastim-
xnst)
Palmetto
L37176
(A56748)
(A54682)
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
White Blood Cell
Colony Stimulating
Factors
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
No

Medications/Drugs (Outpatient/Part B)
Page 16 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Stimufend
®
(pegfilgrastim-
fpgk)
Udenyca
®
(pegfilgrastim-
cbqv)
Ziextenzo
®
(pegfilgrastim-
bmez)
Palmetto
L37176
(A56748)
(A54682)
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
White Blood Cell
Colony Stimulating
Factors
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
No
Cosentyx
®
IV
(secukinumab)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Cosentyx
®
(Secukinumab)
No No No
Crysvita
®
(burosumab-twza)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Crysvita
®
(Burosumab-Twza)
No
No
No
Denosumab
• Xgeva
®
• Prolia
®
NGS
L33394 (A52399)
(A52855)
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Denosumab
(Prolia
®
& Xgeva
®
)
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency
Elevidys
®
(delandistrogene
moxeparvovec-rokl)
None UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Elevidys
™
(Delandistrogene
Moxparvovec-Rokl)
No No No
Enjaymo
™
(sutimlimab-jome)
None UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Enjaymo
®
(Sutimlimab-Jome)
No No No

Medications/Drugs (Outpatient/Part B)
Page 17 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Evenity
®
(Romosozumab-
Aqqg)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Evenity
®
(Romosozumab-
Aqqg)
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
No
Entyvio
®
(vedolizumab)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Entyvio
®
(Vedolizumab)
No
No
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency
Erythropoietin for
Cancer Related
Conditions
NCD for Erythropoiesis
Stimulating Agents
(ESAs) in Cancer and
Related Neoplastic
Conditions (110.21)
Note: Local Coverage
Determinations
(LCDs)/Local Coverage
Articles (LCAs) exist and
compliance with these
policies is required where
applicable. These
LCDs/LCAs are available
at
https://www.cms.gov/me
dicare-coverage-
database/new-
search/search.aspx.
N/A No No
No
Erythropoietin for
Non-cancer Related
Conditions
CGS
L34356 (A56462)
Palmetto
L39237
(A58982)
WPS*
L34633
(A56795)
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Erythropoiesis-
Stimulating Agents
No
No
No

Medications/Drugs (Outpatient/Part B)
Page 18 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Evkeeza
®
(Evinacumab-Dgnb)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Evkeeza
®
(Evinacumab-
Dgnb)
No
No
No
Gemcitabine
Infugem
™
(gemcitabine)
None
UnitedHealthcare
Commercial
Medical Drug
Policy titled
Oncology
Medication Clinical
Coverage
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
No
Gene Therapy (ex
vivo)
Casgevy
™
(exagamglogene
autotemcel)
Lenmeldy
™
(atidarsagene
autotemcel)
Lyfgenia
™
(lovotibeglogene
autotemcel)
Skysona
®
(elivaldogene
autotemcel)
Zynteglo
®
(betibeglogene
autotemcel)
None
Optum Clinical
Guidelines titled
Gene Therapy
No No No
Givlaari
®
(givosiran)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Givlaari
®
(Givosiran)
No
No
No
Gonadotropin
Releasing Hormone
Analogs
Leuprolide
Acetate
NGS
L33394 (A52453)
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Gonadotropin
Releasing
Hormone Analogs
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
No

Medications/Drugs (Outpatient/Part B)
Page 19 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Hemgenix
®
(etranacogene
dezaparvovec-drlb)
None
UnitedHealthcare
Commercial
Medical Drug
Policy titled
Gene
Therapies for
Hemophilia B
No
No
No
Infliximab
Avsola
™
(infliximab-axxq)
Inflectra
®
(infliximab-dyyb)
Infliximab
Remicade
®
(infliximab)
Renflexis
®
(infliximab-abda)
Zymfentra
™
(infliximab-dyyb)
NGS
L33394
(A52423)
Palmetto
L35677
(A56432)
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Infliximab (Avsola
®
,
Inflectra
®
,
Remicade
®
, &
Renflexis
®
)
No Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency
Intravenous Immune
Globulin (IVIG)
Refer to the Intravenous
Immune Globulin (IVIG)
table
N/A
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
No
Intravenous iron
therapy for dialysis
patients
NCD for Intravenous
Iron Therapy (110.10)
N/A
No
No
No
Intravenous iron
therapy for non-
dialysis patients
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Intravenous Iron
Replacement
Therapy
(Feraheme
®
,
Injectafer
®
, &
Monoferric
®
)
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
No

Medications/Drugs (Outpatient/Part B)
Page 20 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Intravitreal vascular
endothelial growth
factor (VEGF)
inhibitors
Cimerli
™
(ranibizumab-
eqrn)
Compounded
Avastin
®
(bevacizu-mab)
Lucentis
®
(ranibizumab)
Eylea
®
(aflibercept)
Eylea
®
HD
(aflibercept)
Beovu
®
(brolucizumab-
dbll)
• Byooviz
™
(ranibizumab-
nuna),
• Susvimo
™
(ranibizumab
injection)
• Vabysmo
™
(faricimab-svoa)
NGS
L33394
(A52370, A52451)
Noridian
A53008, A53009
Palmetto
A53387
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Ophthalmologic
Policy: Vascular
Endothelial Growth
Factor (VEGF)
Inhibitors
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
No
Izervay
™
(avacincaptad pegol
intravitreal solution)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Ophthalmologic
Complement
Inhibitors
No
No
No
Kisunla
™
(donanemab-azbt)
NCD for Monoclonal
Antibodies Directed
Against Amyloid for the
Treatment of
Alzheimer's Disease
(AD) 200.3
For payment rules for
NCDs requiring CED,
refer to the
Medicare
Managed Care Manual,
Chapter 4, §10.7.3 –
Payment for Clinical
Studies Approved Under
Coverage with Evidence
Development (CED)
N/A
Yes
Refer to
Review at
Launch
(RAL)
No
No

Medications/Drugs (Outpatient/Part B)
Page 21 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Krystexxa
®
(Pegloticase)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Krystexxa
®
(Pegloticase)
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
Yes
Refer to the
United-
Healthcare
Commercial
Medical Benefit
Drug Policy titled
Maximum
Dosage and
Frequency
Lantidra
™
(donislecel)
None
Optum Clinical
Guidelines titled
Solid Organ
Transplantation
No No No
Leqembi
™
(lecanemab)
NCD for Monoclonal
Antibodies Directed
Against Amyloid for the
Treatment of
Alzheimer's Disease
(AD) (200.3)
For payment rules for
NCDs requiring CED,
refer to the
Medicare
Managed Care Manual,
Chapter 4, §10.7.3 –
Payment for Clinical
Studies Approved Under
Coverage with Evidence
Development (CED)
N/A
No
No
No
Leqvio
®
(inclisiran) None UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Leqvio
®
(Inclisiran)
No Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
No

Medications/Drugs (Outpatient/Part B)
Page 22 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Leucovorin/
Levoleucovorin
Fusilev
®
(levoleuco-vorin)
Khapzory
™
(levoleuco-vorin)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Oncology
Medication Clinical
Coverage
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
No
Luxturna
™
(voretigene
neparvovec-rzyl)
Palmetto
L37863 (A56419)
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Luxturna
®
(Voretigene
Neparvovec-Rzyl)
No No No
Ocrevus
®
(ocrelizumab)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Ocrevus
®
(Ocrelizumab)
No No No
Omvoh
™
(mirikizumab-mrkz)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Omvoh
™
(Mirikizumab-mrkz)
No
No
No
Onpattro
®
(patisiran) None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
RNA-Targeted
Therapies
(Amvuttra
®
and
Onpattro
®
)
No No
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency
Orencia
®
(abatacept)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Orencia
®
(Abatacept)
Injection for
Intravenous
Infusion
No No
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency

Medications/Drugs (Outpatient/Part B)
Page 23 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Oxlumo
™
(lumasiran)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Oxlumo
®
(Lumasiran) and
Rivfloza
™
(Nedosiran)
No
No
No
Primacor
®
(milrinone)
– use in home
setting
Note: There are
safety and efficacy
issue regarding the
use of Milrinone in
the home setting.
Read the LCDs/
LCAs before
authorizing.
DME MAC LCD for
External Infusion
Pumps
L33794
All states/territories
have LCDs/LCAs
No No No
Qalsody
™
(tofersen)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Qalsody
®
(Tofersen)
No No Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency
Radicava
®
(edaravone)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Radicava
®
(Edaravone)
No
No
No
Rituximab
Riabni
™
(rituximab-aarx)
Rituxan
®
(rituximab)
Ruxience
®
(rituximab-pvvr)
Truxima
®
(rituximab-abbs)
for non-chemo-
therapeutic
indications
CGS
L38920
(A58582)
L38268
(A57160)
NGS
L39297
(A59101)
Palmetto
L35026
(A56380)
WPS*
A55639
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Rituximab
(Riabni
™
, Rituxan
®
,
Ruxience
®
, &
Truxima
®
)
No Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency

Medications/Drugs (Outpatient/Part B)
Page 24 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Rituximab
Riabni
™
(rituximab-aarx)
Rituxan
®
(rituximab)
Rituxan Hycela
®
(rituximab and
hyaluronic-dase)
Ruxience
®
(rituxi
mab-pvvr)
Truxima
®
(rituximab-abbs)
for chemo-
therapeutic
indications
NGS
L39297 (A59101)
Palmetto
L35026
(A56380)
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Oncology
Medication Clinical
Coverage
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency
Rivfloza
™
(nedosiran) None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Oxlumo
®
(Lumasiran) and
Rivfloza
™
(Nedosiran)
No No No
Reblozyl
®
(luspatercept-aamt)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Reblozyl
®
(Luspatercept-
Aamt)
No
No
No
Roctavian
™
(valoctocogene
roxaparvovec-rvox)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Roctavian
™
(Valoctocogene
Roxaparvovec-
Rvox)
No
No
No
Ryplazim
®
(plasminogen,
human-tvmh)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Ryplazim
®
(Plasminogen,
Human-Tvmh)
No No No

Medications/Drugs (Outpatient/Part B)
Page 25 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Rystiggo
®
(rozanolixizumab-
noli)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Neonatal Fc
Receptor Blockers
(Vyvgart
®
, Vyvgart
®
Hytrulo, &
Rystiggo
®
)
No
No
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency
Saphnelo
™
(anifrolumab-fnia)
None UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Saphnelo
®
(Anifrolumab-Fnia)
No Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
No
Skyrizi
®
(Risankizumab-rzaa)
None UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Skyrizi
®
(Risankizumab-
Rzaa)
No No No
Sodium hyaluronate
injections for
osteoarthritis of
knee
NGS
L33394 (A52420)
Palmetto
L39260
(A59030)
WPS*
L39529
NGS
L33394
(A52420)
Palmetto
L39260
(A59030)
WPS*
L39529
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Sodium
Hyaluronate
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
No
Soliris
®
(eculizumab)
NGS
L33394 (A54548)
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Complement
Inhibitors (Soliris
®
& Ultomiris
®
)
No
No
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum

Medications/Drugs (Outpatient/Part B)
Page 26 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Soliris
®
(eculizumab)
NGS
L33394 (A54548)
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Complement
Inhibitors (Soliris
®
& Ultomiris
®
)
No
No
Dosage and
Frequency
Spevigo
®
(spesolimab-sbzo)
None UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Spevigo
®
(Spesolimab-Sbzo)
No No
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency
Spinraza
®
(nusinersen)
Noridian
A58578,
A58579
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Spinraza
®
(Nusinersen)
No
No
No
Subcutaneous
Immune Globulin
(SCIG)
CGS
L33794 (A52507)
Noridian
L33794
(A52507)
WPS*
L34771
(A57554)
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Immune Globulin
(IVIG and SCIG)
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
No
Syfovre
™
(pegcetacoplan
injection)
None UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Ophthalmologic
Complement
Inhibitors
No No
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency

Medications/Drugs (Outpatient/Part B)
Page 27 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Tepezza
®
(teprotumumab-trbw)
First Coast
L34007 (A57778)
Novitas
L35093
(A56786)
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Tepezza
®
(Teprotumumab-
Trbw)
No
No
No
Teplizumab
• Tzield
™
(teplizumab-
mzwv)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Tzield
®
(Teplizumab-
Mzwv)
No
No
No
Testopel
®
(testosterone pellet)
(CPT code 11980
and HCPCS code
J3490)
Refer to the FDA
Warning
Letter/Notice for
Testopel
®
(testosterone pellet).
Noridian
L36569 (A57616)
L36538 (A57615)
Palmetto
L39086
(A58828)
UnitedHealthcare
Commercial Medical
Benefit Drug Policy
titled
Testosterone
Replacement or
Supplementation
Therapy
No
No
No
Tezspire
™
(tezepelumab-ekko)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Tezspire
®
(Tezepelumab-
Ekko)
No
No
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency
Trastuzumab
Herceptin
Hylecta
™
(trastuzumab and
hyaluronidase-
oysk)
Herceptin
®
(trastuzumab)
Herzuma
®
(trastuzumab-
pkrb)
Kanjinti
®
(trastuzumab-
anns)
Ogivri
®
(trastuzumab-
dkst)
First Coast
L34026
(A56660)
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Oncology
Medication Clinical
Coverage
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency

Medications/Drugs (Outpatient/Part B)
Page 28 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Ontruzant
®
(trastuzumab-
dttb)
Trazimera
®
(trastuzumab-
qyyp)
First Coast
L34026 (A56660)
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Oncology
Medication Clinical
Coverage
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency
Ultomiris
®
(ravulizumab)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Complement
Inhibitors (Soliris
®
& Ultomiris
®
)
No No Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency
Uplizna
®
(inebilizumab-cdon)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Uplizna
®
(Inebilizumab-
Cdon)
No
No
No
Vyjuvek
™
(beremagene
geperpavec-svdt)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Vyjuvek
®
(Beramagene
Geperpavec-Svdt)
No
No
No
Vyepti
®
(Eptinezumab- Jjmr)
None UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Vyepti
®
(Eptinezumab-
Jjmr)
No
Yes
Refer to the
United-
Healthcare
Medicare
Advantage
Drug Policy
titled
Medicare
Part B Step
Therapy
Programs
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency

Medications/Drugs (Outpatient/Part B)
Page 29 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Other Examples of Specific Drugs/Medications
Accessed July 1, 2024
*Also refer to the MACs with corresponding States/Territories.
Drug/
Medication
NCD, Medicare
Manual, LCDs/LCAs*
Default Policy for
States Without
LCDs/LCAs
Review at
Launch
(RAL)
Step
Therapy
Maximum
Dosage and
Frequency*
Vyvgart
™
(efgartigimod)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Neonatal Fc
Receptor Blockers
(Vyvgart
®
,
Vyvgart
®
Hytrulo, &
Rystiggo
®
)
No
No
Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency
Vyvgart
®
Hytrulo
(efgartigimod alfa
and hyaluronidase-
qvfc)
None UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Neonatal Fc
Receptor Blockers
(Vyvgart
®
, Vyvgart
®
Hytrulo, &
Rystiggo
®
)
No
No Yes
Refer to the
United-
Healthcare
Commercial
Medical
Benefit Drug
Policy titled
Maximum
Dosage and
Frequency
Zolgensma
®
(onasemnogene
abeparvovec-xioi)
None
UnitedHealthcare
Commercial
Medical Benefit
Drug Policy titled
Zolgensma
®
(Onasemnogene
Abeparvovec-Xioi)
No
No
No
Back to Guidelines
Medicare Administrative Contractors (MACs) with Corresponding States/Territories
MACs
States/Territories
CGS
KY, OH
First Coast
FL, PR, VI
NGS
CT, IL, MA, ME, MN, NH, NY, RI, VT, WI
Noridian
AK, AS, AZ, CA, GU, HI, ID, MT, ND, NV, Northern
Mariana Islands, OR, SD, UT, WA, WY
Novitas AR, AR, CO, DC, DE, LA, MD, MS, NJ, NM, OK, PA, TX
Palmetto AL, GA, NC, SC, TN, VA, WV
WPS* IA, IN, KS, MI, MO, NE
*
Note
: Wisconsin Physicians Service Insurance Corporation Contract Number 05901 - applies only to WPS Legacy
Mutual of Omaha MAC A Providers
DME MACs
States/Territories
CGS (17013)
IL, IN, KY, MI, MN, OH, WI
CGS (18003)
AL, AR, CO, FL, GA, LA, MS, NC, NM, OK, PR, SC, TN,
TX, VA, VI, WV
Noridian (16013) CT, DC, DE, MA, MD, ME, NH, NJ, NY, PA, RI, VT

Medications/Drugs (Outpatient/Part B)
Page 30 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Medicare Administrative Contractors (MACs) with Corresponding States/Territories
DME MACs
States/Territories
Noridian (19003)
AK, AS, AZ, CA, GU, HI, IA, ID, KS, MO, MP, MT, ND,
NE, NV, OR, SD, UT, WA, WY
Back to Guidelines
Intravenous Immune Globulin (IVIG)
Accessed July 1, 2024
LCD/LCA ID LCD/LCA Title Contractor Type Contractor Name
Applicable
States/Territories
L35891
(A56779)
Intravenous Immune
Globulin
Part A and B MAC
CGS Administrators,
LLC
KY, OH
L34007
(A57778)
Immune Globulin
Part A and B MAC
First Coast Service
Options, Inc.
Fl, PR, VI
L39314
(A59105)
Off-Label Use of
Intravenous Immune
Globulin (IVIG)
Part A and B MAC
National Government
Services, Inc.
CT, IL, MA, ME MN, NH, NY,
RI, VT, WI
L34074
(A57194)
Immune Globulin
Intravenous (IVIg)
Part A and B MAC
Noridian Healthcare
Solutions, LLC
AK, AZ, ID, MT, ND, OR, SD,
UT, WA, WY
L34314
(A57187)
Immune Globulin
Intravenous (IVIg)
Part A and B MAC
Noridian Healthcare
Solutions, LLC
CA, AS, GU, HI, MP, NV
L35093
(A56786)
Immune Globulin
Part A and B MAC
Novitas Solutions, Inc.
CO, NM, OK, TX, AR, LA, MS,
DE, DC, MD, NJ, PA
L34580
(A56718)
Intravenous
Immunoglobulin (IVIG)
Part A and B MAC
Palmetto GBA
AL, GA, NC, SC, TN, VA, WV
L34771
(A57554)
Immune Globulins
Part A and B MAC
*Wisconsin Physicians
Service Insurance
Corporation
IA, IN, KS, MI, MO, NE
L33610
(A52509)
Intravenous Immune
Globulin
DME MAC
Noridian Healthcare
Solutions, LLC (16013)
CT, DC, DE, MA, MD, ME,
NH, NJ, NY, PA, RI, VT
CGS Administrators,
LLC (18003)
AL, AR, CO, FL, GA, LA, MS,
NC, NM, OK, PR, SC, TN, TX,
VA, VI, WV
Noridian Healthcare
Solutions, LLC (19003)
AK, AS, AZ, CA, GU, HI, IA,
ID, KS, MO, MT, ND, NE, MP,
NV, OR, SD, UT, WA, WY
CGS Administrators
(17013)
IL, IN, KY, MI, MN, OH, WI
Back to Guidelines
Policy History/Revision Information
Date
Summary of Changes
08/01/2024
Coverage Guidelines
Other Specific Medications
For Oncology Medications
Added reference link to the Medicare Benefit Policy Manual, Chapter 15, §50.4:
Reasonableness and Necessity
For Non-Oncologic Medications
Replaced instruction to “refer to the relevant UnitedHealthcare Commercial Medical Benefit
Drug Policy for all other drugs or biologicals not listed in this Medicare Advantage Coverage
Summary, for which there are no applicable National Coverage Determinations (NCDs), Local
Coverage Determinations (LCDs), or Local Coverage Articles (LCAs)” with “refer to the relevant

Medications/Drugs (Outpatient/Part B)
Page 31 of 31
UnitedHealthcare Medicare Advantage Coverage Summary
Effective 08/01/2024
Proprietary Information of UnitedHealthcare. Copyright 2024 United HealthCare Services, Inc.
Date
Summary of Changes
UnitedHealthcare Commercial Medical Benefit Drug Policy for all other drugs or biologicals
(non-oncologic) not listed in this Medicare Advantage Coverage Summary, for which there are
no applicable NCDs, LCDs, or LCAs”
Other Examples of Specific Drugs/Medications
Added coverage guidelines for Kisunla
™
(donanemab-azbt) to indicate a pre-service review
[Review at Launch (RAL)] is required
Revised coverage guidelines for Beqvez (fidanacogene elaparvovec-dzkt); added instruction to
refer to the UnitedHealthcare Commercial Medical Benefit Drug Policy titled Gene Therapies for
Hemophilia B
Supporting Information
Updated list of applicable LCDs/LCAs to reflect the most current information
Administrative
Archived previous policy version MCS057.31
Instructions for Use
This information is being distributed to you for personal reference. The information belongs to UnitedHealthcare and
unauthorized copying, use, and distribution are prohibited. This information is intended to serve only as a general
reference resource and is not intended to address every aspect of a clinical situation. Physicians and patients should not
rely on this information in making health care decisions. Physicians and patients must exercise their independent clinical
discretion and judgment in determining care. Each benefit plan contains its own specific provisions for coverage,
limitations, and exclusions as stated in the Member’s Evidence of Coverage (EOC)/Summary of Benefits (SB). If there is a
discrepancy between this policy and the member’s EOC/SB, the member’s EOC/SB provision will govern. The information
contained in this document is believed to be current as of the date noted.
The benefit information in this Coverage Summary is based on existing national coverage policy; however, Local
Coverage Determinations (LCDs) may exist and compliance with these policies are required where applicable.
UnitedHealthcare follows Medicare coverage guidelines found in statutes, regulations, NCDs, and LCDs to determine
coverage. The clinical coverage criteria governing the items or services in this coverage summary have not been fully
established in applicable Medicare guidelines because there is an absence of any applicable Medicare statutes,
regulations, NCDs, or LCDs setting forth coverage criteria and/or the applicable NCDs or LCDs include flexibility that
explicitly allows for coverage in circumstances beyond the specific indications that are listed in an NCD or LCD. As a
result, UnitedHealthcare applies internal coverage criteria in the UnitedHealthcare commercial policies referenced in this
coverage summary. The coverage criteria in these commercial policies was developed through an evaluation of the
current relevant clinical evidence in acceptable clinical literature and/or widely used treatment guidelines.
UnitedHealthcare evaluated the evidence to determine whether it was of sufficient quality to support a finding that the
items or services discussed in the policy might, under certain circumstances, be reasonable and necessary for the
diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member.
CPT
®
is a registered trademark of the American Medical Association.
