
Leapfrog Hospital Survey
Hard Copy
QUESTIONS & REPORTING PERIODS
ENDNOTES
MEASURE SPECIFICATIONS
FAQS

2 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Table of Contents
Table of Contents .............................................................................................................................. 2
Welcome to the 2023 Leapfrog Hospital Survey........................................................................................... 8
Important Notes About the 2023 Survey ............................................................................................ 8
Overview of the 2023 Leapfrog Hospital Survey .............................................................................. 10
Pre-Submission Checklist ................................................................................................................ 13
Instructions for Submitting a Leapfrog Hospital Survey ................................................................... 14
Verifying Submission ................................................................................................................... 15
Updating or Correcting a Previously Submitted Leapfrog Hospital Survey ................................ 15
Updating a Survey after Receiving a Help Desk Email ............................................................... 16
Updating a Survey following Virtual On-Site Data Verification ................................................... 16
Making General Updates (for hospitals that have not received a Help Desk email) .................. 16
Deadlines ......................................................................................................................................... 18
Deadlines for the 2023 Leapfrog Hospital Survey ...................................................................... 18
Deadlines for Vermont Oxford Network Data .............................................................................. 18
Deadlines to Join Leapfrog’s NHSN Group ................................................................................ 18
Deadlines Related to the Hospital Safety Grade ........................................................................ 18
Technical Assistance........................................................................................................................ 19
Help Desk .................................................................................................................................... 19
Leapfrog Hospital Survey Webinar Series .................................................................................. 19
Reporting Periods ............................................................................................................................. 20
Page Intentionally Left Blank ...................................................................................................................... 23
HOSPITAL PROFILE .................................................................................................................................. 24
2023 Hospital Profile ............................................................................................................................... 25
Hospital Profile ................................................................................................................................. 26
Facility Information ...................................................................................................................... 26
Demographic Information ............................................................................................................ 26
Contact Information ..................................................................................................................... 27
Page Intentionally Left Blank ...................................................................................................................... 29
SECTION 1: BASIC HOSPITAL INFORMATION ....................................................................................... 30
Section 1: 2023 Basic Hospital Information ............................................................................................. 31
1A: Basic Hospital Information ......................................................................................................... 32
Additional Question (Optional – Fact Finding Only) .................................................................... 33
1B
: Person-Centered Care: Billing Ethics and Health Equity .......................................................... 34
Billing Ethics ................................................................................................................................ 34
Health Equity ............................................................................................................................... 35
1C: Informed Consent ...................................................................................................................... 38

3 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 1: 2023 Basic Hospital Information Reference Information ........................................................ 42
What’s New in the 2023 Survey .................................................................................................. 42
Change Summary Since Release ............................................................................................... 43
Basic Hospital Information Measure Specifications ......................................................................... 44
Basic Hospital Information Frequently Asked Questions (FAQs) .................................................... 45
Basic Hospital Information .......................................................................................................... 45
Billing Ethics ................................................................................................................................ 45
Health Equity ............................................................................................................................... 46
Informed Consent ........................................................................................................................ 48
Page Intentionally Left Blank ...................................................................................................................... 51
SECTION 2: MEDICATION SAFETY ......................................................................................................... 52
Section 2: 2023 Medication Safety .......................................................................................................... 53
2A: Computerized Physician Order Entry (CPOE) ........................................................................... 54
2B: EHR Application Information ...................................................................................................... 56
2C: Bar Code Medication Administration (BCMA) ........................................................................... 57
Additional Questions (Optional – Fact Finding Only) .................................................................. 59
2D: Medication Reconciliation .......................................................................................................... 61
Section 2: 2023 Medication Safety Reference Information ..................................................................... 64
What’s New in the 2023 Survey .................................................................................................. 64
Change Summary Since Release ............................................................................................... 64
Medication Safety Measure Specifications ...................................................................................... 65
Computerized Physician Order Entry (CPOE) ............................................................................ 65
Bar Code Medication Administration (BCMA) ............................................................................. 67
Medication Reconciliation ........................................................................................................... 69
Medication Safety Frequently Asked Questions (FAQs) .................................................................. 75
Computerized Physician Order Entry (CPOE) ............................................................................ 75
Bar Code Medication Administration (BCMA) ............................................................................. 76
Medication Reconciliation ........................................................................................................... 79
Page Intentionally Left Blank ...................................................................................................................... 82
SECTION 3: ADULT AND PEDIATRIC COMPLEX SURGERY ................................................................. 83
Section 3: 2023 Adult and Pediatric Complex Surgery ........................................................................... 84
3A: Hospital and Surgeon Volume ................................................................................................... 85
3B: Surgical Appropriateness ........................................................................................................... 89
3C: Safe Surgery Checklist for Adult and Pediatric Complex Surgery ............................................ 91
Section 3: 2023 Adult and Pediatric Complex Surgery Reference Information ...................................... 94
What’s New in the 2023 Survey .................................................................................................. 94
Change Summary Since Release ............................................................................................... 95
Adult and Pediatric Complex Surgery Measure Specifications ........................................................ 96

4 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Hospital and Surgeon Volume .................................................................................................... 96
Safe Surgery Checklist for Adult and Pediatric Complex Surgery Measure Specifications...... 134
Adult and Pediatric Complex Surgery Frequently Asked Questions (FAQs) ................................. 136
General Questions .................................................................................................................... 136
Hospital Volume ........................................................................................................................ 136
Surgeon Volume ....................................................................................................................... 137
Surgical Appropriateness .......................................................................................................... 138
Safe Surgery Checklist for Adult and Pediatric Complex Surgery ............................................ 139
Page Intentionally Left Blank .................................................................................................................... 140
SECTION 4: MATERNITY CARE ............................................................................................................. 141
Section 4: 2023 Maternity Care ............................................................................................................. 142
4A: Maternity Care Volume and Services ...................................................................................... 143
Maternity Care Volume ............................................................................................................. 143
Maternity Care Services ............................................................................................................ 144
4B: Elective Deliveries.................................................................................................................... 145
4C: Cesarean Birth ......................................................................................................................... 146
Additional Questions (Optional – Fact Finding Only) ................................................................ 147
4D: Episiotomy ............................................................................................................................... 148
4E: Process Measures of Quality ................................................................................................... 149
Newborn Bilirubin Screening Prior to Discharge ....................................................................... 149
Appropriate DVT Prophylaxis in Women Undergoing Cesarean Delivery ................................ 150
4F: High-Risk Deliveries ................................................................................................................. 151
Neonatal Intensive Care Unit(s) – Volume ................................................................................ 152
Neonatal Intensive Care Unit(s) – National Performance Measurement .................................. 152
Section 4: 2023 Maternity Care Reference Information ........................................................................ 155
What’s New in the 2023 Survey ................................................................................................ 155
Change Summary Since Release ............................................................................................. 155
Maternity Care Measure Specifications ......................................................................................... 156
Maternity Care Volume and Services ....................................................................................... 156
Elective Deliveries ..................................................................................................................... 157
Cesarean Birth .......................................................................................................................... 159
Episiotomy ................................................................................................................................. 163
Process Measures of Quality .................................................................................................... 164
High-Risk Deliveries .................................................................................................................. 166
Maternity Care Frequently Asked Questions (FAQs) ..................................................................... 168
Page Intentionally Left Blank .................................................................................................................... 169
SECTION 5: ICU PHYSICIAN STAFFING (IPS) ...................................................................................... 170
Section 5: 2023 ICU Physician Staffing (IPS) Standard ........................................................................ 171

5 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
5: ICU Physician Staffing (IPS) ...................................................................................................... 172
Section 5: 2023 ICU Physician Staffing (IPS) Reference Information................................................... 177
What’s New in the 2023 Survey ................................................................................................ 177
Change Summary Since Release ............................................................................................. 177
ICU Physician Staffing (IPS) Frequently Asked Questions (FAQs) ............................................... 178
General Questions .................................................................................................................... 178
Certification ............................................................................................................................... 178
Telemedicine ............................................................................................................................. 179
Response Time ......................................................................................................................... 179
Rounding ................................................................................................................................... 179
Page Intentionally Left Blank .................................................................................................................... 181
SECTION 6: PATIENT SAFETY PRACTICES ......................................................................................... 182
Section 6: 2023 Patient Safety Practices .............................................................................................. 183
Instructions for Reporting on Section 6: Patient Safety Practices ................................................. 185
6A: NQF Safe Practice #1 – Culture of Safety Leadership Structures and Systems .................... 186
6B: NQF Safe Practice #2 – Culture Measurement, Feedback, and Intervention ......................... 188
6C: Nursing Workforce ................................................................................................................... 190
Nurse Staffing and Skill Level ................................................................................................... 190
NQF Safe Practice #9 – Nursing Workforce ............................................................................. 192
6D: Hand Hygiene .......................................................................................................................... 194
Section 6: 2023 Patient Safety Practices Reference Information ......................................................... 200
What’s New in the 2023 Survey ................................................................................................ 200
Change Summary Since Release ............................................................................................. 200
Patient Safety Practices Measure Specifications ........................................................................... 201
Nursing Workforce .................................................................................................................... 201
Hand Hygiene............................................................................................................................ 205
Patient Safety Practices Frequently Asked Questions (FAQs) ...................................................... 213
General Questions .................................................................................................................... 213
NQF Safe Practice #1 – Leadership Structures and Systems .................................................. 215
NQF Safe Practice #2 – Culture Measurement, Feedback, and Intervention .......................... 217
Nurse Staffing and Skill Level ................................................................................................... 219
NQF Safe Practice #9 – Nursing Workforce ............................................................................. 220
Hand Hygiene............................................................................................................................ 220
Page Intentionally Left Blank .................................................................................................................... 224
SECTION 7: MANAGING SERIOUS ERRORS ........................................................................................ 225
Section 7: 2023 Managing Serious Errors............................................................................................. 226
7A: Never Events ........................................................................................................................... 227
7B: Healthcare-Associated Infections ............................................................................................ 228

6 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 7: 2023 Managing Serious Errors Reference Information ........................................................ 230
What’s New in the 2023 Survey ................................................................................................ 230
Change Summary Since Release ............................................................................................. 230
Healthcare-Associated Infections Measure Specifications ............................................................ 231
Deadlines and Reporting Periods ............................................................................................. 232
Never Events Frequently Asked Questions (FAQs) ....................................................................... 233
Page Intentionally Left Blank .................................................................................................................... 235
SECTION 8: PEDIATRIC CARE ............................................................................................................... 236
Section 8: 2023 Pediatric Care .............................................................................................................. 237
8A: Patient Experience (CAHPS Child Hospital Survey) ............................................................... 238
8B: Pediatric Computed Tomography (CT) Radiation Dose .......................................................... 240
Additional Questions (Optional – Fact Finding Only) ................................................................ 242
Section 8: 2023 Pediatric Care Reference Information ......................................................................... 245
What’s New in the 2023 Survey ................................................................................................ 245
Change Summary Since Release ............................................................................................. 245
Pediatric Care Measure Specifications .......................................................................................... 246
Patient Experience (CAHPS Child Hospital Survey)................................................................. 246
Pediatric Computed Tomography (CT) Radiation Dose ........................................................... 247
Pediatric Care Frequently Asked Questions (FAQs) ..................................................................... 253
Patient Experience (CAHPS Child Hospital Survey)................................................................. 253
Pediatric Computed Tomography (CT) Radiation Dose ........................................................... 253
Page Intentionally Left Blank .................................................................................................................... 255
SECTION 9: OUTPATIENT PROCEDURES ............................................................................................ 256
Section 9: 2023 Outpatient Procedures ................................................................................................ 257
9A: Basic Outpatient Department Information ............................................................................... 258
9B: Medical, Surgical, and Clinical Staff ........................................................................................ 260
9C: Volume of Procedures ............................................................................................................. 262
Gastroenterology ....................................................................................................................... 265
General Surgery ........................................................................................................................ 266
Ophthalmology .......................................................................................................................... 267
Orthopedics ............................................................................................................................... 268
Otolaryngology .......................................................................................................................... 269
Urology ...................................................................................................................................... 270
Neurological Surgery ................................................................................................................. 271
Obstetrics and Gynecology ....................................................................................................... 271
Plastic and Reconstructive Surgery .......................................................................................... 272
9D: Safety of Procedures ............................................................................................................... 274
Patient Follow-up ...................................................................................................................... 274

7 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Patient Selection ....................................................................................................................... 275
Safe Surgery Checklist for Adult and Pediatric Outpatient Procedures .................................... 276
9E: Medication Safety for Outpatient Procedures .......................................................................... 278
9F: Patient Experience (OAS CAHPS) .......................................................................................... 280
Section 9: 2023 Outpatient Procedures Reference Information............................................................ 283
What’s New in the 2023 Survey ................................................................................................ 283
Change Summary Since Release ............................................................................................. 283
Basic Outpatient Department Information Frequently Asked Questions (FAQs) ........................... 285
Medical, Surgical, and Clinical Staff Frequently Asked Questions (FAQs) .................................... 286
Volume of Procedures Measure Specifications ............................................................................. 287
Gastroenterology Measure Specifications ................................................................................ 290
General Surgery Measure Specifications ................................................................................. 290
Ophthalmology Measure Specifications .................................................................................... 290
Orthopedic Measure Specifications .......................................................................................... 291
Otolaryngology Measure Specifications .................................................................................... 291
Urology Measure Specifications................................................................................................ 292
Neurological Surgery Measure Specifications .......................................................................... 292
Obstetrics and Gynecology Measure Specifications ................................................................ 292
Plastic and Reconstructive Surgery Measure Specifications .................................................... 292
Volume of Procedures Frequently Asked Questions (FAQs)......................................................... 293
Safety of Procedures Measure Specifications ............................................................................... 294
Patient Follow-up ...................................................................................................................... 294
Safe Surgery Checklist for Adult and Pediatric Outpatient Procedures .................................... 295
Safety of Procedures Frequently Asked Questions (FAQs)........................................................... 297
General Questions .................................................................................................................... 297
Patient Follow-up ...................................................................................................................... 297
Patient Selection ....................................................................................................................... 297
Safe Surgery Checklist for Adult and Pediatric Outpatient Procedures .................................... 297
Medication Safety for Outpatient Procedures Measure Specifications .......................................... 299
Medication Safety for Outpatient Procedures Frequently Asked Questions (FAQs) ..................... 303
Patient Experience (OAS CAHPS) Measure Specifications .......................................................... 304
Patient Experience (OAS CAHPS) Frequently Asked Questions (FAQs) ..................................... 307
Endnotes ................................................................................................................................................... 308

2023 Leapfrog Hospital Survey – Hard Copy General Information
8 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Welcome to the 2023 Leapfrog Hospital Survey
https://leapfroggroup.org/hospital
Important Notes About the 2023 Survey
1. The Leapfrog Hospital Survey webpages are located at https://leapfroggroup.org/hospital
. Please
bookmark this URL.
2. Note the word “hospital” used throughout this Survey refers to an individual hospital. If your hospital is
part of a multi-hospital health care system or a multi-campus hospital, you will need to complete the
Survey for each individual hospital. Please refer to
Leapfrog’s Multi-Campus Hospital Reporting
Policy.
3. To submit a Survey via the Online Hospital Survey Tool, hospitals are required to complete and affirm
the following six sections: Section 1: Basic Hospital Information, Section 2: Medication Safety,
Section 4: Maternity Care, Section 5: ICU Physician Staffing (IPS), Section 6: Patient Safety
Practices, and Section 7: Managing Serious Errors. However, hospitals are urged to submit all
sections of the Survey and can indicate within a section if a measure does not apply. Hospitals that
would like to be eligible for Top Hospital
must submit all sections of the Survey.
4. Adult and general hospitals that indicate they have a CPOE system in at least one inpatient unit are
asked to demonstrate, via a test, that the inpatient CPOE system can alert prescribers to at least 60%
of frequent serious medication errors known to cause harm to patients. Hospitals will have access to
the CPOE Evaluation Tool immediately after completing the Hospital Profile in the Online Hospital
Survey Tool. Hospitals cannot submit the Survey, including results from the Adult Inpatient CPOE
Test until all the following Survey sections have been completed and affirmed: Section 1: Basic
Hospital Information, Section 2: Medication Safety, Section 4: Maternity Care, Section 5: ICU
Physician Staffing (IPS), Section 6: Patient Safety Practices, and Section 7: Managing Serious Errors.
Hospitals are urged to ensure that the Adult Inpatient CPOE Test is submitted along with the Survey
(i.e., in the same month) to meet the deadlines for the Leapfrog Hospital Survey and Leapfrog’s other
programs such as Top Hospital and the Leapfrog Hospital Safety Grade.
5. Adult and pediatric hospitals reporting on Section 7B: Healthcare-Associated Infections are required
to join Leapfrog’s NHSN Group. Information about teaching status will also be pulled directly from
NHSN. More information, including important deadlines, is available on the
Join NHSN Group
webpage.
6. Leapfrog Hospital Survey Results will be available on the Hospital Details Page beginning July 12
and publicly reported on the public reporting website
on July 25 for hospitals that submit a Survey by
the June 30 Submission Deadline. After July, the Hospital Details Page and public reporting website
will be refreshed monthly within the first five (5) business days of each month to reflect Surveys
submitted or resubmitted between July 1 and November 30 and previously submitted Surveys that
were corrected between December 1 and January 31. Survey Results are frozen from February to
July 25.
7. All questions regarding the Leapfrog Hospital Survey should be submitted to the Help Desk at
https://leapfroghelpdesk.zendesk.com
. Questions submitted to the Help Desk will receive a response
within 1-2 business days (see Help Desk Holiday Schedule for planned closures).
8. For hospitals that would like Leapfrog Hospital Survey Results included in their Leapfrog Hospital
Safety Grade, please visit the “For Hospitals” section of the Hospital Safety Grade website
for
important information on Data Snapshot Dates. A Leapfrog Hospital Survey must be submitted by the
Data Snapshot Date for Survey data to be used in the Hospital Safety Grade.
9. Leapfrog is committed to verifying the accuracy of Leapfrog Hospital Survey Results. Please review
the information on the Data Accuracy webpage
.

2023 Leapfrog Hospital Survey – Hard Copy General Information
9 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
10. The Submission Deadline for the 2023 Leapfrog Hospital Survey is June 30, 2023, and the Late
Submission and Performance Update Deadline is November 30, 2023. Hospitals that do not submit a
Survey or CPOE Evaluation Tool (adult and general hospitals only) before 11:59 pm Eastern Time on
November 30, 2023, will have to wait until the launch of the 2024 Leapfrog Hospital Survey on April
1, 2024 to submit a Survey.

2023 Leapfrog Hospital Survey – Hard Copy General Information
10 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Overview of the 2023 Leapfrog Hospital Survey
The Leapfrog Hospital Survey is divided into nine sections and the Hospital Profile. A description of each
section is listed below. For a more detailed overview of the 2023 Leapfrog Hospital Survey, including a
crosswalk of nationally endorsed measures and a description of how measures are publicly reported, visit
the Survey Overview webpage
.
Section
Number
Section
Title
Brief Description
Hospital
Profile
The Hospital Profile includes questions about demographic and contact
information. The Profile can be accessed and updated anytime throughout
the year via the Survey Dashboard after logging into the
Online Hospital
Survey Tool.
1
Basic
Hospital
Information
Section 1 includes questions about your hospital’s bed size, admissions,
ICU operations, and teaching status. This section also includes questions
on billing practices and health equity. In 2023, the subsection on hospital
information (1A) includes a new optional fact-finding question regarding your
hospital’s environmental services that will not be scored or publicly reported.
The subsection on informed consent (1C) will be scored and publicly
reported.
2
Medication
Safety
Section 2 includes questions about your hospital’s use of CPOE and BCMA,
and (for adult and general hospitals) questions about your hospital’s
medication reconciliation process. The subsection on your hospital’s EHR
application (2B) is only applicable to adult and general hospitals and will not
be scored or publicly reported. In 2023, the subsection on BCMA (2C)
includes new optional fact-finding questions regarding your hospital’s use of
BCMA in pre-op units, PACUs, and the emergency department, and will not
be scored or publicly reported.
3
Adult and
Pediatric
Complex
Surgery
Section 3 includes questions about your hospital volume and process for
privileging surgeons for eleven high-risk procedures, outcomes for mitral
valve repair and replacement, and participation in The Society of Thoracic
Surgeon’s Congenital Heart Surgery Database for hospitals that perform the
Norwood procedure. This section also includes questions about surgical
appropriateness and the implementation of a safe surgery checklist.
4
Maternity
Care
Section 4 includes questions about elective delivery, cesarean birth,
episiotomy, newborn bilirubin screening, and DVT prophylaxis for women
undergoing cesarean delivery. The section also includes questions about
high-risk deliveries, including volume and outcomes. In 2023, the subsection
on maternity care volume (4A) includes new questions about maternity care
services that will be publicly reported. The subsection on cesarean birth
(4C) includes new optional fact-finding questions about cesarean births
stratified by racial and ethnic categories that will not be scored or publicly
reported.
5
ICU
Physician
Staffing
(IPS)
Section 5 includes questions about the management of critical care patients
and the staffing structure of your hospital’s pediatric and adult general
medical and/or surgical ICUs and neuro ICUs.

2023 Leapfrog Hospital Survey – Hard Copy General Information
11 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section
Number
Section
Title
Brief Description
6
Patient
Safety
Practices
Section 6 includes questions about your hospital’s adherence to two
National Quality Forum-endorsed Safe Practices, questions about your
hospital’s nurse staffing and skill mix, and questions about hand hygiene
practices. In 2023, the subsection on nurse staffing and skill mix (6C) will be
scored and publicly reported.
7
Managing
Serious
Errors
Section 7 includes questions about your hospital’s response to Never
Events. In addition, Leapfrog collects information via its NHSN Group about
five healthcare-associated infections (CLABSI, CAUTI, MRSA, C. diff., and
SSI: Colon). Hospitals reporting on Section 7B: Healthcare-Associated
Infections are required to join Leapfrog’s NHSN Group. Important
information and deadlines are available on the Join NHSN Group webpage
.
8
Pediatric
Care
Section 8 includes questions about patient experience (CAHPS Child
Hospital Survey) and Computed Tomography (CT) radiation dose for
pediatric patients. The subsection on CT dose (8B) includes a set of
optional fact-finding questions that will not be scored or publicly reported in
2023.
9
Outpatient
Procedures
Section 9 includes questions about the volume and safety of same-day
procedures performed in hospital outpatient departments, as well as the
experience of patients who had a same-day surgery performed.
Section 1: Basic Hospital Information, Section 2: Medication Safety, Section 4: Maternity Care, Section 5:
ICU Physician Staffing (IPS), Section 6: Patient Safety Practices, and Section 7: Managing Serious Errors
are required to submit a Survey via the Online Hospital Survey Tool. Hospitals are strongly urged to
submit all sections of the Leapfrog Hospital Survey and can indicate within a section if a measure does
not apply.
The hard copy of the Survey and the Online Hospital Survey Tool are organized in the same format for all
nine sections:
• General information about The Leapfrog Group’s standard (included in the hard copy only).
• Reporting periods to provide hospitals with specific periods of time for each set of questions.
• Survey questions which may include references to endnotes or FAQs. The Survey questions
and endnotes match the Online Hospital Survey Tool exactly.
• A
ffirmation of accuracy by your hospital’s CEO/Chief Administrative Officer or by an individual
that has been designated by the hospital CEO. These statements affirm the accuracy of your
hospital’s responses and must be completed in the Online Hospital Survey Tool to submit a
Survey.
• R
eference information which includes “What’s New” and “Change Summaries,” important
measure specifications, answers to frequently asked questions, and other notes that must be
carefully reviewed before responding to any of the Survey questions (included in the hard copy
only).
In addition to the Survey questions, adult and general hospitals that indicate they have a CPOE system in
at least one inpatient unit are asked to demonstrate, via a test, that the inpatient CPOE system can alert
physicians to at least 60% of frequent serious medication errors known to cause harm to patients. Adult
and general hospitals can access the CPOE Evaluation Tool immediately after completing the Hospital
Profile in the Online Hospital Survey Tool. Carefully review the information on the
Prepare for a CPOE
Tool webpage.
Any changes made to the measure specifications after April 1
will be reflected in the hard copy of the
Survey in the Reference Information sections under the “Change Summary” header (see
Table of
Contents). In addition, the updates to the specifications will be highlighted in yellow. If the changes are

2023 Leapfrog Hospital Survey – Hard Copy General Information
12 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
substantial, we will email the Primary Survey Contact your hospital provided in the Hospital Profile of the
Online Hospital Survey Tool. If the notification is sent before your hospital submits a 2023 Leapfrog
Hospital Survey, the email will go to the Primary Survey Contact provided in the previous year’s Survey.
The Leapfrog Group and its participating members are committed to presenting information that is as
current as possible, therefore we allow hospitals to update and resubmit their Survey until the November
30 Late Submission and Performance Update
Deadline. Please carefully review the reporting
periods in each section before updating your Survey. Leapfrog Hospital Survey Results are updated
monthly beginning in July on Leapfrog’s public reporting website. Hospitals are required to update
the
information in their Survey within 30 days of any change in status. We reserve the right to decertify
information that is not current. More information on updating your Survey is available on the Updating
Your Hospital Survey webpage.

2023 Leapfrog Hospital Survey – Hard Copy General Information
13 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Pre-Submission Checklist
Before you complete and submit the Survey via the Online Hospital Survey Tool, there are several steps
you should complete:
Visit the Hospital Survey webpages at https://leapfroggroup.org/hospital.
Make sure you have a 16-digit security code. If you don’t, download a Security Code Request
form. If your hospital is part of a multi-hospital healthcare system, you will need a separate security
code for each individual hospital within the system. Please refer to Leapfrog’s Multi-C
ampus Hospital
Reporting Policy.
Download a hard copy of the Survey (PDF or Word document) on the Survey and CPOE Materials
webpage. Read through the entire document to ensure that you understand what information is
required.
Review the reference information in each section of the Survey document and download other
supporting materials. These documents and tools contain information that you will need to
accurately respond to the Survey questions.
Join Leapfrog’s NHSN Group. Hospitals reporting on Section 7B: Healthcare-Associated Infections
are required to join Leapfrog’s NHSN Group. More information, including important deadlines, is
available on the
Join NHSN Group webpage.
Accept the American Medical Association’s Terms of Use and Download the CPT Code
Workbook. Hospitals reporting on Section 3 Adult and Pediatric Complex Procedures (that perform
bariatric surgery for weight loss, total hip replacement surgery, or total knee replacement surgery)
and Section 9 Outpatient Procedures (that perform outpatient procedures) must accept the American
Medical Association’s Terms of Use and download the CPT Code Workbook via the button on the
Survey Dashboard in Section 9.
Identify individuals from your hospital to help you gather the data you will need to complete the
various sections of the Survey.
Complete a hard copy of the Survey before you log in to the Online Survey Tool. This will
expedite the data entry into the Online Survey Tool and help to avoid the Tool "timing out" after 20
minutes of idle time (a security precaution). Once all the information has been collected and recorded
in the hard copy of the Survey, the CEO or the CEO’s designee can typically complete the online data
entry in less than an hour. Please note, responses must be entered into the Online Survey Tool to be
submitted.
Download and review a copy of the Online Survey Tool Guide on the Get Started webpage and
includes important instructions on how to navigate the Online Hospital Survey Tool, including
instructions on how to verify your hospital has successfully submitted the Survey.
Check Survey deadlines. Carefully review Survey deadlines before you begin. Ensure that you have
enough time to collect the data, complete a hard copy of the Survey, and complete and submit the
Survey via the Online Hospital Survey Tool. In addition, for adult and general hospitals that have
implemented CPOE in at least one inpatient unit, make sure you have enough time to take a
CPOE
Evaluation Tool.
Download and review the 2023 Leapfrog Hospital Survey Scoring Algorithms.
Review Leapfrog’s policies and procedures regarding data accuracy. Detailed information can
be found on the
Data Accuracy webpage.
Leapfrog Hospital Survey Binder: Hospitals should utilize the Leapfrog Hospital
Survey Binder to assist in organizing the documentation used to complete the Survey.
Download a copy of the binder on the
Survey and CPOE Materials webpage.

2023 Leapfrog Hospital Survey – Hard Copy General Information
14 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Instructions for Submitting a Leapfrog Hospital Survey
Important Notes:
Note 1: Please carefully review these instructions and the Online Survey Tool Guide
before you begin.
Note 2: Each section of the Survey must be completed before it can be affirmed in the Online Hospital
Survey Tool. Only sections that are affirmed can be submitted. Hospitals are responsible for ensuring that
each submitted section is accurate.
1. Log into the Online Hospital Survey Tool
using your 16-digit security code.
2. The first time you log into the 2023 Leapfrog Hospital Survey, you will need to complete and
submit the Hospital Profile. The Hospital Profile includes demographic and contact information.
The Hospital Profile should be updated throughout the year if any information changes and can
be accessed at any time. Failure to maintain current contact information could result in
important, time-sensitive information being missed or sent to the wrong person.
3. Once the Hospital Profile has been submitted, you will be taken to the Hospital Survey
Dashboard.
4. You can navigate into sections of the Online Hospital Survey Tool using the linked section names
on the Hospital Survey Dashboard. You can also access the CPOE Evaluation Tool immediately
after completing the Hospital Profile using the Take CPOE Tool button on the Hospital Survey
Dashboard. More information about navigating within the Online Hospital Survey Tool is available
in the Online Survey Tool Guide
.
5. Within sections, you can enter responses to questions and/or update responses to previously
submitted sections. The Online Hospital Survey Tool will automatically save your responses as
you enter them. There is no “save” button.
6. Once you have completed each section, you will need to return to the Hospital Survey Dashboard
to affirm each section of the Survey. Please remember that if you are making updates, all
updated sections must be re-affirmed. Please note that affirmed sections are not yet submitted,
please review Step 7 below to ensure successful submission.
7. Before you can submit the Survey (select the “submit affirmed sections” button on the Hospital
Survey Dashboard), you will need to “check for data review warnings.” When you select the
“check for data review warnings” button, the sections of your Survey that have been affirmed will
be scanned for potential reporting errors. If any errors are identified, a data review warning
message will be generated and will appear on the Hospital Survey Dashboard.
8. If any data review warnings
are generated, you will still be able to submit your Survey. However,
you will need to address the potential reporting errors identified during the scan or risk having the
related sections of your Survey decertified (publicly reported as “Declined to Respond”). Please
note this may not be a comprehensive list – you may still receive additional data verification
messages via email.
9. Once you have checked for data review warnings, you can select the “submit affirmed sections”
button to submit the Survey. Please review the Section Status column on the Hospital Survey
Dashboard to verify which sections have been submitted.
10. Use the “Print Last Submitted Survey” button on the Hospital Survey Dashboard to print a copy of
your Last Submitted Survey and review it for accuracy and completeness. Remember, sections
that are not affirmed will not be submitted.
11. Review the 2023 Leapfrog Hospital Survey Scoring Algorithms
to see how your Survey
responses will be scored and publicly reported by Leapfrog.
12. Review your Survey Results on the Hospital Details Page or public reporting website. Hospitals
that submit by June 30 can preview their Survey Results on the Hospital Details Page beginning
on July 12, before Leapfrog
publicly reports Survey Results beginning on July 25. After July, the

2023 Leapfrog Hospital Survey – Hard Copy General Information
15 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Hospital Details Page and public reporting website will be refreshed monthly within the first five
(5) business days of each month following your (re)submission.
13. Adult and general hospitals submitting a CPOE Evaluation Tool should carefully review the
instructions, scoring information, and FAQs available on the
Survey and CPOE Materials
webpage (see CPOE Tool Instructions under Other Supporting Materials).
14. Leapfrog is committed to verifying the accuracy of Leapfrog Hospital Survey Results. Please
review our data accuracy protocols on the Data Accuracy webpage
.
15. Responses can be updated or corrected, and the Survey can be resubmitted at any point during
the Survey Cycle (April 1 – November 30). Please remember that if you are making updates, all
updated sections must be re-affirmed. More information on updating your Survey is available on
the Updating Your Hospital Survey webpage
.
Verifying Submission
Use the following steps to verify that your submission was completed and that the appropriate sections
were submitted:
• Check the Hospital Survey Dashboard: Refer to the “Section Status” column on the Hospital
Survey Dashboard. All submitted sections will be marked as “Submitted.”
• Check your email: You will receive a Survey submission confirmation email within five minutes
of submitting a Survey. Please Note: This email will not specify which sections were submitted –
you will need to use the other steps to determine which sections were submitted.
• Print Last Submitted Survey: The Survey submission date will be listed at the top of the page
under the heading “Submitted Survey.” Be sure to check the submission date, review each
section for accuracy and completeness, and check that each affirmation is complete (Sections 1-
9).
• Review the Hospital Details Page: Your Survey Results will be available beginning July 12 via
the Hospital Details Page link on the Hospital Survey Dashboard. Carefully review your results,
including data from VON for high-risk deliveries, and your NHSN information for applicable
healthcare-associated infections.
• Check your publicly reported results: Always check your Leapfrog Hospital Survey Results on
the public reporting website
. Results are posted on July 25 and are updated within the first 5
business days of the month following your submission starting in August.
Updating or Correcting a Previously Submitted Leapfrog Hospital Survey
Hospitals can update or correct previously submitted Survey responses at any point during the Survey
Cycle (April 1 to November 30). Please review the Survey Deadlines webpage
. Most updates or
corrections are made:
• At the request of Leapfrog:
o Following Leapfrog’s Extensive Monthly Data Verification
, the Primary Survey Contact,
Secondary Survey Contact, and System Survey Contact will receive an email from the
Help Desk detailing potential reporting errors.
• Following Virtual On-Site Data Verification:
o Hospitals selected for Virtual On-Site Data Verification will receive a report which will
indicate any responses that need to be updated or corrected.
• At the discretion of the hospital:
o To correct a data entry or reporting error.
o To reflect a change in status or performance on a measure (e.g., closed a unit, stopped
performing a procedure, implemented a new policy, etc.).
o To provide more current responses based on the reporting periods outlined in the hard
copy of the Survey.
Following any updates, hospitals should always use the steps for verifying submission provided
above
.

2023 Leapfrog Hospital Survey – Hard Copy General Information
16 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Updating a Survey after Receiving a Help Desk Email
Leapfrog conducts Extensive Monthly Data Verification
of responses submitted to the Leapfrog Hospital
Survey starting with Surveys submitted by the June 30 Submission Deadline and monthly thereafter until
the Online Survey Tool is taken offline on January 31. Following the Extensive Monthly Data Verification,
the Primary Survey Contact, Secondary Survey Contact, and System Contact are notified by email of
any Survey responses that need to be reviewed and/or updated by the hospital.
If you receive a Data Verification email, you are required to document that your original responses were
correct or update/correct your previously submitted Leapfrog Hospital Survey by the end of the same
month using the original reporting period that was used for that section of the Survey in the original
submission. For example, if a hospital submitted a Survey for the first time on August 20, then received a
Data Verification email at the beginning of September, they would update their responses based on the
reporting period used in the August 20 submission.
Hospitals that receive a Category A
Data Verification message at the beginning of the month for any
measure will have until the end of that same month to contact the Help Desk to either (1) document that
the original response was correct or (2) correct the data entry or reporting error, or they will be publicly
reported as “Pending Leapfrog Verification” for that measure. This term is used to indicate that the
hospital has self-reported Survey responses that are under further review by Leapfrog.
If any Category A Data Verification messages are not resolved by January 31 (when the Online Hospital
Survey Tool is taken offline), the entire Survey will be decertified, and the hospital will be publicly reported
as “Declined to Respond” for the entire Leapfrog Hospital Survey.
Updating a Survey following Virtual On-Site Data Verification
Hospitals that are selected for Virtual On-Site Data Verification will receive a findings report. If the findings
report details any responses that need to be updated or corrected, please contact the Help Desk
.
Making General Updates (for hospitals that have not received a Help Desk email)
Leapfrog offers hospitals multiple reporting periods so that they can report the most current data. Except
for Section 4F: High-Risk Deliveries (VON data only), Section 7B: Healthcare-Associated Infections, and
Section 9D: Patient Follow-up (OP-32), updating a Survey is optional. However, we do recommend that if
your performance or if a structure has changed significantly, you update your Survey within 30 days. In
addition, hospitals should update their Surveys if they become aware of any reporting errors or data
inaccuracies in their previous submission.
Hospitals may update one or more sections of the Survey without updating the entire Survey. In addition,
hospitals are not required to retake the CPOE Evaluation Tool if making updates to Section 2A: CPOE
questions #3 and #4.
General updates and corrections can be made at any point during the Survey Cycle (April 1 – November
30). The months of December and January are reserved for correcting data entry (i.e., correcting data
entry errors) or reporting errors (i.e., in response to Leapfrog’s Extensive Monthly Data Verification
) to
previously submitted sections of the Survey. Any updates made to reflect a change in performance must
be made prior to the November 30 Late Submission and Performance Update
Deadline. Updates made
to reflect a change in performance after November 30 will not be scored or publicly reported. New
sections of the Survey submitted after November 30 will not be scored or publicly reported.
Hospitals that are submitting general updates should:
• Use the stated reporting period
at the top of each section selected based on the date of your
resubmission.
• For Section 4: Maternity Care and Section 6A-6B: NQF Safe Practice #1 and #2, update
responses to ALL questions within the section they wish to update using the same reporting

2023 Leapfrog Hospital Survey – Hard Copy General Information
17 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
period. For example, if a hospital submitted a Survey for the first time in June and then wanted to
update the responses for the Elective Deliveries questions in subsection 4B in November, they
would update the entire Section 4: Maternity Care based on the updated reporting period for
November.
For information on Leapfrog’s automatic updates to the VON data in Section 4F: High-Risk Deliveries, the
NHSN data in Section 7B: Healthcare-Associated Infections, or the CMS data in Section 9D: Patient
Follow-up, please review the Section 4F: High-Risk Deliveries Measure Specifications, the
Join NHSN
Group webpage, and the Section 9D: Patient Follow-up Measure Specifications.
Quick Tip: Remember to re-affirm any section of the Survey that has been updated,
check for data review warnings, and then resubmit the entire Survey. Always print a
copy of your Last Submitted Survey and review it for accuracy and completeness. Check
your updated Survey Results within the first 5 business days of the month following your
resubmission on the public reporting website
and the Hospital Details Page.

2023 Leapfrog Hospital Survey – Hard Copy General Information
18 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Deadlines
Deadlines for the 2023 Leapfrog Hospital Survey
The 2023 Leapfrog Hospital Survey, including the CPOE Evaluation Tool (if applicable), opens on April 1
and has a Submission Deadline of June 30, 2023. The Late Submission and Performance Update
Deadline is November 30, 2023. Surveys and Adult Inpatient CPOE Tests must be submitted before
11:59 pm Eastern Time on November 30. The CPOE Evaluation Tool will not be available after
November 30.
Corrections to Surveys submitted by November 30 must be submitted by the January 31, 2024
Corrections Deadline. The Online Hospital Survey Tool will not be available after January 31, 2024. Find
detailed information about the 2023 Leapfrog Hospital Survey Deadlines, including deadlines for receiving
free Competitive Benchmarking Summary Reports and consideration for Top Hospital Awards on the
Deadlines webpage
.
Deadlines for Vermont Oxford Network Data
Hospitals participating in the Vermont Oxford Network (VON) may opt to report on the VON National
Performance Measure in Section 4F: High-Risk Deliveries. Instructions, deadlines, and reporting periods
can be reviewed in the VON National Performance Measure Specifications
.
Deadlines to Join Leapfrog’s NHSN Group
Hospitals reporting on Section 7B: Healthcare-Associated Infections are required to join Leapfrog’s NHSN
Group. Please visit our webpage
for instructions on how to join the group as well as information about
important deadlines.
Deadlines Related to the Hospital Safety Grade
Hospitals that would like Leapfrog Hospital Survey Results used in their Leapfrog Hospital Safety Grade
must submit a Survey and an Adult Inpatient CPOE Test by the Data Snapshot Dates
. The Leapfrog
Hospital Survey and the Hospital Safety Grade are distinct programs administered by The Leapfrog
Group. Though some measures from the Leapfrog Hospital Survey are used in the Hospital Safety
Grade, the grade also utilizes publicly available data from other data sources. Find answers to Frequently
Asked Questions in the “For Hospitals” section of the Hospital Safety Grade
website.

2023 Leapfrog Hospital Survey – Hard Copy General Information
19 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Technical Assistance
Help Desk
Leapfrog operates a free online Help Desk to provide hospitals with technical assistance and answers to
content-related Survey questions. The Help Desk is staffed Monday-Friday from 9:00 am to 5:00 pm ET.
Help Desk support staff typically respond to inquiries within 1-2 business days, but we do ask you to plan
ahead and allow ample time to fulfill security code requests and other urgent tickets before Survey
deadlines. Review the Help Desk Holiday Schedule
for planned closures.
Hospitals can also submit feedback regarding the questions, measure specifications, and FAQs to the
Help Desk.
Tickets can be submitted electronically at https://leapfroghelpdesk.zendesk.com
. You will receive a
confirmation email and response from support@leapfroghelpdesk.zendesk.com. To ensure that you
receive our emails, please work with your IT Team to add the following to your safe sender list:
• @leapfrog-group.org
• @leapfroghelpdesk.zendesk.com
• @em8434.leapfrog-group.org
• IP address: 159.183.167.150
You may also create an account with Zendesk and log in there to correspond with the Help Desk.
Leapfrog Hospital Survey Webinar Series
The Leapfrog Hospital Survey Webinar Series is designed for Survey coordinators, hospital leaders, and
others who would benefit from a more interactive presentation of Survey materials and information.
The Webinar Series is held monthly from March to December and includes monthly office hours in
addition to monthly webinars. The one-hour webinars focus on specific topics related to the Survey,
including new measure. Throughout each webinar, participants can ask questions via the Zoom Q&A
function and take part in a live question-and-answer session at the end.
The monthly 30-minute office hours are designed to give participants another regular, interactive touch
point with Leapfrog’s Help Desk, so Survey coordinators always have the information they need, when
they need it.
Join now and start benefiting from:
• Monthly office hours with the Leapfrog Help Desk: 30 minutes to get real-time technical
assistance, answers to your questions, and help staying on top of upcoming deadlines. Staffed by
Leapfrog’s expert Help Desk.
• Monthly webinars: Timely and focused presentations on Survey and CPOE Evaluation Tool
measures and specifications, scoring information, frequently asked questions, technical
assistance, and public reporting. Each webinar concludes with a 20-minute open Q & A session.
• Archive Library: Slides and recordings from each webinar are archived in an online portal that
participants can access from March to January.
The annual registration fee is $500 per individual.
For a schedule of events and to register, please visit the Leapfrog
Hospital Survey Webinar Series
webpage.

2023 Leapfrog Hospital Survey – Hard Copy General Information
20 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Reporting Periods
Important Note: Reporting periods should be selected based on the date of Survey or section
submission. However, hospitals do not need to use the same reporting period throughout the Survey.
Survey Submitted Prior to
September 1
Survey (Re)Submitted on o
r
After September 1
Survey Section/
Measure
Reporting Period Reporting Period
1A Basic Hospital Information
12 months ending 12/31/2022 12 months ending 06/30/2023
1B Person-Centered Care: Billing
Ethics and Health Equity
N/A N/A
1C Informed Consent
N/A N/A
2A Computerized Physician Order
Entry (CPOE)
Latest 3 months prior to
Survey submission
Latest 3 months prior to
Survey submission
2B EHR Application Information N/A N/A
2C Bar Code Medication
Administration (BCMA)
Latest 3 months prior to
Survey submission
Latest 3 months prior to
Survey submission
2D Medication Reconciliation
Latest 6 months prior to
Survey Submission
Latest 6 months prior to
Survey submission
3A Hospital and Surgeon Volume
Volume:
12 months or 24-month annual
average ending 12/31/2022
Volume:
12 months or 24-month annual
average ending 06/30/2023
STS MVRR Composite Score:
Latest 36-month report
STS MVRR Composite Score:
Latest 36-month report
3B Surgical Appropriateness
Latest 12 months prior to
Survey submission
Latest 12 months prior to
Survey submission
3C Safe Surgery Checklist for Adult
and Pediatric Complex Surgery
Latest 6 months prior to
Survey submission
Latest 6 months prior to
Survey submission
4A Maternity Care Volume and
Services
12 months ending 12/31/2022 12 months ending 06/30/2023
4B Elective Deliveries
12 months ending 12/31/2022 12 months ending 06/30/2023
4C Cesarean Birth
12 months ending 12/31/2022 12 months ending 06/30/2023
4D Episiotomy
12 months ending 12/31/2022 12 months ending 06/30/2023
4E Process Measures of Quality
12 months ending 12/31/2022 12 months ending 06/30/2023

2023 Leapfrog Hospital Survey – Hard Copy General Information
21 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Survey Submitted Prior to
September 1
Survey (Re)Submitted on o
r
After September 1
Survey Section/
Measure
Reporting Period Reporting Period
4F High-Risk Deliveries*
Volume:
12 months ending 12/31/2022
Volume:
12 months ending 06/30/2023
VON:
2021 report
VON:
2022 report
5 ICU Physician Staffing (IPS)
Latest 3 months prior to
Survey submission
Latest 3 months prior to
Survey submission
6A NQF Safe Practice #1 – Culture of
Safety Leadership Structures and
Systems
Latest 12 months prior to
Survey submission
Latest 12 months prior to
Survey submission
6B NQF Safe Practice #2 – Culture
Measurement, Feedback, and
Intervention
Latest 12 or 24 months prior to
Survey submission (see
individual safe practice for
specific reporting period)
Latest 12 or 24 months prior to
Survey submission (see
individual safe practice for
specific reporting period)
6C Nursing Workforce
Nurse Staffing and Skill Level:
12 months ending 12/31/2022
Nurse Staffing and Skill Level:
12 months ending 06/30/2023
Percentage of RNs who are
BSN-Prepared:
N/A
Percentage of RNs who are
BSN-Prepared:
N/A
NQF Safe Practice #9:
Latest 12 months prior to
Survey submission
NQF Safe Practice #9:
Latest 12 months prior to
Survey submission
6D Hand Hygiene
N/A N/A
7A Never Events
N/A N/A
7B Healthcare-Associated Infections**
June and August Data
Downloads:
01/01/2022 – 12/31/2022
October and December Data
Downloads:
07/01/2022 – 06/30/2023
8A Patient Experience (CAHPS Child
Hospital Survey)
Latest 12 months prior to
Survey submission
Latest 12 months prior to
Survey submission
8B Pediatric Computed Tomography
(CT) Radiation Dose
12 months ending 12/31/2022 12 months ending 06/30/2023
9A Basic Outpatient Department
Information
12 months ending 12/31/2022 12 months ending 06/30/2023
9B Medical, Surgical, and Clinical
Staff
Latest 3 months prior to
Survey submission
Latest 3 months prior to
Survey submission
9C Volume of Procedures 12 months ending 12/31/2022 12 months ending 06/30/2023

2023 Leapfrog Hospital Survey – Hard Copy General Information
22 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Survey Submitted Prior to
September 1
Survey (Re)Submitted on o
r
After September 1
Survey Section/
Measure
Reporting Period Reporting Period
9D Safety of Procedures***
Patient Follow-up:
Latest 24 months prior to
Survey submission
Patient Follow-up:
Latest 24 months prior to
Survey submission
Patient Selection:
N/A
Patient Selection:
N/A
Safe Surgery Checklist:
Latest 6 months prior to
Survey submission
Safe Surgery Checklist:
Latest 6 months prior to
Survey submission
9E Medication Safety for Outpatient
Procedures
12 months ending 12/31/2022 12 months ending 06/30/2023
9F Patient Experience (OAS CAHPS)
Latest 12 months prior to
Survey submission
Latest 12 months prior to
Survey submission
*Leapfrog will update VON data 3 times per Survey Cycle for all hospitals that have provided an accurate
VON Transfer Code in the Hospital Profile, submitted a Data Sharing Authorization
letter to VON,
selected “VON National Performance Measure” in Section 4F, and submitted the 2023 Leapfrog Hospital
Survey.
**Adult and pediatric hospitals reporting on Section 7B: Healthcare-Associated Infections are required to
join Leapfrog’s NHSN Group. More information, including important deadlines, is available on the
Join
NHSN Group webpage. Leapfrog will update data 4 times for all members of our NHSN group that have
provided an accurate NHSN ID in the Hospital Profile and submitted the 2023 Leapfrog Hospital Survey.
***Adult and pediatric hospitals reporting on Section 9D: Patient Follow-up are required to provide an
accurate CMS Certification Number (CCN) in the Hospital Profile. Leapfrog will update data 3 times per
Survey Cycle for all hospitals that have provided an accurate CCN in the Hospital Profile and submitted
Section 9: Outpatient Procedures.

2023 Leapfrog Hospital Survey – Hard Copy Hospital Profile
24 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
HOSPITAL PROFILE
Hospitals must complete and submit a Hospital Profile via the Online Hospital Survey Tool before
accessing the Hospital Survey Dashboard for the first time. The Profile is available year-round and should
be updated as needed.

2023 Leapfrog Hospital Survey – Hard Copy Hospital Profile
25 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
2023 Hospital Profile
The Hospital Profile includes questions about demographic and contact information. The Profile can be
accessed and updated anytime throughout the year after logging into the Online Hospital Survey Tool
.
The Hospital Profile must be completed and submitted before you can access Sections 1-9 and the
CPOE Evaluation Tool on the Survey Dashboard.

2023 Leapfrog Hospital Survey – Hard Copy Hospital Profile
26 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Hospital Profile
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Notes:
Note 1: Leapfrog uses an administration system that links contacts shared by hospitals (i.e., CEOs,
Survey Contacts, System Contacts, and Public Relations Contacts). Only one phone number and email
address will be maintained for each contact, meaning that if this shared contact’s information is updated
in one hospital’s Profile, it will be updated for all hospitals associated with the contact.
Note 2: The Primary Survey Contact, Secondary Survey Contact, and System Contact will be notified at
the beginning of each month if Leapfrog finds any error in your Survey that needs to be corrected.
Facility Information
Organization Name
This is the name that will appear on Leapfrog’s public
reporting website.
CMS Certification Number (CCN)
1
If the CCN displayed in the Online Hospital Survey Tool
is not correct, contact the Leapfrog Help Desk
immediately.
Does your hospital share this CCN with another
facility?
Yes
No
NHSN ID
2
Vermont Oxford Network (VON) Transfer Code
3
Federal Tax Identification Number (TIN)
4
National Provider Identifier (NPI)
5
If the NPI displayed in the Online Hospital Survey Tool
is not correct, contact the Leapfrog Help Desk
immediately.
Does your hospital share this NPI with another
facility?
Yes
No
Demographic Information
Physical Address
(used for public reporting)
Mailing Address
(used to send important communications)
Street Address
Street Address or P.O. Box
City
City
State
6
State
Zip Code
Zip Code
Zip Code Suffix
Zip Code Suffix
Main Phone Number

2023 Leapfrog Hospital Survey – Hard Copy Hospital Profile
27 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Hospital Website Address
7
(So consumers can learn more about your hospital’s
efforts in the area of patient safety and quality
improvement)
Contact Information
Chief Executive Officer (CEO)
Chairperson of the Board
First Name
First Name
Last Name
Last Name
Email Address
(required for emailing of security codes and Top
Hospital notification)
Primary Survey Contact
Secondary Survey Contact
First Name
First Name
Last Name
Last Name
Title
Title
Phone Number
Phone Number
Phone Number Extension
Phone Number Extension
Email Address
Email Address
Hospital Public Relations Contact
(required so that Leapfrog may provide information on
Leapfrog accolades, such as Top Hospital notification,
and announcements)
First Name
Last Name
Phone Number
Phone Number Extension
Email Address
Opt-Out
Opt-out of having information in the “Contact
Information” subsection shared with third parties.
Opt-out

2023 Leapfrog Hospital Survey – Hard Copy Hospital Profile
28 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Health System Information
Is this hospital part of a healthcare system or
Integrated Delivery Network
8
?
System Public Relations Contact First Name
Yes
No
If yes, provide contact information.
Name of the healthcare system or Integrated
Delivery Network
System Public Relations Contact Last Name
System Contact First Name
System Public Relations Contact Phone Number
System Contact Last Name System Public Relations Contact Phone Number
Extension
System Contact Email Address
System Public Relations Contact Email Address

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
30 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
SECTION 1: BASIC HOSPITAL INFORMATION
This section includes questions and reference information for Section 1: Basic Hospital Information.
Please carefully review the questions, endnotes, and reference information (e.g., measure specifications,
notes, and frequently asked questions) before you begin. Failure to review the reference information
could result in inaccurate responses.

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
31 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 1: 2023 Basic Hospital Information
Billing Ethics Bibliography and Fact Sheet:
https://ratings.leapfroggroup.org/measure/hospital/2023/billing-ethics
Health Equity Bibliography:
https://ratings.leapfroggroup.org/measure/hospital/2023/whats-new-2023
Informed Consent Bibliography and Fact Sheet:
https://ratings.leapfroggroup.org/measure/hospital/2023/informed-consent
Section 1 includes questions about your hospital’s bed size, admissions, ICU operations, and teaching
status. This section also includes questions on billing practices and health equity. In 2023, the subsection
on hospital information (1A) includes a new optional fact-finding question regarding your hospital’s
environmental services that will not be scored or publicly reported. The subsection on informed consent
(1C) will be scored and publicly reported.
Each hospital achieving the standard for Billing Ethics:
1) Provides every patient with a billing statement within 30 days after final claims adjudication that
includes all 10 required elements listed in question #1, and
2) Gives patients instructions for contacting a billing representative with access to an interpretation
service to communicate in the patient’s preferred language and has the authority to do all three
required elements in question #2, and
3) Does NOT take legal action against patients for late or insufficient payment.
Each hospital achieving the standard for Informed Consent:
1) Has a training program on informed consent that tailors different training topics to different staff
roles and has made the training required for newly hired staff and existing staff who were not
trained, and
2) Ensures that as part of the process for obtaining informed consent, clinicians explain expected
difficulties, recovery time, pain management, and restrictions after a test, treatment, or procedure,
and give the patient an opportunity to ask questions, and
3) Ensures every consent form used by the hospital includes the name of the clinician performing
the test, treatment, or procedure, whether the clinician is expected to be absent, and whether any
assistants or trainees will be involved, and
4) Ensures every consent form is written at a 6
th
grade reading level or lower, and
5) Prior to conducting the informed consent discussion, identifies the patient/legal guardian’s
preferred language and provides a medical interpreter, and has a place in the consent form that
indicates whether an interpreter was used, and
6) Requires clinicians at the hospital to use the “teach back method” with patients/legal guardians.
Download the 2023 Leapfrog Hospital Survey Scoring Algorithm on the
Scoring and Results
webpage.

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
32 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
1A: Basic Hospital Information
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Specifications: See Basic Hospital Information Measure Specifications
in the Reference Information
beginning on page 44.
1) Reporting period used:
01/01/2022 – 12/31/2022
07/01/2022 – 06/30/2023
2) Total number of licensed acute-care
9
beds.
_____
3) Total number of staffed acute-care
10
beds.
_____
4) Total number of adult acute-care admissions
11
to your hospital
during the reporting period.
_____
5) Total number of pediatric acute-care admissions
12
to your hospital
during the reporting period.
_____
6) Does your hospital operate any adult or pediatric general medical
and/or surgical or neuro ICUs?
If “no” to question #6, skip questions #7-9 and continue to question
#10.
Yes
No
7) Total number of licensed ICU
13
beds in adult and pediatric general
medical/surgical ICU(s) and neuro ICU(s).
_____
8) Total number of staffed ICU
14
beds in adult and pediatric general
medical/surgical ICU(s) and neuro ICU(s).
_____
9) Total number of admissions to adult and pediatric general
medical/surgical ICUs and neuro ICUs
15
during the reporting
period.
_____
10) Does your hospital operate any of the following specialty ICUs:
medical cardiac, respiratory, surgical cardiothoracic, burn, trauma,
pediatric cardiothoracic, oncology, or any level neonatal ICU?
If “no” to question #10, skip question #11 and continue to question
#12.
Yes
No
11) Total number of admissions to any level neonatal ICU
16
during the
reporting period.
_____
12) Is your hospital a Major or Graduate teaching hospital for
physicians and/or physicians-in-training?
No response required here.
Determined automatically
based on NHSN 2022 Patient
Safety Component – Annual
Hospital Survey.
Reporting Period: 12 months
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o 07/01/2022 – 06/30/2023
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted Surveys
only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission and
Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or publicly
reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
33 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Additional Question (Optional – Fact Finding Only)
15) How are environmental services (EVS) and
facilities engineering integrated into your
hospital’s quality and safety structures?
Select all that apply.
EVS leadership participates in and shares
relevant data as part of the organization’s
quality and safety committees
Facilities engineering leadership participates in
and shares relevant data as part of the
organization’s quality and safety committees
EVS and facilities engineering staff are surveyed
as part of the hospital’s culture of safety survey
and leaders conduct debriefings with the EVS
and facilities engineering staff around the
team’s survey results
EVS and facilities engineering staff are included
in the hospital’s daily patient safety huddles
Other
Not applicable, EVS and facilities engineering
are not integrated into the hospital’s quality and
safety structures
13) To help ensure that patients are cared for by well-trained
physicians and other providers (e.g., certified registered nurse
anesthetists, certified midwives, or certified nurse-midwives, etc.),
do your medical staff by-laws or hospital-wide policies require all
physicians and providers who have privileges to provide care at
your hospital to be board certified or board eligible?
Yes
No
14) Does your hospital include performance on the Leapfrog Hospital
Survey, Leapfrog Hospital Safety Grade, or Leapfrog Top Hospital
in performance reviews and/or compensation incentives for senior
administrative leadership?
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
34 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
1B: Person-Centered Care: Billing Ethics and Health Equity
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Notes:
Note 1: Health Equity (questions #6-18) will not be scored or publicly reported.
Note 2: Hyperlinks throughout this subsection refer to the
Person-Centered Care: Billing Ethics and
Health Equity FAQs beginning on page 45, not to endnotes. These hyperlinks are not included in the
Online Survey Tool.
Billing Ethics
1) Within 30 days of the final claims adjudication (or within 30 days
from date of service for patients without insurance), does your
hospital provide every patient, either by mail or electronically, with a
billing statement and/or master itemized bill for facility services that
includes ALL the following?
a. Name and address of the facility where billed services
occurred
b. Date(s) of service
c. An individual line item for each service or bundle of
services performed
d. Description of services billed that accompanies each
line item or bundle of services performed
e. Amount of any principal, interest, or fees (e.g., late or
processing fees), if applicable
f. Amount of any adjustments to the bill (e.g., health plan
payment or discounts), if applicable
g. Amount of any payments already received (from the
patient or any other party), if applicable
h. Instructions on how to apply for financial assistance
i. Instructions in the patient’s preferred language on how
to obtain a written translation or oral interpretation of
the bill
j. Notification that physician services will be billed
separately, if applicable
If any one of the elements above are only provided upon
request, select “Only upon request.” If any one of the elements
above are not ever provided, select “No.”
Yes
No
Only upon request
Reporting Period: Answer questions #1-18 based on the practices currently in place at the time you
submit this section of the Survey.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted Surveys
only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission and
Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or publicly
reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
35 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
2) Does your hospital give patients instructions for contacting a billing
representative with:
• access to an interpretation service to communicate in the
patient’s preferred language, and
• the authority to (a) initiate an investigation into errors on the bill,
(b) offer a price adjustment or debt forgiveness based on
hospital policy, and (c) offer a payment plan do all of the
following within 10 business days of being contacted by the
patient or patient representative?
If “no” to question #2, skip question #4.
Yes
No
3) Does your hospital take legal action against patients for late
payment or insufficient payment of a medical bill?
This question does not include patients with whom your hospital
has entered into a written agreement specifying
a good faith
estimate for a medical service.
Yes
No
Additional Questions (Optional – Fact Finding Only)
4) Based on a quantified analysis of response times, do the billing
representatives from question #2 (a) initiate investigations into
errors on the bill, (b) offer a price adjustment or debt forgiveness
based on hospital policy, and (c) offer payment plans within 10
business days at least 95% of the time?
Yes
No
Not applicable, our hospital
did not conduct a quantified
analysis of response times
5) Does your hospital notify patients when their outstanding unpaid
balance is closed (e.g., due to the hospital’s charity care program or
the bill having been written off as unrecoverable debt) within 30
days?
Yes
No
Not applicable, our hospital
does not close outstanding
unpaid balances
Health Equity
6) Which of the following patient self-identified demographic data
does your hospital collect directly from its patients
(or patient’s
legal guardian) prior to or while registering a patient for a hospital
visit?
Select all that apply.
If “none of the above,” skip the remaining questions in Section 1B
and continue to the next subsection.
Race
Ethnicity
Spoken language
preferred for healthcare
(patient or legal guardian)
Written language
preferred for healthcare
(patient or legal guardian)
Sexual orientation
Gender identity
None of the above
7) Which of the following methods does your hospital use to collect the
demographic data in question #6 directly from patients (or patient’s
legal guardian)?
Select all that apply.
Online Patient Portal
Paper Registration Forms
Over the Phone
At Registration (in-person)
8) Does your hospital train staff responsible for registering patients
either in-person or over the phone on how to collect self-identified
demographic data in question #6 from its patients (or patient’s legal
guardian) at both:
• the time of onboarding, and
•
annually thereafter?
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
36 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
9) Does your hospital routinely take any of the following steps to
ensure the accuracy of the patient self-identified demographic data
collected directly from its patients (or patient’s legal guardian) in
question #6?
Select all that apply.
Ensure appropriate data
collection fields are
available in EHR
Use analytic tools to
assess completion rates of
data collection fields in
EHR
Compare data collected
from patient experience
surveys with EHR data
Compare data collected
through patient portals
with EHR data
Compare data collected
with community data
provided by state or
county or Community
Health Needs Assessment
(CHNA)
Compare data collected to
census data for the
hospital’s service area
Other
None of the above
10) Does your hospital use the patient self-identified demographic data
it collects directly from patients (or patient’s legal guardian) in
question #6 to stratify any quality measure(s) with the aim of
identifying health care disparities?
If “no” to question #10, skip questions #11-15 and continue to
question #16.
Yes
No
11) Which type(s) of quality measure(s) does your hospital stratify?
Select all that apply.
Clinical process measures
Clinical outcome
measures
CAHPS measures (i.e.,
Adult HCAHPS, OAS
CAHPS, CAHPS Child
Hospital Survey, etc.)
Other patient experience
measures
Other
12) What types of patient self-identified demographic data selected in
question #6 did your hospital use to stratify the quality measures
selected in question #11?
Select all that apply based on items that were also selected in
question #6.
Race
Ethnicity
Spoken language
preferred for healthcare
(patient or legal guardian)
Written language
preferred for healthcare
(patient or legal guardian)
Sexual orientation
Gender identity

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
37 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
13) Prior to using the patient self-identified demographic data selected
in question #12 to stratify quality measures, did your hospital do
any of the following to ensure the quality of the data?
Select all that apply.
Periodically (e.g., monthly
for first six months, and
quarterly thereafter)
review data set to identify
critical issues with data
quality
Set targets for missing
data (e.g., 10%) to use as
a benchmark for
performance
Monitor “other” response
rates to identify issues
with existing data
collection categories or
methods
Include the data collection
team (i.e., registration
team) when addressing
data quality issues
None of the above
14) By stratifying the measure(s) selected in question #11, has your
hospital identified any disparities among its patients based on the
demographic data selected in question #12?
If “no, disparities were not identified” or “inadequate data available
to determine if disparities exist,” skip question #15 and continue to
question #16.
Yes, disparities were identified
No, disparities were not
identified
Inadequate data available to
determine if disparities exist
15) In the past 12 months, has your hospital used the data and
information obtained through question #14 to update or revise its
policies or procedures?
OR
In the past 12 months, has your hospital developed a written action
plan that describes how it will address at least one of the health
care disparities identified through question #14?
Yes
No
16) Does your hospital share information on its efforts to identify and
reduce health care disparities based on race, ethnicity, spoken
language preferred for healthcare (patient or legal guardian), written
language preferred for healthcare (patient or legal guardian), sexual
orientation, or gender identity and the impact of those efforts on its
public website?
Yes
No
17) Does your hospital report out and discuss efforts related to
identifying and addressing disparities with the Board at least
annually?
Yes
No
18) Does your hospital make unconscious and implicit bias training
available to all hospital staff?
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
38 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
1C: Informed Consent
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Notes:
Note 1: In this subsection, questions regarding the informed consent process and consent forms concern
ALL tests, treatments, and procedures for which your hospital obtains consent, EXCEPT anesthesia.
Note 2: Hyperlinks throughout this subsection refer to the Informed Consent FAQs beginning on page 48
,
not to endnotes. These hyperlinks are not included in the Online Survey Tool.
Policies and Training
1) Does your hospital have a training program on informed consent
that tailors different training topics to different staff roles
, including
hospital leaders, MD/NP/PA, nurses and other clinical staff,
administrative staff, and interpreters, and has your hospital made
the training:
• a required component of onboarding for the appropriate
newly hired staff, and
• required for the appropriate existing staff who were not
previously trained?
Yes
No
Content of Informed Consent Forms
2) As part of your hospital’s process for obtaining informed consent,
does:
• the clinician explain expected difficulties, recovery time,
pain management, and restrictions after a test, treatment,
or procedure, in the facility and post-discharge, if
applicable,
• the patient have the opportunity to ask questions, and
• the consent form document that these two elements of
the process have taken place?
Yes
No
3) Do ALL your hospital’s consent forms include:
• the name(s) of the clinician(s) performing the test,
treatment, or procedure,
• whether the clinician is expected to be absent from
portions of the test, treatment, or procedure (e.g.,
opening, closing), and
• whether any assistants or trainees will be involved in the
test, treatment, or procedure?
Yes
No
Reporting Period: Answer questions #1-6 based on the practices currently in place at the time you
submit this section of the Survey.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
39 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
4) Are ALL your hospital’s consent forms written at a 6
th
grade
reading level or lower?
The procedure name and description can be excluded from the
reading level assessment
.
Yes, all forms are written at a
6
th
grade reading level or
lower
At least one form is written at
a 6
th
grade reading level or
lower
No forms are written at a 6
th
grade reading level or lower
Process for Gaining Informed Consent
5) Prior to the informed consent discussion, does your hospital:
• ask what the patient/legal guardian’s preferred language
for medical decision-making is,
• where needed, provide the patient/legal guardian access
to a qualified medical interpreter,
• use a consent form or notation in the medical record to
document whether a qualified medical interpreter was
used to conduct the informed consent process, and
• have the medical interpreter sign the consent form (either
in-person, electronically, or by documenting the use of an
interpreter in the medical record)?
If anyone other than a qualified medical interpreter is ever
used to translate (e.g., caregiver or family member), select
“No.”
Yes
No
6) As part of the informed consent discussion, do clinicians at your
hospital use the “teach back method” with patients/legal
guardians, where patients/legal guardians are asked to describe,
in their own words, what they understand will be performed, why it
will be performed, and what are the primary risks?
Yes
No
Additional Questions (Optional – Fact Finding Only)
7) Does your hospital’s written policy on informed consent reference
a list, or a defined set of guidelines, so the appropriate staff know
which tests, treatments, and procedures require patient/legal
guardian consent, with any exceptions noted?
Yes
No
8) As part of your hospital’s process for obtaining informed consent,
does:
• the clinician explain all the patient’s test or treatment
choices (including the choice of declining to go through
with the test, treatment, or procedure), including the
severity and probability of the risks and benefits of each
choice, if applicable,
• the patient have the opportunity to ask questions, and
• the consent form document that these two elements of
the process have taken place?
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
40 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
9) As part of your hospital’s process for obtaining informed consent,
does:
• the clinician explain the clinical rationale (i.e., condition-
specific justification) for why the test, treatment, or
procedure is being performed,
• the patient have the opportunity to ask questions, and
• the consent form document that these two elements of the
process have taken place?
Yes
No
10) Which clinician is responsible for conducting the informed consent
process at your hospital?
The clinician primarily
responsible for performing
the procedure
Another clinician on the
procedure team
A clinician not involved with
performing the procedure
Other
11) As part of the informed consent discussion, do clinicians at your
hospital tell patients/legal guardians how many times a year, on
average, they perform the test, treatment, or procedure?
Yes
No
12) For tests, treatments, and procedures that are scheduled a week
(i.e., seven calendar days) or more in advance, is the consent
form shared with the patient at least three calendar days before
the patient’s test, treatment, or procedure?
Yes
No
13) For tests, treatments, and procedures that are scheduled a week
(i.e., seven calendar days) or more in advance, do clinicians
discuss the consent form with the patient/legal guardian at least
one calendar day before the patient’s test, treatment, or
procedure, and is the patient/legal guardian provided with an
opportunity to ask questions?
Yes
No
14) At least once a year, does your hospital solicit feedback from
patients/legal guardians about your hospital’s informed consent
process to understand how it can be improved over time?
Yes
No
15) At least once a year, does your hospital complete an audit of the
informed consent process to evaluate its efficacy and provide
feedback to staff on opportunities for improvement?
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
41 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Affirmation of Accuracy
As the hospital CEO or as an employee of the hospital to whom the hospital CEO has delegated
responsibility, I have reviewed this information pertaining to the Basic Hospital Information Section at our
hospital, and I hereby certify that this information is true, accurate, and reflects the current, normal
operating circumstances at our hospital. I am authorized to make this certification on behalf of our
hospital.
The hospital and I understand that The Leapfrog Group, its members, the public and entities and persons
who contract or have other business dealings with The Leapfrog Group are relying on the truth and
accuracy of this information. The hospital and I also understand that The Leapfrog Group will make this
information and/or analyses of this information public through the Survey Results public reporting website,
The Leapfrog Group’s Hospital Safety Grade, and/or other Leapfrog Group products and services. This
information and/or analyses and all intellectual property rights therein shall be and remain the sole and
exclusive property of The Leapfrog Group in which The Leapfrog Group retains exclusive ownership. This
information does not infringe upon any third-party intellectual property rights or any other third-party rights
whatsoever and is free and clear of all encumbrances and liens of any kind. The hospital and I
acknowledge that The Leapfrog Group may use this information in a commercial manner for profit. The
hospital shall be liable for and shall hold harmless and indemnify The Leapfrog Group from any and all
damages, demands, costs, or causes of action resulting from any inaccuracies in the information or any
misrepresentations in this Affirmation of Accuracy. The Leapfrog Group and its members and entities and
persons who contract or have other business dealings with The Leapfrog Group reserve the right to omit
or disclaim information that is not current, accurate or truthful.
Affirmed by _____________________, the hospital’s ___________________________,
(first name and last name) (title)
on _______________________.
(date)

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
42 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 1: 2023 Basic Hospital Information Reference Information
What’s New in the 2023 Survey
Section 1A: Basic Hospital Information
In recognition of the published evidence and guidelines documenting the importance of environmental
hygiene on infection prevention, Leapfrog is exploring the development of a new standard around
environmental hygiene and will be consulting with experts in advance of the 2024 Leapfrog Hospital
Survey. Environmental hygiene topics under consideration include cleaning and disinfecting surfaces, air-
handling, ventilation, and water quality. For the 2023 Leapfrog Hospital Survey, we are adding an
optional, fact-finding question to Section 1A: Basic Hospital Information to assess how hospitals are
integrating environmental services and facilities engineering into their quality and safety structures. Based
on public comments and feedback received from 2023 Leapfrog Hospital Survey pilot participants, the
response options to this question have been updated. This optional, fact-finding question will not be used
in scoring or public reporting in 2023.
Section 1B: Person-Centered Care
Billing Ethics
In response to hospital feedback, an analysis of responses submitted to the 2022 Leapfrog Hospital
Survey, and feedback from researchers in the field, Leapfrog is making the following updates to Section
1B: Billing Ethics:
• Updating question #1, regarding a master itemized bill, to clarify that hospitals must provide
instructions on how to obtain a written translation or oral interpretation of the bill in the patient’s
preferred language.
• Updating question #2, regarding access to billing representatives and timely resolution of billing
issues, to give billing representatives 10 days, rather than 5 days, to initiate an investigation into
errors on a bill, offer a price adjustment or debt forgiveness based on hospital policy, or offer a
payment plan. We are also updating question #2 to require that billing representatives have
access to a translation service to help communicate information in the patient’s preferred
language.
• Leapfrog is also adding two optional fact-finding questions regarding additional aspects of
hospital billing practices that will not be used in scoring or public reporting in 2023.
Additionally, we are adding Frequently Asked Questions (FAQs) regarding alternatives to taking legal
action against patients and defining a “good faith estimate.” There are no changes to the scoring
algorithm.
Health Equity
To date, Leapfrog has focused questions in this subsection on the collection of patient self-reported
demographic information. Based on feedback from Leapfrog’s National Advisory Committee and an
analysis of responses submitted to the 2022 Leapfrog Hospital Survey, Leapfrog is revising the questions
to focus on methods that hospitals are using to stratify measures by race, ethnicity, preferred language,
sexual orientation, and gender identity. These questions are required, but responses will not be scored or
publicly reported in 2023.
Section 1C: Informed Consent
Based on an analysis of responses submitted to the 2022 Leapfrog Hospital Survey and consultation with
Leapfrog’s Patient and Family Caregiver Expert Panel
, Leapfrog will score and publicly report Section 1C:
Informed Consent in 2023. We are updating Section 1C as follows:

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
43 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
First, the following six questions, identified by the expert panel as being the most relevant to patients and
family caregivers, will be scored and publicly reported in 2023:
• One (1) question from the Policies and Training domain that focuses on staff training on the
hospital’s informed consent policies.
• Three (3) questions from the Content of the Informed Consent Forms domain that focus on:
detailing expected difficulties with the procedure; naming individuals who will be involved with the
procedure, including trainees and assistants; and ensuring consent forms are at a 6
th
grade
reading level.
• Two (2) questions from the Process for Gaining Informed Consent domain that focus on providing
medical interpretation in the patient/legal guardian’s preferred language, where needed, when
discussing informed consent and using the “teach back method” with patients to ensure they
understand what is being explained to them.
Based on public comments and feedback received from 2023 Leapfrog Hospital Survey pilot participants,
Leapfrog is making several updates to the originally proposed questions.
Second, we are removing questions focused on the hospital having a written policy on informed consent,
the hospital explicitly offering patients the opportunity for a care partner to participate in the informed
consent process, and the use of high-quality decision aids when discussing treatment options.
Finally, we are retaining several questions from the 2022 Leapfrog Hospital Survey but making them
optional and for fact finding purposes only; they will not be scored or publicly reported in 2023. As we
conduct additional research on these and other important, evidence-based practices related to the
informed consent process, additional questions may be scored and publicly reported in the future.
Change Summary Since Release
None. If substantive changes are made to this section of the Survey after release on April 1, 2023, they
will be documented in this Change Summary section.

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
44 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Basic Hospital Information Measure Specifications
Important Note: Hospitals that share a CMS Certification Number are required to report by facility.
Please carefully review Leapfrog’s Multi-Campus Reporting Policy
.
Teaching Status is obtained directly from CDC’s National Healthcare Safety Network (NHSN) using a
hospital’s response to the following questions within the “Hospital Facility” section of the 2022 Patient
Safety Component – Annual Hospital Survey:
Is your hospital a teaching hospital for physicians and/or physicians-in-training? [Yes, No]
If Yes, what type: [Major, Graduate, Undergraduate]
For the purposes of the 2023 Leapfrog Hospital Survey and Leapfrog’s Top Hospital program, Leapfrog
will consider the following types a “teaching hospital:” Major and Graduate. NHSN’s definitions for types
of teaching hospitals may be reviewed here
. This designation will also be used for the purposes of
assigning hospitals to a teaching or non-teaching hospital cohort for the purposes of scoring the Total
Nursing Care Hours per Patient Day, RN Hours per Patient Day, and Nursing Skill Mix measures in
Section 6C: Nursing Workforce.
In order for Leapfrog to obtain teaching status from NHSN, hospitals must complete the following steps:
1) Join Leapfrog’s NHSN Group and review/accept Leapfrog’s Data Rights Template by the
published deadlines
*,
2) Enter a valid NHSN ID in the Profile of their 2023 Leapfrog Hospital Survey, and
3) Submit a 2023 Leapfrog Hospital Survey by the published deadlines
.
*Hospitals are not required to “re-join” Leapfrog’s NHSN Group if they joined and conferred rights in
previous Leapfrog Hospital Survey Cycles. However, all hospitals in Leapfrog’s NHSN Group must review
their Rights Acceptance Report at least annually by the published join-by deadlines
.
Instructions for joining or verifying that you are in Leapfrog’s NHSN Group are available here
(See “NHSN
Guidance: Join the Group, Data Rights Template, and Downloading Reports”). These instructions also
include information for verifying the teaching status response to the 2022 Patient Safety Component –
Annual Hospital Survey.

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
45 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Basic Hospital Information Frequently Asked Questions (FAQs)
Basic Hospital Information
1. How does Leapfrog define board certified and board eligible?
For physicians:
• Board certified means that the physician has been awarded certification from the American Board
of Medical Specialties (ABMS) or the American Osteopathic Association (AOA).
• B
oard eligible indicates that the physician has completed their initial training/fellowship but has not
yet passed an existing board-certifying exam in a specialty. Leapfrog adheres to the ABMS and
AOA Board Eligibility Policy for all specialties, which may be reviewed here:
https://www.abms.org/board-certification/board-certification-requirements/board-eligibility/ and
https://certification.osteopathic.org/about/, respectively. These eligibility periods provide the
physician with an adequate window to take their boards and re-take if necessary.
For CRNAs:
• Board certified means that the RN has been awarded certification from The National Board of
Certification and Recertification for Nurse Anesthetists (NBCRNA).
Billing Ethics
2. To meet the criteria for item “i” in question #1, does our hospital have to translate the billing
statement and/or master itemized bill to every language spoken by our patients?
Hospitals must provide instructions, in the patient’s preferred language, on how to obtain a written
translation or oral interpretation of the bill if the language constitutes 5% (and at least 50 patients) or
1,000 patients (whichever is less) of the population eligible to be served or likely to receive care at the
hospital.
3. What does Leapfrog mean by “legal action” in question #3?
Legal action can include, but is not limited to, a lawsuit, wage garnishment, filing to take a patient’s
money out of their tax return, seizing or placing a lien on a patient’s personal property, and selling or
transferring a patient’s debt to a debt collection agency that will take legal action against the patient. If
the debt collection agency is prevented from taking legal action against patients by their contract with
the hospital, selling or transferring a patient’s debt to that debt collection agency would not be
considered legal action.
Patients with whom your hospital has entered into a written agreement specifying a good faith
estimate for a medical service would not be included in this question. A patient's insurance being
accepted by the hospital, or publicly available prices for a procedure, do NOT constitute a written
agreement specifying a set price for a procedure.
In addition, other legal proceedings where patients may be named as defendants for causes other
than late or non-payment of a medical bill are not included in this standard (e.g., filing a lien after an
auto accident, or misappropriation of an insurance reimbursement).
4. What are alternatives to legal action against patients?
To ensure that patients are not being pursued when they no longer have the means to pay, some
healthcare providers partner with nonprofits such as RIP Medical Debt, a nonprofit that uses
philanthropically raised funds to acquire bad debt from health systems solely for the purpose of debt
relief. They use credit analytics to locate patients with financial hardship and help notify the patient
that the debt is abolished. Hospitals can contact RIP Medical Debt here:
https://ripmedicaldebt.org/hospitals/
.
5. What is a “good faith estimate” as referred to in question #3?
A good faith estimate includes an itemized list of expected charges for the primary item or service the
patient will receive, and any other items or services provided as part of the same scheduled episode
of care. The final bill must be no more than $400 over the amount of the good faith estimate. The

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
46 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Centers for Medicare and Medicaid Services have published an example template for providing good
faith estimates: https://www.cms.gov/files/document/good-faith-estimate-example.pdf
.
6. What procedure should we follow to conduct the quantified analysis of response times for
billing representatives as described in question #4?
Although this question is not scored or publicly reported this year, Leapfrog has identified preliminary
parameters for conducting the audit of response times. First, a minimum of 30 patient contacts with
the hospital billing department should be evaluated against the standard. Hospitals can begin logging
patient contacts (either by phone or email) with the billing department until at least 30 contacts from
patients have been identified. Exclude patient contacts that are unrelated to errors on the bill, or
where the patient is not asking for a payment plan or a price adjustment or debt forgiveness. Also
exclude contacts where the patient was determined to be ineligible for any price adjustment or
payment plan, or if the patient withdrew their request. To calculate whether the timeframe was met,
count the number of business days in between when the patient first contacted the billing department,
and when an investigation was initiated into errors on the bill, or when a payment plan, price
adjustment, or debt forgiveness was first offered to the patient, as applicable. If the interval is under
10 days, the billing representative met the required timeframe.
Health Equity
7. Our hospital is just starting to explore the collection and use of demographic data. What are
some resources or tools we can use to collect demographic data?
Hospitals that are starting their data collection journey can refer to the Toronto Measuring Health
Equity website which includes training videos, manuals, and presentations on how to collect
demographic data from patients, including the modeling of interactions between health-care staff and
patients. Additional resources such as scripting for staff and addressing patient concerns can be
found here: https://ifdhe.aha.org/hretdisparities/how-use-hret-disparities-toolkit
.
8. What types of demographic data should hospitals be collecting?
Regarding patient self-identified race and ethnicity, at a minimum, hospitals should collect ethnic and
racial categories as outlined by the Office of Management and Budget (OMB) in their Standards for
the Classification of Federal Data on Race and Ethnicity. Ethnic categories include Hispanic or Other
Latino. Racial categories include American Indian or Alaska Native, Asian, Black or African American,
Native Hawaiian or Other Pacific Islander, and White. If your hospital has its own categories that go
above and beyond OMB classifications, you can still respond to question #6.
In addition, race and ethnic categories should be presented as separate questions, and patients
should be able to select multiple racial categories. More information is available at
https://www.govinfo.gov/content/pkg/FR-1997-10-30/pdf/97-28653.pdf
.
Regarding patient self-identified gender identity and sexual orientation, the Centers for Disease
Control and Prevention has issued helpful guidance for providers and facilities, including a list of
questions that can be asked at registration. More information is available at
https://www.cdc.gov/hiv/clinicians/transforming-health/health-care-providers/collecting-sexual-
orientation.html.
9. To select any of the patient self-identified demographic data in question #6, does a hospital
need to collect the data in a particular way?
Hospitals should be collecting the information directly from the patient or, for pediatric and other
patients who cannot communicate the information themselves, the legal guardian present. Patients or
their legal guardians should have the opportunity to provide the information either verbally (in-person
or over the phone) or via a paper form or online portal. Information should NOT be collected through
observation or other documents (i.e., state issue ID).

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
47 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
10. Our hospital staff collects some of the demographic data in question #6 outside of
registration, and during the nursing admission assessment/process. Can we still select the
demographic data in question #6 if it is collected during a nursing assessment?
Yes, hospitals may select the appropriate patient self-identified demographic data that is collected
during a nursing admission assessment. Hospitals should ensure they are collecting the self-identified
demographic data directly from the patient or legal guardian present, as applicable. See more
information about collecting the demographic data in FAQ #9.
11. Does Leapfrog have a definition of sexual orientation or an example of what needs to be
reflected in the data to select “sexual orientation” in question #6?
If your hospital does not have an established set of questions to use to collect patient self-identified
sexual orientation information at registration or during the visit, we would recommend that you refer to
information on the CDC's webpage - Collecting Sexual Orientation and Gender Identity Information at
https://www.cdc.gov/hiv/clinicians/transforming-health/health-care-providers/collecting-sexual-
orientation.html. This webpage includes a list of questions that hospitals can ask to obtain the
information, and some helpful tips on collecting and using the data.
12. What are some ways in which hospitals can ensure the accuracy of the demographic data they
collect from patients?
One method to ensure the accuracy of demographic data is to compare a patient’s self-identified
demographic information in the “About You” of the HCAHPS to the patient’s demographic data in the
hospital’s EHR (e.g., via online patient portal or paper forms) and see if the patient agrees. Hospitals
that offer self-check-in available through “kiosks” can ask the patient to confirm their demographic
data and offer the opportunity to update it. In addition, CAHPS vendors such as Press Ganey offer
free tools and resources to their clients. More information is available at
https://info.pressganey.com/cahps-regulatory
.
13. How does Leapfrog define “health care disparities?”
Leapfrog defines health care disparities as differences in the quality of health care that are not due to
access-related factors or clinical needs, preferences, and appropriateness of intervention. More
information is available at
http://www.ihi.org/communities/blogs/making-sense-of-health-equity-
terminology#:%7E:text=Health%20care%20disparity%3A%20%E2%80%9CRacial%20or,by%20the%
20Institute%20of%20Medicine.
14. Our hospital uses demographic data it collects from patients to stratify quality measures for
research purposes. Can we respond “yes” to question #10?
Yes, hospitals doing this work for research purposes or internal quality measurement/ improvement
can respond “yes” to this question.
15. Can our hospital report on measures that CMS is stratifying based on dual eligibility in
question #10?
No, hospitals should report on measures that they are stratifying themselves using the patient self-
identified demographic data they are collecting directly from patients. For example, to select “Clinical
process measures” a hospital could explore disparities in their cancer screening measures based on
patient race.
16. In question #14, what does Leapfrog mean by “inadequate data available to determine if
disparities exist”?
Hospitals may find that they cannot determine if a health care disparity exists due to small sample
sizes (i.e., fewer than 25 patients able to be measured) or not enough additional data such as data on
income or education.
17. Can we report on health system level data in question #14 if we do not have adequate data to
determine if disparities exist?
Yes, individual hospitals that do not have enough data to identify disparities among their patients
based on the demographic data selected in question #12, can answer “yes” to question #14 if they

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
48 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
are aggregating their data for the purposes of analysis with other facilities that are part of their
system.
18. In question #16, is Leapfrog asking whether our hospital is publicly reporting the measures we
stratify in question #11 on our website?
No. We are trying to assess the extent to which hospitals are sharing any information on their efforts
to identify and reduce health care disparities (based on race, ethnicity, spoken language preferred for
healthcare (patient or legal guardian), written language preferred for healthcare (patient or legal
guardian), sexual orientation, and gender identity) and the impact of those efforts. This may take the
form of sharing quantitative or qualitative data. It may also include a description of the types of
demographic data collected and the analyses performed, which in some cases demonstrated no
apparent health care disparities. One example can be found on the Massachusetts General Hospital
website, available at https://www.massgeneral.org/quality-and-safety/about/care-equity
.
19. What does Leapfrog mean by unconscious and implicit bias training in question #18?
Unconscious or implicit bias is generally understood to mean the unintentional and unconscious bias
in your behaviors, attitudes, or beliefs towards a group of people. More specifically, implicit or
unconscious bias is defined as “the bias in judgment and/or behavior that results from subtle cognitive
processes (e.g., implicit attitudes and implicit stereotypes) that often operate at a level below
conscious awareness and without intentional control”, as defined in
Achieving Health Equity: A guide
for Health Care Organizations. Your facility should offer some form of training to staff that addresses
an implicit bias such as race, gender, sexual orientation, or gender identity. Please see examples of
unconscious and implicit bias training in FAQ #21.
20. Can we answer “yes” to question #18 even if unconscious and implicit bias training isn’t
mandatory for all hospital staff?
Yes. Leapfrog is not asking if hospitals require unconscious and implicit bias training. Hospitals
should, however, provide staff with an opportunity or option to take unconscious and implicit bias
training. Please see more information in FAQ #21 for an example of unconscious and implicit bias
training.
21. What is an example of unconscious and implicit bias training?
An example of unconscious and implicit bias training can be found on the American Hospital
Association Institute for Diversity and Health Equity (IFDHE) website at
https://ifdhe.aha.org/unconscious-bias-training-two-approaches-equity-care-honorees
. The IFDHE
includes a webinar that highlights various methods to train staff on how to identify and address
unconscious bias in the workplace.
Informed Consent
22. In cases where hospitals do not have information about informed consent processes that take
place at a clinician’s (e.g., surgeon’s) office, or if informed consent processes are conducted
by clinicians that are not employed by the hospital, or in other cases where the hospital does
not have visibility into the informed consent process, how should hospitals respond to
questions in this section?
Hospitals that do not have input over the consent form or visibility into the informed consent process
for tests, treatments, and procedures performed at a hospital, should select “No” for the questions in
this section.
If the hospital does have input over the consent form and visibility into the informed consent process
for tests, treatments, and procedures performed at the hospital, but the consent forms and the
consent process are being completed at the clinician(s)'s office, the hospital can work with those
offices to implement the requirements outlined in the questions and, via an annual audit, verify that
the forms and process meet the criteria to respond “Yes” to the questions in this section. All
documentation should be maintained throughout the Survey Cycle.

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
49 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
23. Are there any examples of patients for whom the informed consent questions would not
apply?
When responding to the questions in this subsection, you can exclude patients who are unable to
communicate and for whom no family member or medical proxy has been identified at least one week
prior to the test, treatment, or procedure being performed.
24. When answering questions that ask about “all your hospital’s consent forms,”, are any
consent forms excluded?
Hospitals can exclude their general consent to treat and anesthesia consent forms when responding
to questions regarding “all your hospital’s consent forms.”
25. What roles and staff levels need to be included in the training program on informed consent
included in question #1? What types of training can we use?
As described on page 98 of the
AHRQ’s Making Informed Consent an Informed Choice – Training for
Health Care Leaders, the appropriate roles for training include all the following: hospital leaders,
physicians/independent nurse practitioners/independent physician assistants, nurses or other clinical
staff, administrative staff, and interpreters. The training may be tailored to only include relevant
materials based on the staff role. The goal is for each responsible staff person to be trained in their
applicable domains. For example:
• For hospital leaders, training on the definition and principles of informed consent and specifics on
the hospital’s informed consent policy is appropriate.
• Clinical staff such as physicians and nurses should also be trained in strategies for clear
communication, for presenting choices, and for documentation.
• For administrative staff and interpreters, participating in the informed consent process should also
be trained in documentation.
Staff that are not directly employed by the hospital (e.g., medical interpreters who are employed by a
contractor) do not need to be trained by the hospital.
Trainings do not need to be exclusive to informed consent and can be included as components or
modules in other trainings. Examples of trainings include computer-based training, one-on-one
precepting, webinars, and staff meeting presentations, as well as other modalities where learning can
be assessed after the content is delivered to the trainee.
26. Why has Leapfrog selected a 6th-grade reading level target for consent forms, and what are
some strategies we can use to meet this?
Just over half of U.S. adults have a reading level that permits them to understand and synthesize
information from a complex text. According to a Gallup analysis
, 54% of Americans between the ages
of 16 and 74 read below the equivalent of a sixth-grade level. Leapfrog has included an exemption in
the standard for the procedure name and description, that may include highly technical language that
would affect the reading level.
Leapfrog hosted two Town Hall Calls last year led by AHRQ describing techniques for reducing the
written complexity of consent forms. The slides are available on Leapfrog’s Town Hall Calls webpage
;
please refer to slide 40-47 for more information in the “Informed Consent” slide deck and slides 40-45
in the “Health Literacy” deck. Additional resources include:
• AHRQ Training Module
• The Patient Education Materials Assessment Tool (PEMAT)
• Clear Communication Index (CCI)
• CMS Toolkit for Making Written Material Clear and Effective
27. Should each consent form be customized to include patient- and procedure-specific details to
explain expected difficulties and recovery time (question #2), treatment choices (question #8),
or the clinical rationale (question #9)?
No. Instead, the consent form must document that the conversation between the patient and the
clinician that covered these topics took place, and that the patient had the opportunity to ask
questions. For example, such language might read: “I acknowledge my treatment choices (including

2023 Leapfrog Hospital Survey – Hard Copy Section 1: Basic Hospital Information
50 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
the severity and probability of the risks and benefits of each choice) were explained to me, and that
expected difficulties as a result of undergoing the Procedure were explained to me, including recovery
time, pain management, and restrictions in the hospital and after I leave the hospital. I also
acknowledged that I understand why the Procedure is being performed.”
28. How should the reading level of the consent form be assessed?
There are software tools available to assess reading level. For example, consent forms can be edited
in Microsoft Word, where a readability tool can be used to make this assessment by: (1) on the “File”
tab, click the “Options” button; (2) on the “Proofing” tab, under “When correcting spelling and
grammar in Word”, make sure “Check grammar with spelling” is selected; (3) under “When correcting
spelling and grammar in Word”, select the “Show readability statistics” check box. After the grammar
check is complete, Word displays a message box showing you the readability grade-level.
29. Regarding the process for soliciting patient feedback in question #14, or the audit included in
question #15, what parameters should these processes follow? Are there a minimum number
of instances that should be audited, or a patient feedback evaluation form that should be
used?
Leapfrog has not established any specific requirements or forms for either the process audit, or the
solicitation of patient feedback. Results from both activities can be used to improve processes. Note
that although Leapfrog may specify a minimum sample for audits in subsequent Survey Cycles,
Leapfrog does not have plans at this time to designate a threshold rate for patient satisfaction or
consistent implementation of the informed consent policies assessed in the audit.

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
52 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
SECTION 2: MEDICATION SAFETY
This section includes questions and reference information for Section 2: Medication Safety. Please
carefully review the questions, endnotes, and reference information (e.g., measure specifications, notes,
and frequently asked questions) before you begin. Failure to review the reference information could result
in inaccurate responses.

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
53 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 2: 2023 Medication Safety
Computerized Physician Order Entry (CPOE) Fact Sheet and Bibliography:
https://ratings.leapfroggroup.org/measure/hospital/2023/safe-medication-ordering
Bar Code Medication Administration (BCMA) Fact Sheet and Bibliography:
https://ratings.leapfroggroup.org/measure/hospital/2023/safe-medication-administration
Medication Reconciliation Fact Sheet:
https://ratings.leapfroggroup.org/measure/hospital/2023/medication-reconciliation
In Section 2, pediatric hospitals should only respond to questions in Sections 2A and 2C.
Sections 2B and 2D do not apply to pediatric hospitals. Additionally, pediatric hospitals are not
required to complete the CPOE Evaluation Tool.
Section 2 includes questions about your hospital’s use of CPOE and BCMA, and (for adult and general
hospitals) questions about your hospital’s medication reconciliation process. The subsection on your
hospital’s EHR application (2B) is only applicable to adult and general hospitals and will not be scored or
publicly reported. In 2023, the subsection on BCMA (2C) includes new optional fact-finding questions
regarding your hospital’s use of BCMA in pre-op units, PACUs, and the emergency department, and will
not be scored or publicly reported.
Each hospital achieving the standard for Computerized Physician Order Entry (CPOE):
1. Assures that prescribers enter at least 85% of inpatient medication orders via a computer system
that includes decision support software to reduce prescribing errors, and
2. For adult and general hospitals, demonstrates, via a test, that its inpatient CPOE system can alert
physicians to at least 60% of frequent serious medication errors known to cause harm to patients.
Each hospital achieving the standard for Bar Code Medication Administration:
1. Has implemented the use of BCMA at the bedside in 100% of applicable units, and
2. Has achieved at least 95% compliance with scanning patients and medications during
administration in applicable units where BCMA is implemented, and
3. Has a BCMA system that includes all of the following types of decision support: wrong patient,
wrong medication, wrong dose, wrong time, and second nurse check needed, and
4. Has structures in place to monitor and reduce workarounds, which include having a formal
committee that meets routinely to review data reports on BCMA system use, having back-up
systems for hardware failures, having a help desk that provides timely responses to urgent BCMA
issues in real-time, conducting real-time observations of users using the BCMA system, and
engaging nursing leadership at the unit level on BCMA use. Additionally, information from these
structures is used to implement quality improvement projects or monitor previous quality
improvement projects focusing on the hospital’s BCMA system. Results from the quality
improvement projects are evaluated and demonstrate that these projects have resulted in higher
adherence to standard medication administration processes. Finally, resolution of system
deficiencies and/or problems that may have contributed to the workaround are communicated
back to the end user.
Each hospital achieving the standard for Medication Reconciliation:
1. Uses a nationally endorsed protocol to collect data on the accuracy of its medication
reconciliation process for at least 30 sampled patients and reported the data collected to
Leapfrog, and
2. Has a rate of unintentional medication discrepancies that
is lower than the 50th percentile (where
lower performance is better).
Download the 2023 Leapfrog Hospital Survey Scoring Algorithm on the
Scoring and Results
webpage.

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
54 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
2A: Computerized Physician Order Entry (CPOE)
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Notes:
Note 1: “Prescribers” used throughout this subsection refers to all licensed clinicians who are authorized
by the state in which the hospital is located to order medications for patients. This includes residents and
interns who are authorized to order medications under their own authority.
Note 2: Adult and general hospitals must complete an Adult Inpatient Test at least once per Survey Cycle
(April to November). Hospitals are encouraged to ensure that the Adult Inpatient CPOE Test is submitted
along with the Survey to meet the deadlines for Leapfrog’s Programs such as Top Hospital, Leapfrog
Hospital Safety Grade, etc.
Note 3: Hospitals will have access to the CPOE Evaluation Tool immediately after completing the Hospital
Profile in the Online Hospital Survey Tool.
Specifications: See Computerized Physician Order Entry (CPOE) Measure Specifications
in the
Reference Information beginning on page 65.
1) What is the latest 3-month reporting period for which your hospital is
submitting responses to this section? 3-month reporting period ending:
______
Format: Month/Year
2) Does your hospital have a functioning CPOE system in one or more
inpatient units of the hospital that:
• includes decision support software to reduce prescribing errors; and,
• is linked
17
to pharmacy, laboratory, and admitting-discharge-transfer
(ADT) information in your hospital?
If “no” to question #2, skip the remaining questions in Section 2A and
Section 2B, and continue to Section 2C. The hospital will be scored as
“Limited Achievement.”
Yes
No
3) Total number of inpatient medication orders, including orders made in
units that do NOT have a functioning CPOE system.
_____
4) Total number of inpatient medication orders in question #3 that
licensed prescribers entered via a CPOE system that meets the criteria
outlined in question #2.
_____
If “yes” to question #2 and you are an adult or general hospital, use the Take CPOE Tool button on the
Survey Dashboard to access the CPOE Evaluation Tool. Question #5 does not apply to pediatric
hospitals.
Reporting Period: 3 months
Answer questions #1-5 for the latest 3-month period prior to the submission of this section of the Survey.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted Surveys
only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission and
Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or publicly
reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
55 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
5) Hospital’s score when it tested its CPOE system using
the Leapfrog CPOE Evaluation Tool:
Adult Inpatient Test must be completed between
April 1 and November 30, 2023.
No response required here. Determined
automatically based on separately
completing a test using the Leapfrog
CPOE Evaluation Tool.

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
56 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
2B: EHR Application Information
This section is not applicable to pediatric hospitals.
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Note: This subsection will not be scored or publicly reported. The information collected in this
subsection is used by the CPOE Evaluation Tool developers for research and CPOE Evaluation Tool
improvements.
1) Which EHR source is your hospital currently using?
If your hospital purchased a third-party vendor
system and substantially altered it on
implementation, select “Homegrown App,” skip
questions #2-4, and continue to question #5.
Vendor Application
Homegrown Application
2) Which EHR vendor is your hospital currently using?
Allscripts/Eclipsys
CareCast
Cerner
CPSI
Epic
MEDHOST
MEDITECH
Quadramed
Other (please specify): _____
3) What EHR version is your hospital currently using?
_____
4) What is the name of the EHR product that your
hospital is currently using?
_____
5) When was the EHR initially installed at the hospital?
Enter the month and year the EHR was installed at
the hospital.
______
Format: Month/Year
6) Which EHR Medication Reference Database is your
hospital currently using?
Homegrown
First DataBank (FDB)
Gold Standard / Elsevier
Lexicomp
Medi – Span
Multum
Other (please specify): _____
Reporting Period: Answer questions #1-6 based on the EHR application currently in place at the
time you submit this section of the Survey.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.
2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
57 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
2C: Bar Code Medication Administration (BCMA)
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Specifications: See Bar Code Medication Administration (BCMA) Measure Specifications
in the
Reference Information beginning on page 67.
1) What is the latest 3-month reporting period for which your hospital is submitting
responses to questions #2-15? 3-month reporting period ending:
______
Format: Month/Year
2) Does your hospital use a Bar Code Medication Administration (BCMA) system
that is linked to the electronic medication administration record (eMAR) when
administering medications at the bedside in at least one inpatient unit?
If “no” to question #2, skip questions #3-30 and continue to the next subsection.
The hospital will be scored as “Limited Achievement.”
Yes
No
3) Does your hospital operate Intensive Care Units
18
(adult, pediatric, and/or
neonatal)?
If “no” to question #3, skip questions #4-5 and continue to question #6.
Yes
No
4) If “yes,” how many of this type of unit are open and staffed in the hospital? _____
5) How many of the units in question #4 utilized the BCMA/eMAR system when
administering medications at the bedside?
_____
6) Does your hospital operate Medical and/or Surgical Units (including
telemetry/step-down/progressive units)
19
(adult and/or pediatric)?
If “no” to question #6, skip questions #7-8 and continue to question #9.
Yes
No
7) If “yes,” how many of this type of unit were open and staffed in the hospital? _____
8) How many of the units in question #7 utilized the BCMA/eMAR system when
administering medications at the bedside?
_____
9) Does your hospital operate a Labor and Delivery Unit
20
?
If “no” to question #9, skip questions #10-11 and continue to question #12.
Yes
No
10) If “yes,” how many of this type of unit were open and staffed in the hospital? _____
Reporting Period: 3 months
Answer questions #1-15 for the latest 3-month period prior to the submission of this section of the
Survey.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
58 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
11) How many of the units in question #10 utilized the BCMA/eMAR system when
administering medications at the bedside?
_____
If “no” to questions #3, #6, and #9 above, skip questions #12-15 and continue to question #16. Your
hospital will be scored as “Does Not Apply.”
12) The number of scannable inpatient medication administrations during the
reporting period in those units that utilize BCMA as indicated in questions #5, #8,
and #11 above:
_____
13) The number of medication administrations from question #12 that had both the
patient and the medication scanned during administration with a BCMA system
that is linked to the electronic medication administration record (eMAR):
_____
14) What types of decision support does your hospital’s BCMA system provide to users of the system?
a) Wrong patient
Yes
No
b) Wrong medication
Yes
No
c) Wrong dose
Yes
No
d)
Wrong time (e.g., early/late warning; warning that medication cannot be
administered twice within a given window of time)
Yes
No
e) Second nurse check needed
Yes
No
15) Which of the following mechanisms does your hospital use to reduce and understand potential
BCMA system “workarounds”?
a)
Has a formal committee that meets routinely to review data reports on BCMA
system use
Yes
No
b) Has back-up systems for BCMA hardware failures
Yes
No
c)
Has a Help Desk that provides timely responses to urgent BCMA issues in
real-time
Yes
No
d)
Conducts real-time observations of users at the unit level using the BCMA
system
Yes
No
e) Engages nursing leadership at the unit level on BCMA use
Yes
No
f)
In the past 12 months used the data and information obtained through items a-
e to implement quality improvement projects that have focused on improving
the hospital’s BCMA performance
OR
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
59 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
In the past 12 months used the data and information obtained through items a-
e to monitor a previously implemented quality improvement project focused on
improving the hospital’s BCMA performance
Cannot respond “yes” to this question, unless “yes” to either 15d or 15e.
g)
In the past 12 months evaluated the results of the quality improvement projects
(from f) and demonstrated that these projects have resulted in higher
adherence to your hospital’s standard medication administration process
OR
In the past 12 months evaluated the results of the quality improvement projects
(from f) and demonstrated continued adherence to your hospital’s standard
medication administration process
Cannot respond “yes” to this question, unless “yes” to 15f.
Yes
No
h)
Communicated back to end users the resolution of any system deficiencies
and/or problems that may have contributed to workarounds
Cannot respond “yes” to this question, unless “yes” to either 15d or 15e.
Yes
No
Additional Questions (Optional – Fact Finding Only)
16) Does your hospital operate pre-operative units?
If your hospital has a combined pre-operative and post anesthesia care unit
(PACU), select “yes” to question #16 and report the data for the combined unit
in questions #17-20.
If “no” to question #16, skip questions #17-20 and continue to question #21.
Yes
No
17) If “yes,” how many of this type of unit are open and staffed in the hospital? _____
18) How many of the units in question #17 utilized the BCMA/eMAR system when
administering medications at the bedside?
_____
19) The number of scannable medication administrations during the reporting
period in those pre-operative units that utilize BCMA as indicated in question
#18 above:
_____
20) The number of medication administrations from question #19 that had both the
patient and the medication scanned during administration with a BCMA system
that is linked to the electronic medication administration record (eMAR):
_____
21) Does your hospital operate post anesthesia care units (PACUs)?
If your hospital has a combined pre-operative and post anesthesia care unit
(PACU), select “no” to question #21 and instead report on questions #16-20.
If “no” to question #21, skip questions #22-25 and continue to question #26.
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
60 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
22) If “yes,” how many of this type of unit were open and staffed in the hospital? _____
23) How many of the units in question #22 utilized the BCMA/eMAR system when
administering medications at the bedside?
_____
24) The number of scannable medication administrations during the reporting
period in those PACUs that utilize BCMA as indicated in question #23 above:
_____
25) The number of medication administrations from question #24 that had both the
patient and the medication scanned during administration with a BCMA system
that is linked to the eMAR:
_____
26) Does your hospital operate an emergency department?
If “no” to question #26, skip questions #27-30 and continue to the next
subsection.
Yes
No
27) If “yes,” how many of this type of unit were open and staffed in the hospital? _____
28) How many of the units in question #27 utilized the BCMA/eMAR system when
administering medications at the bedside?
_____
29) The number of scannable medication administrations during the reporting
period in those emergency departments that utilize BCMA as indicated in
question #28 above:
_____
30) The number of medication administrations from question #29 that had both the
patient and the medication scanned during administration with a BCMA system
that is linked to the eMAR:
_____

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
61 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
2D: Medication Reconciliation
This section is not applicable to pediatric hospitals.
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Specifications: See Medication Reconciliation Measure Specifications
in the Reference Information
beginning on page 69.
Sufficient Sample: See Medication Reconciliation Measure Specifications
for instructions on
identifying a sufficient sample for question #3.
1) What is the latest 6-month reporting period for which your hospital is submitting
responses to this section? 6-month reporting period ending:
______
Format: Month/Year
2) During the 6-month reporting period, did your hospital conduct a random sample
of adult patients and have a pharmacist or certified pharmacy technician
21
complete the following steps for each patient included in the sample:
• Interview Patient and Obtain the Gold Standard Medication History
(pharmacist or certified pharmacy technician
21
)
• Complete a Medication Reconciliation Worksheet for each sampled
patient (pharmacist or certified pharmacy technician
21
)
• Compare Gold Standard Medication History to Admission Orders
(pharmacist only)
• Compare Gold Standard Medication History to Discharge Orders
(pharmacist only)
If “no” to question #2, skip the remaining questions in Section 2D and go to the
Affirmation of Accuracy. The hospital will be scored as “Limited Achievement.”
If “no, had fewer than 30 admissions to medical or med/surg units” to question
#2, skip the remaining questions in Section 2D and go to the Affirmation of
Accuracy. The hospital will be scored as “Does Not Apply.”
Yes
No
No, had fewer
than 30
admissions to
medical or
med/surg units
For questions #3-8, if the sample is less than 30 patients from the 6-month period, the hospital will be
scored as “Some Achievement” and the rate of unintentional discrepancies will not be publicly reported.
3) Number of adult patients that your hospital sampled
22
. _____
Reporting Period: 6 months
Answer questions #1-8 for the latest 6-month period prior to the submission of this section of the Survey.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted Surveys
only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission and
Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or publicly
reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
62 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
4) Number of patients from question #3 who had zero (0) Gold Standard
Medications.
If more than 10 out of 30 patients (or one-third) included in the sample had zero
(0) Gold Standard Medications, the hospital will be scored as “Unable to
Calculate Score.”
_____
5) Total number of medications obtained by the pharmacist or certified pharmacy
technician
21
from the Gold Standard Medication History
23
for the adult patients
included in the sample.
_____
6) Total number of unintentional discrepancies in admission and discharge among
the gold standard medications
24
in question #5 identified by the pharmacist.
_____
7) Total number of unintentionally ordered additional medications
25
for the adult
patients included in the sample on admission and/or discharge identified by the
pharmacist.
_____
8) Total number of discrepancies due to unintentionally ordered additional
medications
26
in question #7 identified by the pharmacist.
_____

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
63 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Affirmation of Accuracy
As the hospital CEO or as an employee of the hospital to whom the hospital CEO has delegated
responsibility, I have reviewed this information pertaining to the Medication Safety Section at our hospital,
and I hereby certify that this information is true, accurate, and reflects the current, normal operating
circumstances at our hospital. I am authorized to make this certification on behalf of our hospital.
The hospital and I understand that The Leapfrog Group, its members, the public and entities and persons
who contract or have other business dealings with The Leapfrog Group are relying on the truth and
accuracy of this information. The hospital and I also understand that The Leapfrog Group will make this
information and/or analyses of this information public through the Survey Results public reporting website,
The Leapfrog Group’s Hospital Safety Grade, and/or other Leapfrog Group products and services. This
information and/or analyses and all intellectual property rights therein shall be and remain the sole and
exclusive property of The Leapfrog Group in which The Leapfrog Group retains exclusive ownership. This
information does not infringe upon any third-party intellectual property rights or any other third-party rights
whatsoever and is free and clear of all encumbrances and liens of any kind. The hospital and I
acknowledge that The Leapfrog Group may use this information in a commercial manner for profit. The
hospital shall be liable for and shall hold harmless and indemnify The Leapfrog Group from any and all
damages, demands, costs, or causes of action resulting from any inaccuracies in the information or any
misrepresentations in this Affirmation of Accuracy. The Leapfrog Group and its members and entities and
persons who contract or have other business dealings with The Leapfrog Group reserve the right to omit
or disclaim information that is not current, accurate or truthful.
Affirmed by _____________________, the hospital’s ___________________________,
(first name and last name) (title)
on _______________________.
(date)

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
64 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 2: 2023 Medication Safety Reference Information
What’s New in the 2023 Survey
We are combining Section 8: Medication Safety, which includes Section 8A: Bar Code Medication
Administration (BCMA) and Section 8B: Medication Reconciliation, with Section 2: Medication Safety.
Section 2: Medication Safety now includes the following subsections:
• Section 2A: Computerized Physician Order Entry (CPOE)
• Section 2B: EHR Application Information
• Section 2C: Bar Code Medication Administration (BCMA)
• Section 2D: Medication Reconciliation
Because Section 2 is required for submission, hospitals are required to complete subsections 2C: Bar
Code Medication Administration (BCMA) and 2D: Medication Reconciliation to submit the Survey.
Section 2B: EHR Application Information
Leapfrog is removing questions regarding Medicare Promoting Interoperability Program scores. As a
reminder, the remaining questions in this subsection are not scored or publicly reported.
CPOE Evaluation Tool
Leapfrog is making the CPOE Evaluation Tool available immediately upon completion of the Hospital
Profile. This allows adult and general hospitals to complete a CPOE Test at their earliest convenience
starting on April 1, without having to first complete and affirm Section 2. CPOE Test results will continue
to be scored and publicly reported only once the Survey has been submitted.
Additionally, Leapfrog is making several content updates for the Adult Inpatient Test. First, we are
updating the Test Order library as appropriate based on the latest published literature. Second, we are
removing the Drug Allergy Order Checking Category due to sustained high performance in this category
across all hospitals over multiple years. Finally, we are combining the Drug Dose (Single) and Drug Dose
(Daily) Order Checking Categories into a single Order Checking Category renamed Excessive Dose
which includes both single and daily dose testing scenarios.
Although the total number of Test Orders is decreasing, there are no changes to the scoring algorithm for
the Adult Inpatient Test.
Section 2C: Bar Code Medication Administration
There are no changes to the questions used in scoring or the scoring algorithm for this subsection.
However, based on feedback received from hospitals as well as guidance from Leapfrog’s Bar Code
Medication Administration Expert Panel, Leapfrog is updating the optional, fact-finding questions included
in this subsection to focus on BCMA utilization and compliance in the following areas: pre-operative units,
post anesthesia care units (PACUs), and emergency departments. Based on questions received during
the public comment period, Leapfrog is adding clarifying information to instruct hospitals that have a
combined pre-operative and post anesthesia care unit to report their combined data in the pre-operative
unit questions. These new questions will not be scored or publicly reported in 2023.
Change Summary Since Release
None. If substantive changes are made to this section of the Survey after release on April 1, 2023, they
will be documented in this Change Summary section.

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
65 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Medication Safety Measure Specifications
Computerized Physician Order Entry (CPOE)
Important Notes:
Note 1: Hospitals that share a CMS Certification Number are required to report by facility. Please carefully
review Leapfrog’s Multi-Campus Reporting Policy
.
Note 2: “Licensed prescriber” refers to all licensed clinicians who are authorized by the state in which the
hospital is located to order medications for patients. This includes residents and interns who are
authorized to order medications under their own authority.
Source: The Leapfrog Group
Reporting Period: The latest 3-month period prior to the submission of this section of the Survey.
Medications: For the purposes of this measure, a medication is a substance that is taken into, or placed
onto, the body of a person for one or more of the following reasons:
• as a placebo
• to prevent a disease (e.g., flu vaccine)
• to make a diagnosis (e.g., contrast dye)
• to test for the possibility of an adverse effect
• to modify a physiological, biochemical, or anatomical function or abnormality (e.g.,
heparin/heparin flushes, statins, antihypertensives, etc.)
• to replace a missing factor (e.g., blood product)
• to ameliorate a symptom (e.g., aspirin)
• to treat a disease or condition (including topicals, nasal sprays, eye drops, compounds,
inhalants, injectables, patches, etc.)
• to induce anesthesia
• to stabilize or hydrate during medical treatment or procedures (e.g., IV fluids, normal
saline, lactated ringers, etc.)
• to provide nutrition (e.g., enteral nutrition products, parenteral nutrition products, breast
milk [breast milk applies to BCMA measure only])
• as a supplement (e.g., iron for a patient with iron deficiency anemia or calcium/vitamin D
for a patient with osteoporosis)
For the purposes of this measure, the following are not considered medications:
• Pre-filled saline flushes and pre-filled heparin flushes
Question #3 (denominator): Total number of inpatient medication orders, including medication orders
made in inpatients units that do NOT have a functioning CPOE system.
Include:
• Medications ordered for any patient with an inpatient status, including those with an inpatient
status who may be in an outpatient unit during an overflow situation.
• Medications ordered for any neonatal ICU patient.
• Medications ordered by any individual, including nurses and pharmacists.
• Medications ordered electronically, verbally, or via paper.
• Medications ordered verbally during a rapid response (prevention of serious injury, cardiac arrest,
and respiratory arrest).
Exclude:
• Medications ordered for any newborn in a nursery or mother’s room.

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
66 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
• New medication orders that have been modified from the original order but maintain the intent of
the original order (e.g., “new” medication orders generated when a dose, route, frequency, or
brand are changed).
• Medications ordered verbally during a “code blue” (i.e., medications ordered verbally when a
patient requires resuscitation or needs immediate medical attention, most often as the result of a
respiratory arrest or cardiac arrest).
• Medications ordered in an operating room or procedural area.
Question #4 (numerator): Total number of inpatient medication orders in question #3 that licensed
prescribers entered via a CPOE system that meets the criteria outlined in question #2.
Include:
• Medication orders entered in to the CPOE system by a licensed prescriber
• In addition, the following types of medication orders entered in to a CPOE system by a non-
licensed prescriber, such as a nurse or pharmacist, can also be included:
o Per protocol medication orders (i.e., medication orders with detailed guidance on how to
administer, dose or adjust a medication) approved by a medical committee where the
implementation of the protocol was authorized by a
licensed prescriber and orders were
initiated in the CPOE system.
o Standing medication orders (i.e., orders which can be initiated based on medical staff
approval of a screening criteria and indication for all patients that meet the screening
criteria) approved by a medical committee that were initiated in the CPOE system.
o Med
ication orders entered in to the CPOE system that required either verbal read back of
alerts to the licensed prescriber or a co-signature by a licensed prescriber prior to
administration to ensure that the licensed prescriber was made aware of all
advice/information generated by the CPOE system.
Exclude:
• Medications ordered verbally without readback of alerts to a licensed prescriber or medications
ordered via paper.
• Medication orders entered into the CPOE system by a non-licensed prescriber, such as a nurse
or pharmacist, that were NOT per protocol medication orders or standing medication orders.
• Medication orders entered in to the CPOE system by a non-licensed prescriber, such as a nurse
or pharmacist, that DID NOT require either verbal read back of alerts or a co-signature of a
licensed prescriber prior to administration.
See FAQs
for additional information about responding to the questions in this section.

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
67 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Bar Code Medication Administration (BCMA)
Important Note: Hospitals that share a CMS Certification Number are required to report by facility.
Please carefully review Leapfrog’s Multi-Campus Reporting Policy
.
Source: The Leapfrog Group
Reporting Period: The latest 3-month period prior to the submission of this section of the Survey.
Medications: For the purposes of this measure, a medication is a substance that is taken into, or
placed onto, the body of a person for one or more of the following reasons:
• as a placebo
• to prevent a disease (e.g., flu vaccine)
• to make a diagnosis (e.g., contrast dye)
• to test for the possibility of an adverse effect
• to modify a physiological, biochemical or anatomical function or abnormality (e.g.,
heparin/heparin flushes, statins, antihypertensives, etc.)
• to replace a missing factor (e.g., blood product)
• to ameliorate a symptom (e.g., aspirin)
• to treat a disease or condition (including topicals, nasal sprays, eye drops, compounds,
inhalants, injectables, patches, etc.)
• to induce anesthesia
• to stabilize or hydrate during medical treatment or procedures (e.g., IV fluids, normal saline,
lactated ringers, etc.)
• to provide nutrition (e.g., enteral nutrition products, parenteral nutrition products, breast milk
[breast milk applies to BCMA measure only])
• as a supplement (e.g., iron for a patient with iron deficiency anemia or calcium/vitamin D for a
patient with osteoporosis)
For the purposes of this measure, the following are not considered medications:
• Pre-filled saline flushes and pre-filled heparin flushes
Question #12 (denominator): Total number of scannable inpatient medication administrations during
the reporting period in those units that utilize BCMA as indicated in questions #5, #8, and #11.
Include: All scannable inpatient medication administrations in intensive care units (adult, pediatric,
and/or neonatal), medical and/or surgical units (including telemetry/step-down/progressive units) (adult
and/or pediatric), and labor and delivery units that utilize a BCMA system that is linked to the electronic
medication administration record (eMAR)
Exclude:
• Medication administrations in units that are not specified in question #5, #8, or #11
• Medication administrations in units that do not utilize a BCMA system that is linked to the eMAR
• Medication administrations in units that have not been open or staffed for the entire 3-month
reporting period
• Administrations of medications that are not “scannable,” i.e., medications that do not have a bar
code and could not be scanned if BCMA were in use
Question #13 (numerator): Total number of medication administrations from question #12 that had
both the patient and the medication scanned during administration with a BCMA system that is linked to
the eMAR.
Include: Medication administrations where both the patient and medication were scanned during
administration with a BCMA system that is linked to the eMAR
Exclude: Medication administrations where neither patient nor medication were scanned during
administration or only the patient or only the medication were scanned with a BCMA system that is
linked to the eMAR

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
68 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Question #19, #24, and #29 (denominator): Total number of scannable medication administrations
during the reporting period in those units that utilize BCMA as indicated in questions #18 (pre-operative
units), #23 (post anesthesia care units), or #28 (emergency department units)
Include: All scannable medication administrations in the specific unit identified in the question (#19: pre-
operative care units, #25: post anesthesia care units, #29: emergency department units) that utilize a
BCMA system that is linked to the electronic medication administration record (eMAR)
Exclude:
• Medication administrations in units that are not specified in question #18, #24, or #28
• Medication administrations in units that do not utilize a BCMA system that is linked to the eMAR
• Medication administrations in units that have not been open or staffed for the entire 3-month
reporting period
• Administrations of medications that are not “scannable,” i.e., medications that do not have a bar
code and could not be scanned if BCMA were in use
Question #20, #25, and #30 (numerator): Total number of medication administrations from question
#19, #24, or #29 that had both the patient and the medication scanned during administration with a
BCMA system that is linked to the eMAR.
Include: Medication administrations where both the patient and medication were scanned during
administration with a BCMA system that is linked to the eMAR
Exclude: Medication administrations where neither patient nor medication were scanned during
administration or only the patient or only the medication were scanned with a BCMA system that is
linked to the eMAR
See FAQs for additional information about responding to the questions in this section.

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
69 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Medication Reconciliation
Important Notes:
Note 1: This section does not apply to pediatric hospitals.
Note 2: Hospitals that share a CMS Certification Number are required to report by facility. Please carefully
review Leapfrog’s Multi-Campus Reporting Policy
.
Note 3: A hospital pharmacist or certified pharmacy technician
21
plays two important roles in data
collection for this measure. First, the pharmacist or certified pharmacy technician is responsible for
obtaining the Gold Standard Medication History from each sampled patient. Second, the pharmacist is
responsible for identifying the unintentional discrepancies by comparing the Gold Standard Medication
History to admission orders and discharge orders.
Source: Brigham and Women’s Hospital (NQF #2456)
Reporting Period: The latest 6-month period prior to submission of this section of the Survey.
Materials for Data Collection:
To complete the data collection for this subsection and respond to questions #3-8 hospitals should
download four important tools:
• The Medication Reconciliation Workbook
(Excel) includes 3 tabs: Instructions, Sampling, and
Data Entry. This can be used to identify patients to sample and collect data from, as well as
calculate the responses to enter into the Online Hospital Survey Tool from the completed
Worksheets.
• The Medication Reconciliation Worksheet (Word) can be used by the pharmacist to identify the
number of unintentional discrepancies at admission and/or discharge for each sampled patient
(question #6). The Medication Reconciliation Worksheet can also be used to track additional
medications that were ordered unintentionally at admission and/or discharge (questions #7-8).
• The MARQUIS 2 Best Possible Medication History: Quick Tips tri-fold (PDF) includes important
information on how the pharmacist or certified pharmacy technician
21
should obtain the Gold
Standard Medication History
• Identifying Discrepancies Flow Chart (PDF) includes important information on how the
pharmacist should identify discrepancies between the Gold Standard Medication History and
admission and discharge orders.
All of these tools are available on the Survey and CPOE Materials webpage
and should be used when
reporting on this measure.
The intent of this measure is to calculate your hospital’s rate of unintentional medication discrepancies
per medication. Data collection requirements for this measure can be met in two ways:
1. Hospitals that started and continued to sample 15 patients on a quarterly basis using the 2022
Leapfrog Hospital Survey measure specifications, can use those data when reporting on this section
of the Survey (i.e., 30 patients over a 6-month reporting period).
2. Hospitals that did not start sampling patients in 2022, can sample in real-time (i.e., sampling occurs
after April 1) and start data collection anytime during the Survey Cycle (April 1 – November 30).
Sampling can be performed over the entire reporting period, or within a shorter reporting period (i.e.,
a few weeks).
Follow the steps below to complete data collection for this measure:

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
70 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Step 1: Identify Patients to Include in the Sample (Survey Coordinator)
a) To be scored on the rate of unintentional medication discrepancies, hospitals are required to sample
at least 30 patients from a 6-month reporting period. Hospitals that sample fewer than 30 patients
during the reporting period will be scored and publicly reported as “Some Achievement.”
b) All hospitals should exclude patients under 18 years old, patients who were discharged or expired
before the Gold Standard Medication History could be obtained, and patients that do not have
discharge orders written during the reporting period.
c) Hospitals should sample patients from medical and med-surg units to reduce the number of sampled
patients with zero (0) Gold Standard Medications. However, hospitals may expand their sampling to
patients in additional units of the hospitals.
d) Hospitals should sample patients from different days of the week, including patients admitted on the
weekend.
e) On the day of data collection, obtain a list of patients that were admitted the day before to medical
and/or med-surg units, in the order that they were admitted.
f) Follow the instructions in the Medication Reconciliation Workbook to randomly sample admitted
patients.
g) Send the list of sampled patients to the pharmacist, pharmacy resident, or pharmacy technician so
they can schedule an in-person, video, or phone interview with each patient to obtain their Gold
Standard Medication History.
Step 2: Interview Patients and Obtain the Gold Standard Medication History (Pharmacist or
Certified Pharmacy Technician
21
)
a. A trained pharmacist, pharmacy resident, or certified pharmacy technician
21
must interview the
patients from Step 1 and obtain the Gold Standard Medication History within 24 hours after
admission, typically the morning after admission. Note that this is in addition to, and separate from,
any pre-admission medication list that was created as part of normal care.
b. The pharmacist, pharmacy resident, or certified pharmacy technician
21
can customize the following
script to explain to the patient the reason for the interview: “Hi, I’m (pharmacist’s name), a pharmacist
at (name of hospital). I know (care team member who may have collected a pre-admission
medication list or PAML) already asked you about the medications you were taking before you were
admitted to the hospital. I’m here to ask you about these medications again. Our hospital has asked
me to collect this information again so I can use it to measure how well we are doing in gathering
medication histories. What we learn will help us improve our processes of care in the future and make
sure we manage patients’ medications safely when they come into and after they leave the hospital.”
c. The Gold Standard Medication History is the list of medications that the patient was taking prior to
admission. Best practices for collecting the Gold Standard Medication History can be found in the
“Other Supporting Materials” for Section 2 on the Survey and CPOE Materials webpage
.
i. Pharmacists, pharmacy residents, and certified pharmacy technicians
21
should try to use two
sources of information (see MARQUIS 2 Best Possible Medication History: Quick Tips and
Medication Reconciliation Implementation Toolkit for examples) and explore any
discrepancies (e.g., errors related to dose, route, timing, etc.) before finalizing the Gold
Standard Medication History. Once the pharmacist, pharmacy resident, or certified pharmacy
technician
21
has completed the patient interview, they can utilize support from non-certified
pharmacy technicians, medical assistants, or nurses to investigate second or third
information sources (e.g., EHR list, pharmacy list, primary or specialty provider information,
etc.). However, the pharmacist, pharmacy resident, or
certified pharmacy technician
21
must
be the one to obtain and finalize the Gold Standard Medication History.
ii. Medications that a patient is completely non-adherent to, meaning the patient has not taken
the medication for at least 30 days, should be excluded from the Gold Standard Medication
History.
iii. If a patient has been taking a medication differently to how it was prescribed, then the Gold
Standard Medication History should list the medication as the patient was taking it.
iv. Exclude PRN medications unless they are clinically relevant. This includes topical locations
and creams, saline nasal spray and artificial tear eye drops, herbals and supplements, and
vitamins. Two examples of clinically relevant medications that should not be excluded from

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
71 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
the gold standard pre-admission medication list would be iron for a patient with iron-
deficiency anemia or calcium/vitamin D for a patient with osteoporosis.
v. Include PRN medications that are clinically relevant. This includes inhalers, nitroglycerin,
analgesics (opioid and non-opioid), muscle relaxants, and sedatives, including medications
where sedation is a common side effect (e.g., sedation occurs in more than 10% of
individuals). This also includes topical medications with systemic actions (e.g., lidocaine,
triamcinolone, sulfadiazine, etc.).
vi. Clinically relevant is defined as any PRN medication that treats a medical condition on the
patient’s problem list and/or condition they are being treated for during the visit (i.e., reason
for the hospital stay).
Step 3: Complete a Medication Reconciliation Worksheet for each sampled patient (Pharmacist or
Certified Pharmacy Technician
21
)
a. Once the Gold Standard Medication History is complete, print out the Medication Reconciliation
Worksheet for each sampled patient.
b. Page 1 needs to be printed out for each sampled patient. Page 2 needs to be printed for each Gold
Standard Medication the patient was taking.
c. Complete the “Gold Standard Medication” column highlighted in yellow for each Gold Standard
Medication on the list:
d. If possible, wait until after the patient has been discharged to complete the next steps.
Step 4: Compare Gold Standard Medication History to Admission Orders (Pharmacist Only)
a. After the patient has been discharged, obtain the admission and discharge orders for the patient
(instructions for comparing the discharge orders are in Step 5 below). Admission orders include all
orders written from the time of admission until 8:00 a.m. the following morning or up until 12 hours
after the time of admission, whichever comes first.
b. Compare the admission orders to each Gold Standard Medication on the Medication Reconciliation
Worksheet. Note any differences.
i. Review the records for the patient to determine if the differences were intentional or
unintentional. Use the Identifying Discrepancies Flow Charts
to help with this step.

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
72 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ii. If the discrepancy was unintentional, then check the box next to “Yes” highlighted in
orange. Otherwise, check “No.”
c. Review the admission orders for any medications that were not listed in the Gold Standard
Medication History. If any of the additional medications were ordered unintentionally, list it on the
first page of the Medication Reconciliation Worksheet in the blue-highlighted column. You may need
to contact the Provider to determine if the medication was ordered unintentionally or not.
d. If the pharmacist identifies an unintentional discrepancy in the admission orders prior to discharge
and alerts the physician so that the unintentional discrepancy can be corrected prior to discharge, this
should be recorded on the Medication Reconciliation Worksheet as an unintentional discrepancy in
the Admission Comparison and Discharge Comparison columns of the worksheet.
Step 5: Compare Gold Standard Medication History to Discharge Orders (Pharmacist Only)
a. Obtain the discharge orders for the patient.
b. Perform the comparison between the Gold Standard Medications and the discharge orders using the
same steps as the admission comparison. Remember to use the
Identifying Discrepancies Flow
Charts to help with this step.
c. Review the discharge orders for any medications that were not listed in the Gold Standard Medication
History. If any of the additional medications were ordered unintentionally, list them on the first page
of the Medication Reconciliation Worksheet in the blue-highlighted column.

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
73 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
i. If an unintentionally ordered additional medication was only ordered on admission, then
check the “Admission” box in the pink-highlighted column.
ii. If an unintentionally ordered additional medication was only ordered on discharge, then
check the “Discharge” box in the pink -highlighted column.
iii. If the unintentionally ordered additional medication was ordered on both admission and
discharge, then check the “Both” box in the pink-highlighted column.
Step 6: Sum the number of medications and discrepancies (Survey Coordinator)
a. The top of the first page of the Medication Reconciliation Worksheet contains four spaces for you to
list the data for the patient.
i. Find the number of unintentionally ordered additional medications from the blue highlighted
column on the same page. Enter in the first space, also highlighted in blue.
ii. Find the number of discrepancies due to unintentionally ordered additional medications
from the pink highlighted column on the same page.
1. If a medication was only ordered on admission or only ordered on discharge,
then this counts as one discrepancy.
2. If a medication was ordered on both admission and discharge, then this counts
as two discrepancies.
3. Sum the number of discrepancies across all unintentionally ordered additional
medications and enter this number in the pink-highlighted space.
iii. Find the number of Gold Standard Medications and enter this number in the yellow-
highlighted space.
iv. Find the number of discrepancies in the admission and discharge orders for the Gold
Standard Medications
1. Review the Medication Reconciliation Worksheet for each Gold Standard Medication.
2. Sum the number of times the orange highlighted “Yes” box is checked, indicating an
unintentional discrepancy in admission or discharge orders.
3. Enter this sum into the orange-highlighted space on the first page of the Medication
Reconciliation Worksheet.
Step 7: Contact providers if necessary (Pharmacist Only)
If you found any serious discrepancies that could cause the patient harm, you will need to contact the
providers. The provider may need to reach out to the patient, PCP, or pharmacies to have the issue
corrected.
Step 8: Enter data into Excel Workbook and Online Hospital Survey Tool (Survey Coordinator)
a. Collect all the Medication Reconciliation Worksheets from the pharmacists. Open the Data Entry Tab
of the Medication Reconciliation Excel Workbook.
i. Enter the numbers from the top of each Medication Reconciliation Worksheet into the
corresponding columns of the Excel Workbook, for the sampled patient, one patient per
row.

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
74 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ii. As you enter the data, Row 6 (in red) will automatically sum the values entered for each
patient. Once you have entered the data for all sampled patients, you will have the final
values to enter into the Online Hospital Survey Tool.
iii. On the right, your hospital’s rate of unintentional medication discrepancies per medication
will automatically be calculated based on the data entered.
Step 9: Use your hospital’s results in quality improvement (Survey Coordinator and Pharmacist)
The developer of this measure has two toolkits available for hospitals that wish to implement a medication
reconciliation program:
• Medication Reconciliation Implementation Toolkit
(free)
• The MARQUIS Collaborative (fee to participate)
See FAQs
for additional information about responding to the questions in this section.

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
75 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Medication Safety Frequently Asked Questions (FAQs)
Computerized Physician Order Entry (CPOE)
Information specific to the CPOE Evaluation Tool, including instructions and FAQs, is available on
the Survey and CPOE Materials webpage and the Prepare for CPOE Tool webpage.
1. What 3-month reporting period should be used when reporting on this section?
When responding to the questions in Section 2A, hospitals should use the most recent three
months prior to submitting the Survey for which they have complete data. For example, if
submitting Section 2A in the month of June, the most recent 3-month reporting period for which
you have complete data may be February, March, and April or March, April, and May. Hospitals
cannot submit data collected for less than three full months.
2. My hospital transitioned to a new CPOE system, and we have not been on the new system
for a full 3 months. How should we respond to the questions in Section 2A-2B?
When responding to questions #3 and #4 in Section 2A, hospitals can combine inpatient
medication data from their old CPOE system with their new CPOE system to have three full
months of data. However, hospitals should respond to the questions in Section 2B based on the
new CPOE system and take the CPOE Evaluation Tool using the new CPOE system. When
taking the CPOE Evaluation Tool, hospitals should select a licensed prescriber that is fully trained
on the new system. All hospitals are urged to take a Sample Test before starting the Adult
Inpatient Test.
3. Should we include medications ordered verbally or via paper during system downtime
when responding to question #3 (denominator)?
Yes. Count medication orders entered during system downtime when responding to question #3.
Exclude these orders when responding to question #4.
4. We can’t exclude medication orders that originated from an operating room or procedural
area from the CPOE report that we are using to respond to questions #3 (denominator) and
#4 (numerator). Can we include these medication orders?
Yes, if you are unable to exclude medication orders for inpatients that were ordered from an
operating room or procedural area, you can count these orders when responding to questions #3
and #4.
5. What are examples of per protocol orders?
Examples of per protocol orders include vancomycin dosing per protocol and heparin drip per
protocol. Per protocol orders refer to orders that the medical staff has approved the use of, based
on evidence-based guidance, in advance of its use. Per protocol orders are often identified by the
Pharmacy and Therapeutics (P&T) committee and then recommended to the Medical Executive
Committee (MEC) for approval. Once approved, per protocol orders are initiated by a
licensed
prescriber, but then acted on by pharmacy, nursing, radiology, respiratory therapy, etc. Count per
protocol orders when responding to questions #3 (denominator) and #4 (numerator).
6. What are examples of standing orders?
Examples of standing orders include influenza vaccine, pneumonia vaccine, hepatitis B vaccine,
or erythromycin eye ointment. Standing orders are usually rare, limited use situations. Count
standing orders when responding to questions #3 (denominator) and #4 (numerator).
7. My hospital requires readback for all phone orders, but there is no way to document that
the readback occurred. How can we include these orders when responding to question #4
(numerator)?
If your hospital cannot document that verbal readback on phone orders occurred, you should
develop a written policy that outlines training and implementation of this practice and perform a

2023 Leapfrog Hospital Survey – Hard Copy Section 2: Medication Safety
76 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
random audit at least annually to ensure the policy is being followed. Maintain documentation of
the audit that demonstrates compliance with the policy as Leapfrog performs monthly
documentation requests starting with June 30
submissions and continuing until the Survey closes
on January 31.
8. When should we take the CPOE Evaluation Tool?
The CPOE Evaluation Tool is a core element of Leapfrog’s CPOE Standard. Hospitals are urged
to ensure that the Adult Inpatient CPOE Test is submitted along with the Survey (i.e., in the same
month) in order to meet the deadlines
for the Leapfrog Hospital Survey and Leapfrog’s other
programs such as Top Hospital and the Leapfrog Hospital Safety Grade.
Hospitals that submit the Survey, but do not submit an Adult Inpatient Test via the CPOE
Evaluation Tool, are scored and publicly reported as “Limited Achievement” on the CPOE
measure.
Within a Survey Cycle (April 1 – November 30), a hospital cannot retake a CPOE Evaluation Tool
until at least 120 days have passed since their last test.
9. Should hospitals take the CPOE Test more than once per Survey Cycle?
Not necessarily. Hospitals have the option of re-taking a test after 120 days, but if there were no
changes made to your CPOE system after the first test there is likely no reason to retake the test.
Hospitals that switch vendors, perform major upgrades, or make significant changes to their
decision support settings and/or formulary (e.g., expanding from a limited formulary to a large
formulary) should re-take the test to ensure the results reflect their current system.
10. If we update our responses to questions #3 and #4, do we need to re-take the CPOE Test?
No. Hospitals don’t need to retake the CPOE Evaluation Tool after submitting updates to Section
2 via the Online Survey Tool. Retaking a test is only recommended for hospitals that switched
vendors, performed major upgrades, or made significant changes to their decision support
settings and/or formulary.
Bar Code Medication Administration (BCMA)
General Questions
11. Why does the Bar Code Medication Administration system have to be connected to an
electronic medication administration record (eMAR)?
An eMAR serves as the communication interface that automatically documents the administration
of medication into certified Electronic Health Record (EHR) technology. By linking BCMA with the
eMAR, information on medication administration is captured in a much timelier manner than a
manual documentation process can accomplish.
Units
12. Should a unit that has only been open for part of the 3-month reporting period be
included?
No. Only include those units that have been opened and staffed for the entire 3-month reporting
period. For example, if you open a new unit that has only been open and staffed for 1-month out
of the 3-month reporting period, you will not include that unit when responding to the questions in
this section.
13. How should a hospital report if they had BCMA implemented in some units but not all
during the reporting period?
To answer “yes” to question #2, your hospital must have a Bar Code Medication Administration
(BCMA) system that is linked to the electronic medication administration record (eMAR) when
administering medications at the bedside in at least one inpatient unit. You should only report on

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Medication Safety
77 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
inpatient units that have been in operation for the entire 3-month reporting period selected in
question #1. If you have 3 surgical units and are only using BCMA in 1 of the 3 units, you will
respond 3 in question #7 and 1 in question #8. You should only include inpatient medication
administrations that were ordered and scannable in the open and staffed units in which a BCMA
system was implemented (as indicated in questions #5, #8, and #11) when reporting on
compliance in questions #12-13.
Compliance
14. What is considered a “scannable” medication?
Any medication (see measure specifications
) that has a bar code and could be scanned if BCMA
were in use would be considered “scannable.” In question #12, #19, #24, and #29, hospitals
should only include ordered and “scannable” medications from the units where they indicated a
BCMA system was implemented.
15. Should we exclude medications that were administered in units that are not currently
implementing BCMA?
Yes. Questions #12, #19, #24, and #29 are asking about inpatient medication administrations
ordered and scannable in those units that are open and staffed and that have implemented a
BCMA system. You should include all scannable medications ordered and administered to
inpatients in the open and staffed units (from questions #5, #8, #11, #18, #23, and #28) in which
a BCMA system was implemented. Questions #12, #19, #24, and #29 are used as the
denominator to calculate the rate of compliance and should include administrations whether the
medication and/or patient was scanned during the administration. Questions #13, #20, #25, and
#30 are used as the numerator and are where you would report the number of inpatient
medication administrations from these units where the patient and medication were scanned
during the administration. We would not expect these two responses to be the same.
16. Should we be excluding medications that are given during emergencies when responding
to questions #12-13, #19-20, #24-25, and #29-30?
No. If the medications are considered "scannable" e.g., any medication that has a bar code and
could be scanned if BCMA were in use, they should be included in questions #12, #19, #24, and
#29 (the denominator) but would not be counted in questions #13, #20, #25, and #30 (the
numerator) if the patient and medication were not scanned during administration, even if due to
an emergency.
Leapfrog's target rate for compliance is 95% to allow for emergency cases such as these. Please
note that questions #16-30 are not used in scoring or public reporting.
17. Should we exclude medications that are given during a system downtime when
responding to questions #12-13, #19-20, #24-25, and #29-30?
No. Medications administered during a system downtime should be included in questions #12,
#19, #24, and #29 (the denominator) but would not be counted in questions #13, #20, #25, and
#30 (the numerator) if the patient and medication were not scanned during administration
because your BCMA system was down.
18. Our vendor report provides medication and patient scanning rates separately. How can we
report on our compliance if our reports do not provide the number of administrations that
had both the medication and patient scanned?
If your vendor’s report only provides the percentage of patients scanned and the percentage of
medications scanned separately but does not provide the rate of administrations where both the
patient and medication were scanned, please report the lower of the two rates (supply the
denominator in questions #12, #19, #24, and #29 and numerator in questions #13, #20, #25, and
#30), as that would be the maximum possible rate of having both scanned.
Moving forward, we do ask that your hospital work with your vendor to report out the rate of
administrations where both the patient and medication are scanned. It is only when both are
scanned that safe medication administration can be ensured.

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Medication Safety
78 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
19. Is manual scanning (e.g., in lieu of scanning the patient’s wristband, typing in the patient’s
number) something we can count in our BCMA scans?
No. The problem is that the user may type in the wrong patient number, negating the safety
benefits. The best practice is to scan the wristband that is on the wrist of the patient.
20. Our process is to scan the patient once and then scan each medication. Questions #13,
#20, #25, and #30 seem to want each medication and patient scanned with each
medication. Can you clarify?
Hospitals should report on the number of administrations where both the patient and the
medication were scanned during the administration. During administrations where multiple
medications are administered sequentially, the patient should be scanned first, but does not need
to be rescanned before each medication is administered.
21. In our hospital some medications are ordered and scheduled, but not administered.
Should medications that are ordered and scheduled, but not administered be included
when responding to questions #12-13, #19-20, #24-25, and #29-30?
No, medications that are not administered should not be included in questions #12-13, 19-20,
#24-25, and #29-30.
Decision Support
22. If an alert is part of the eMAR, but not the Bar Code Medication Administration system,
should we respond “yes” to the decision support elements in question #14a - e?
If the provider and pharmacist are notified or alerted (e.g., second nurse check), but the nurse or
provider administering the medication does not receive an alert at the point of administration, then
your hospital should answer “no” to these questions about decision support.
23. My hospital’s EHR workflow for medication administration is designed in such a way that
our system will never generate a “wrong patient” alert. How should we answer the
question in the Survey about whether we have that type of decision support?
If your hospital’s EHR workflow is designed so that the nurse scans the patient first, and then the
medications, such that the nurse would never receive a “wrong patient” alert, for purposes of the
Survey, your hospital should indicate that it has ‘wrong patient’ decision support. The goal of
including a "wrong patient” alert is to acknowledge that as a safe practice and to drive
organizations to validate the “right patient” in the medication administration process. The workflow
described helps ensure that a ‘wrong patient’ is not encountered.
Workarounds
24. Must a hospital establish a separate committee to meet solely to review data reports on
BCMA system use?
While establishing a committee that has the sole purpose of reviewing data reports on BCMA
system use is encouraged, it is not required to meet Leapfrog’s standard. At a minimum, a pre-
existing standing committee that meets on a regular basis could be given the responsibility of
reviewing these reports. The committee chosen to review the reports must include individuals
whose roles reflect each part of the BCMA process (e.g., pharmacists, nurses, IT personnel, etc.).
25. What are some examples of “back-up systems” for hardware failures?
Examples of “back-up systems” include extra BCMA scanners, portable computers, batteries, and
mice that are easily accessible to nurses experiencing equipment malfunctions. Quickly replacing
malfunctioning equipment is essential to prevent workarounds.
26. What are some examples of “engaging nursing leadership at the unit level on BCMA use?”
Engaging nursing leadership on BCMA use should be an active, ongoing process. An engaged
leader would actively use BCMA data to coach staff towards safe or desired behaviors. Examples
of activities in which nursing leadership could be engaged include, but are not limited to:

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Medication Safety
79 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
• Education sessions in units
• Review of policies regarding use and non-use of BCMA
• Investigating problems with BCMA specific to the unit
• Providing a forum for users to report BCMA problems and reasons for workarounds
• Providing suggestions for improvements to both technology and process
27. The Leapfrog Survey asks whether our hospital conducts real-time observations of users
at the unit level using the BCMA system. How often should hospitals conduct these real-
time observations and what do ‘best practices’ look like?
At a minimum, the observations should be conducted on each unit at least biannually (2x/year)
and should be 30 minutes or 30 medication administrations in length, whichever is shorter. If
observations are done by a central team, they should ensure each type of unit is observed within
that 6-month period. More frequent observations are appropriate for hospitals that have recently
introduced BCMA and/or when safety reports indicate a problem, where observations can be
helpful in understanding the root cause of an incident or near miss.
The observations should be direct nurse-BCMA observations, watching how the nurse uses the
BCMA system as part of the bedside medication administration process. The observer should
note if nurses are using any workarounds to compensate for system issues. In addition to
observing the nurse-BCMA interaction, the observer should also have conversations with the
nurses, as their comments are often a good source for understanding the causes of workarounds.
The observations collected from the different units should be aggregated together to understand
trends across the hospital and where the hospital should place a priority for addressing BCMA
system issues.
28. What are some appropriate mechanisms for communicating back to end users for
question #15h?
Appropriate mechanisms for communicating back to end users would include staff meetings,
safety briefings or unit huddles, or sharing in a Nursing Informatics eNewsletter, through a
Medication Safety Team, or Quality Committee newsletter/flyer.
Additional Questions
29. How should hospitals report on the additional fact-finding questions if they have a
combined pre-operative and post anesthesia care unit (PACU)?
Hospitals that have a combined pre-operative unit and PACU should report on the combined unit
in questions #16-20 and answer “no” to question #21.
Medication Reconciliation
Sampling
30. Are we required to use Leapfrog's random sampling methodology?
No. You may use a different sampling methodology from the methodology provided in the
Medication Reconciliation Workbook. However, you should be sure to sample from different days
of the week, including weekends.
31. If the first few patients included in the sample have zero gold standard medications,
should we sample more than 30 patients?
Yes, hospitals can sample more than 30 patients to ensure their rate of unintentional
discrepancies per medication is scored and publicly reported. While 30 is the minimum sample
size, hospitals are welcome to sample more than this.

2023 Leapfrog Hospital Survey – Hard Copy
80 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Gold Standard Medication History
32. A pharmacist, pharmacy resident, or certified pharmacy technician creates the pre-
admission Medical List as part of normal care. Can this be used as the Gold Standard
Medication List?
No, a different trained pharmacist, pharmacy resident, or certified pharmacy technician
21
should
collect the Gold Standard Medication List when collecting data for this measure.
33. Does the same pharmacist, pharmacy resident, or certified pharmacy technician who
obtains the Gold Standard Medication List also need to perform the review to identify
unintentional medication discrepancies?
No, different pharmacists or pharmacy residents can play different roles in the data collection
process. Only pharmacists and pharmacy residents are able to compare the Gold Standard
Medication List to admission and discharge orders to identify unintentional medication
discrepancies.
34. We have two differing records of what dose the patient was taking prior to admission, one
from the patient and one from the outpatient pharmacy. What do we record as the correct
dose for the Gold Standard Medication?
In cases where the medication history obtained from interviewing the patient does not match
other written records, the pharmacist will need to go back to the patient with the pharmacy
records in hand and figure out the source of the discrepancy (e.g., the patient systematically
takes it differently than prescribed vs. the patient was just mistaken about the strength of the pill,
etc.). If the discrepancy can’t be resolved, then another source will be needed (e.g., caregiver,
PCP).
Admission and Discharge Orders
35. Are there any types of admission orders that can or should be excluded?
Yes, (a) Medication orders that are clearly related to the chief complaint (e.g., levofloxacin for
pneumonia when pneumonia is the admitting diagnosis), (b) Medication orders that are clearly
documented (e.g., Lovenox for DVT prophylaxis), and (c) Standard PRN orders at your hospital
(e.g., Tylenol PM if that is in the standard order set at your hospital).
36. Should admission orders that are discontinued prior to discharge be included?
Yes. Some of these orders may end up being counted in question #7 (additional medications that
were unintentionally ordered).
Identifying Discrepancies
37. If a dose and a route discrepancy are found for the same medication, does it count as one
or two in the number of unintentional discrepancies?
The number of unintentional discrepancies is a count of medication orders where an unintentional
discrepancy occurred. A medication order may have several errors associated with it (e.g., dose,
route, timing, etc.). You should not count the number of errors associated with the same
medication order. However, discrepancies in admission orders and discharge orders are counted
separately. For example, if a medication on the Gold Standard Medication List is ordered for a
patient on admission with the incorrect dose, this counts as one discrepancy. If this medication is
ordered on discharge with the same incorrect dose, this would count as a second discrepancy.
But a medication with a dose and frequency discrepancy in admission orders counts as one
discrepancy.
38. Do all the additional medications that were ordered unintentionally in question #7 count as
unintentional discrepancies in #8?
Yes. If a medication is unintentionally ordered at admission, then this counts as one discrepancy.
If the same medication is unintentionally ordered at discharge, then this counts as a second
discrepancy. If an unintentionally ordered medication in question #7 was ordered on both

2023 Leapfrog Hospital Survey – Hard Copy
81 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
admission and discharge, then this would count as two discrepancies in question #8 (but counts
as one medication in question #7).
39. If there is no documentation of systematic or sporadic nonadherence, why does the
pharmacist have to count unintentional discrepancies on admission and discharge?
Systematically non-adherent means the patient consistently takes the medication differently than
prescribed. Lack of appropriate documentation of systematic non-adherence can lead to an
adverse drug event. For example, if a patient is prescribed hydralazine 25mg three times a day,
but always takes 25mg two times a day this must be documented in the clinical record so the care
team can decide how to order the medication on admission and discharge (i.e., recorded on the
Med Rec Worksheet as an intentional discrepancy). If the systematic non-adherence is not
documented and the care team is unaware of how the patient has been taking the medication,
they could order and administer it as prescribed and cause, in this example, hypotension in the
patient.
Sporadically non-adherent means that the patient takes the medication as prescribed but, on
average, misses two or more doses per week. Lack of appropriate documentation of sporadic
non-adherence can lead to an adverse drug event. For example, if a patient is prescribed four
antihypertensives, but misses two or more doses per week, this must be documented in the
clinical record so the care team can decide how to order the medication on admission and
discharge (i.e., recorded on the Med Rec Worksheet as an intentional discrepancy). If the
sporadic non-adherence is not documented and the care team is unaware of how the patient has
been taking the medication, they could order and administer it as prescribed and cause, in this
example, hypotension in the patient.
40. A patient had two medications that were not on our hospital’s formulary. They were not
substituted. Do these two medications count as errors of omission?
Yes. If a Gold Standard Medication was not ordered or appropriately substituted on admission
and discharge, it would count as two unintentional medication discrepancies for each medication
that was omitted.
Scoring
41. What is an example of a data entry or reporting error that would lead to being scored as
“Some Achievement?”
Data reported in Section 2D will be reviewed for potential data reporting errors that suggest the
data was collected incorrectly or a data entry error was made. For example, hospitals that
reported an extremely large sample or reported zero medications from the Gold Standard
Medication History, are scenarios that will be flagged in the Extensive Monthly Data Verification
and scored as “Some Achievement” until the data is corrected.
42. What questions are used to calculate the numerator and denominator that results in the
rate of unintentional medication discrepancies per medication?
The rate of unintentional medication discrepancies per medication is calculated using the
following formula:
(Question #6 + Question #8)
= Rate of unintentional medication discrepancies per medication
(Question #5 + Question #7)
This rate is calculated for you in the Med Rec Workbook provided on the
Survey Materials
webpage.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
83 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
SECTION 3: ADULT AND PEDIATRIC COMPLEX
SURGERY
This section includes questions and reference information for Section 3: Adult and Pediatric Complex
Surgery. Please carefully review the questions, endnotes, and reference information (e.g., measure
specifications, notes, and frequently asked questions) before you begin. Failure to review the reference
information could result in inaccurate responses.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
84 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 3: 2023 Adult and Pediatric Complex Surgery
Adult and Pediatric Complex Surgery Fact Sheet and Bibliography:
https://ratings.leapfroggroup.org/measure/hospital/2023/complex-adult-and-pediatric-surgery
Section 3 is applicable to all hospitals. Hospitals that do not perform the high-risk procedures
included in Section 3A should select “None of the above” in question #2 and move on to the
Affirmation of Accuracy. Your hospital’s results will be displayed as “Does Not Apply” on
Leapfrog’s public reporting website. Pediatric hospitals should only report on the Norwood
procedure, if applicable.
Section 3 includes questions about your hospital volume and process for privileging surgeons for eleven
high-risk procedures, outcomes for mitral valve repair and replacement, participation in The Society of
Thoracic Surgeon’s Congenital Heart Surgery Database for hospitals that perform the Norwood
procedure. This section also includes questions about surgical appropriateness and the implementation of
a safe surgery checklist.
Each hospital achieving the standard for each of the nine applicable high-risk procedures:
1. Meets the minimum hospital volume standard for the procedure, and
2. Has a process for privileging surgeons that includes the surgeon meeting or exceeding the
minimum annual surgeon volume standard for the procedure.
Each hospital achieving the standard for mitral valve repair and replacement procedures:
1. Meets the minimum hospital volume standard for the procedure, and
2. Has a process for privileging surgeons that includes meeting or exceeding the minimum annual
surgeon volume standard for the procedure, and
3. Participates in the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database (ACSD),
and
4. Has an STS Mitral Valve Repair/Replacement Composite Score of 3 Stars.
Each hospital achieving the standard for Norwood procedures:
1. Meets the minimum hospital volume standard for the procedure, and
2. Has a process for privileging surgeons that includes meeting or exceeding the minimum annual
surgeon volume standard for the procedure, and
3. Participates in the Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database
(CHSD).
Each hospital achieving the standard for the safe surgery checklist:
1. Uses a safe surgery checklist on all patients undergoing an applicable procedure (reported on in
Section 3A) that includes all safe surgery checklist elements, and
2. Verbalizes all elements of the checklist in the presence of the appropriate personnel, and
3. Completes an audit on a sufficient sample of patients to document adherence to the checklist,
and
4. Has documented adherence to the checklist for at least 90% of the patients included in the audit.
Download the 2023 Leapfrog Hospital Survey Scoring Algorithm on the
Scoring and Results
webpage.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
85 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
3A: Hospital and Surgeon Volume
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Notes:
Note 1: This subsection is applicable to all hospitals. Hospitals that do not perform the procedures
included in this subsection should select “None of the above” in question #2 and move on to the
Affirmation of Accuracy. Your hospital’s results will be displayed as “Does Not Apply” on Leapfrog’s public
reporting website.
Note 2: Pediatric hospitals should only respond to questions regarding the Norwood procedure, if
applicable. If the Norwood procedure is not applicable, select “None of the above” in question #2 and
move on to the Affirmation of Accuracy. Your hospital’s results will be displayed as “Does Not Apply” on
Leapfrog’s public reporting website.
Specifications: See Hospital and Surgeon Volume Measure Specifications
in the Reference
Information beginning on page 96.
1) 12-month or 24-month reporting period
used:
01/01/2022 – 12/31/2022 (12-month count)
01/01/2021 – 12/31/2022 (24-month annual average)
07/01/2022 – 06/30/2023 (12-month count)
07/01/2021 – 06/30/2023 (24-month annual average)
2) Check all procedures that your hospital
performs as defined in the Adult and
Pediatric Complex Surgery Reference
Information.
Select all that apply.
Do not check the box for a procedure if your
hospital ONLY performs the procedure on
an emergency basis or when a patient is too
unstable for safe transfer.
If “none of the above,” skip the remaining
questions in Section 3 and go to the
Affirmation of Accuracy. The hospital will be
scored as “Does Not Apply.”
Carotid endarterectomy
Mitral valve repair and replacement
Open aortic procedures
Lung resection for cancer
Esophageal resection for cancer
Pancreatic resection for cancer
Rectal cancer surgery
Bariatric surgery for weight loss
Total knee replacement
Total hip replacement
Norwood procedure
None of the above
Respond to questions #3-11 based on the procedures selected in question #2.
Reporting Period: 12 months or optionally 24 months (annual average)
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022 (12-month count) or 01/01/2021 – 12/31/2022 (24-month annual
average)
• Surveys (re)submitted on or after September 1:
o 07/01/2022 – 06/30/2023 (12-month count) or 07/01/2021 – 06/30/2023 (24-month annual
average)
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
86 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
3) Total hospital volume for each selected procedure during the reporting period:
Volume should represent a 12-month count or 24-month annual average consistent with the
reporting period selected in question #1.
Procedure Hospital Volume Standard
Total Hospital Volume
(12-month count or 24-month annual
average)
Carotid endarterectomy 20
Mitral valve repair and replacement 40
Open aortic procedures 10
Lung resection for cancer
40
Esophageal resection for cancer
20
Pancreatic resection for cancer
20
Rectal cancer surgery
16
Bariatric surgery for weight loss
50
Total knee replacement
50
Total hip replacement 50
Norwood procedure 8
4) Does the total hospital volume for total knee replacement, total hip replacement, and/or bariatric
surgery for weight loss reported on in question #3 above include procedures performed on an
inpatient basis, outpatient basis, or both?
Total knee replacement
Inpatient
Outpatient
Both (inpatient and outpatient)
Total hip replacement
Inpatient
Outpatient
Both (inpatient and outpatient)
Bariatric surgery for weight loss
Inpatient
Outpatient
Both (inpatient and outpatient)
5) Does your hospital’s privileging process include the surgeon meeting or exceeding the minimum
annual surgeon volume standard listed below?
Procedure Annual Surgeon Volume Standard
Carotid endarterectomy 10
Yes
No
Mitral valve repair and replacement 20
Yes
No
Open aortic procedures 7
Yes
No
Lung resection for cancer 15
Yes
No
Esophageal resection for cancer 7
Yes
No
Pancreatic resection for cancer 10
Yes
No
Rectal cancer surgery 6
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
87 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Bariatric surgery for weight loss 20
Yes
No
Total knee replacement 25
Yes
No
Total hip replacement 25
Yes
No
Norwood procedure 5
Yes
No
If your hospital performs mitral valve repair and replacement (selected the checkbox in question #2),
continue to question #6. Otherwise, skip questions #6-9 and continue to question #10.
6) Does your hospital participate in the
Society of
Thoracic Surgeons (STS) Adult Cardiac Surgery
Database (ACSD) and did your hospital submit data
for all applicable procedures during the most recent
36-month period for which performance reports are
available?
If “no” to question #6, skip questions #7-9 and
continue to question #10.
Yes
No
7) What is the most recent 36-month reporting period
for which STS performance reports are available?
Reporting period ending:
_________
Format: Month/Year
8) Does your hospital choose to report data from the
most recent performance report to this Survey?
If “no” or “yes, but did not meet the STS
Data
Completeness Requirement
27
during the reporting
period,” skip question #9 and continue to question
#10.
Yes
No
Yes, but did not meet the STS Data
Completeness Requirement during the
reporting period
9) What is your hospital’s Mitral Valve
Repair/Replacement Composite Score?
1 star – lower-than-expected performance
2 stars – as-expected performance
3 stars – higher-than-expected performance
If your hospital performs the Norwood procedure (selected the checkbox in question #2), continue to
question #10. Otherwise, skip questions #10-11 and continue to the next subsection.
Reporting Period: Base your responses on the latest 36-month report received from the Society of
Thoracic Surgeons (STS) Adult Cardiac Surgery Database (ACSD) for the Mitral Valve
Repair/Replacement Composite Score.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
88 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
10) Does your hospital participate in the Society of
Thoracic Surgeons (STS) Congenital Heart Surgery
Database (CHSD) and did your hospital submit data
for the Norwood procedure during the most recent
48-month period for which performance reports are
available?
If “no” to question #10, skip question #11 and
continue to the next subsection.
Yes
No
11) What is the most recent 48-month reporting period
for which STS performance reports are available?
Reporting period ending:
_________
Format: Month/Year

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
89 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
3B: Surgical Appropriateness
This section is not applicable to pediatric hospitals. Pediatric hospitals should select “None of the
above” in question #1 and #6 if they reported on Norwood procedures in Section 3A.
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
1) Does your hospital have appropriateness criteria
28
for any of the following procedures:
Select all that apply.
If your hospital does not perform any of the
procedures listed in this question (did not select
them in Section 3A question #2), select “none of the
above.”
If “none of the above,” skip questions #1b-5 and
continue to question #6.
Carotid endarterectomy
Mitral valve repair and replacement
Bariatric surgery for weight loss
Total knee replacement
Total hip replacement
None of the above
1b) Did your hospital do any of the following in
developing the appropriateness criteria:
Select all that apply.
Use the latest evidence and clinical
guidelines
Solicit input from employed
surgeons, and if applicable, non-
employed surgeons
Incorporate relevant Choosing
Wisely lists
Review, and if appropriate, update
the criteria on an annual basis
None of the above
2) Does your hospital have processes or structures in
place to promote ongoing adherence to the
appropriateness criteria for any of the following
procedures:
Select all that apply.
Carotid endarterectomy
Mitral valve repair and replacement
Bariatric surgery for weight loss
Total knee replacement
Total hip replacement
None of the above
3) Does your hospital conduct regular retrospective
reviews of surgical cases to evaluate the extent to
which your appropriateness criteria are met or not
met by each surgeon for any of the following
procedures:
Select all that apply.
Carotid endarterectomy
Mitral valve repair and replacement
Bariatric surgery for weight loss
Total knee replacement
Total hip replacement
None of the above
Reporting Period: 12 months
Answer questions #1-6 for the latest 12-month period prior to the submission of this section of the
Survey.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
90 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
4) Does your hospital have a process in place for
communicating with surgeons, surgical leadership,
and administrative leadership when a surgeon’s
trend or pattern suggests challenges adhering to
your appropriateness criteria and work to
understand potential barriers to meeting the criteria
for any of the following procedures:
Select all that apply.
Carotid endarterectomy
Mitral valve repair and replacement
Bariatric surgery for weight loss
Total knee replacement
Total hip replacement
None of the above
5) Does your hospital report annually to its Board the
findings from the retrospective reviews and plans to
improve adherence to the appropriateness criteria
for any of the following procedures:
Select all that apply.
Carotid endarterectomy
Mitral valve repair and replacement
Bariatric surgery for weight loss
Total knee replacement
Total hip replacement
None of the above
6) Does your hospital have national accreditation from
the American College of Surgeons
(applies to rectal
cancer surgery only)
OR
Does your hospital have a multidisciplinary tumor
board that prospectively reviews cancer cases to
ensure surgical appropriateness for any of the
following procedures:
Select all that apply.
If your hospital does not perform any of the
procedures listed in this question (did not select
them in Section 3A question #2), select “none of the
above.”
Lung resection for cancer
Esophageal resection for cancer
Pancreatic resection for cancer
Rectal cancer surgery
None of the above

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
91 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
3C: Safe Surgery Checklist for Adult and Pediatric Complex Surgery
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Notes:
Note 1: The elements required for each stage of the safe surgery checklist are adapted from the
WHO
Surgical Safety Checklist and the AHRQ Endoscopy Checklist.
Note 2: Hyperlinks throughout this subsection refer to the
Safe Surgery Checklist for Adult and Pediatric
Complex Surgery FAQs beginning on page 139, not to endnotes. These hyperlinks are not included in the
Online Survey Tool.
Specifications: See Safe Surgery Checklist for Adult and Pediatric Complex Surgery
Measure
Specifications in the Reference Information beginning on page 134.
Sufficient Sample: See
Safe Surgery Checklist for Adult and Pediatric Complex Surgery Measure
Specifications for instructions on identifying a sufficient sample for question #6.
1) What is the latest 6-month reporting period for which your hospital
is submitting responses to questions #2-8? 6-month reporting
period ending:
_________
Format: Month/Year
2) Does your hospital utilize a safe surgery checklist on every patient
every time one of the applicable procedures in Section 3A is
performed?
If “no” to question #2, skip the remaining questions in Section 3C
and go to the Affirmation of Accuracy. The hospital will be scored
as “Limited Achievement.”
Yes
No
3) Before the induction of anesthesia, is a safe surgery checklist
that includes all the following elements read aloud in the presence
of the anesthesia professional and nursing personnel:
• Patient ID
• Confirmation of procedure
• Patient consent
• Site marked, if applicable
• Anesthesia/medication check
• Allergies assessed
• Difficult airway/aspiration risk
• Risk of blood loss (only applicable if risk of blood loss is
>500ml for adults or > 7ml/kg for children)
• Availability of devices on-site, if applicable?
Yes
No
Reporting Period: 6 months
Answer questions #1-8 for the latest 6-month period prior to submission of this section of the Survey.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
92 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
4) Before the skin incision and/or before the procedure begins,
is a safe surgery checklist that includes all the following elements
read aloud in the presence of the whole surgical team:
• Clinical team introduction
• Confirmation of patient name, procedure, and, if applicable,
surgical/incision site
• Antibiotic prophylaxis, if applicable
• Anticipated Critical Events (i.e., non-routine steps, length of
procedure, blood loss, patient-specific concerns, sterility)
• Equipment check/concerns
• Essential imaging available, if applicable?
Yes
No
5) Before the patient leaves the operating room and/or
procedure room, is a safe surgery checklist that includes all the
following elements read aloud in the presence of the whole
surgical team:
• Confirmation of procedure performed
• Instrument/supply counts
• Specimen labeling, if applicable
• Equipment concerns
• Patient recovery/management concerns?
Yes
No
If “no” to question #3, #4, or #5, skip the remaining questions in Section 3C, and go to the Affirmation of
Accuracy. The hospital will be scored as “Limited Achievement.”
Hospitals performing the audit in Section 3C question #6 and the audit in Section 9D question #10 should
audit 15 cases who underwent a procedure included in Section 3A and 15 cases who underwent a
procedure included in Section 9C. Hospitals only performing the audit in Section 3C question #6 and not
in Section 9D question #10 should audit 30 cases who underwent a procedure included in Section 3A.
6) Did your hospital perform an audit (either in-person or via the
medical record or other EHR data) on a sufficient sample of
patients who underwent a procedure included in Section 3A and
measure adherence to the safe surgery checklist?
Free-standing pediatric hospitals that perform the Norwood
Procedure and hospitals that reported a combined total hospital
volume of less than the sufficient sample for all the procedures in
Section 3A can sample any patients that had a procedure
performed under general anesthesia.
If “no” to question #6, skip the remaining questions in Section 3C
and go to the Affirmation of Accuracy. The hospital will be scored
as “Limited Achievement.”
Yes
No
7) Which method was used to perform the audit on a sufficient
sample in question #6?
In-person observational audit
Retrospective audit of
medical records or EHR data
Both
8) Based on your hospital’s audit (either in-person or via the medical
record or other EHR data) on a sufficient sample of patients who
underwent a procedure included in Section 3A, what was your
hospitals documented rate of adherence to the safe surgery
checklist (e.g., what percentage of the sampled cases had all
elements in questions #3, #4, and #5 completed)?
90%-100%
75%-89%
50%-74%
Less than 50%

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
93 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Affirmation of Accuracy
As the hospital CEO or as an employee of the hospital to whom the hospital CEO has delegated
responsibility, I have reviewed this information pertaining to the Adult and Pediatric Complex Surgery
Section at our hospital, and I hereby certify that this information is true, accurate, and reflects the current,
normal operating circumstances at our hospital. I am authorized to make this certification on behalf of our
hospital.
The hospital and I understand that The Leapfrog Group, its members, the public and entities and persons
who contract or have other business dealings with The Leapfrog Group are relying on the truth and
accuracy of this information. The hospital and I also understand that The Leapfrog Group will make this
information and/or analyses of this information public through the Survey Results public reporting website,
The Leapfrog Group’s Hospital Safety Grade, and/or other Leapfrog Group products and services. This
information and/or analyses and all intellectual property rights therein shall be and remain the sole and
exclusive property of The Leapfrog Group in which The Leapfrog Group retains exclusive ownership. This
information does not infringe upon any third-party intellectual property rights or any other third-party rights
whatsoever and is free and clear of all encumbrances and liens of any kind. The hospital and I
acknowledge that The Leapfrog Group may use this information in a commercial manner for profit. The
hospital shall be liable for and shall hold harmless and indemnify The Leapfrog Group from any and all
damages, demands, costs, or causes of action resulting from any inaccuracies in the information or any
misrepresentations in this Affirmation of Accuracy. The Leapfrog Group and its members and entities and
persons who contract or have other business dealings with The Leapfrog Group reserve the right to omit
or disclaim information that is not current, accurate or truthful.
Affirmed by _____________________, the hospital’s ___________________________,
(first name and last name) (title)
on _______________________.
(date)

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
94 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 3: 2023 Adult and Pediatric Complex Surgery Reference Information
What’s New in the 2023 Survey
Section 3A: Hospital and Surgeon Volume
Open Aortic Procedures
Leapfrog is adding one ICD-10 procedure code to Open Aortic Procedures. This procedure code was
added by CMS in 2022 and meets our definition of an Open Aortic Procedure: A procedure where the
surgeon exposes the aorta (thoracic or abdominal), clamps it, and sews on the aorta.
Bariatric Surgery for Weight Loss
Leapfrog is expanding the list of procedure codes for Bariatric Surgery for Weight Loss to include
outpatient procedures captured using Current Procedural Terminology (CPT) codes. The CPT Codes will
be available via the Online Survey Tool when the Survey opens on April 1, 2023. Due to the American
Medical Association’s Terms of Use, hospitals must complete the Terms of Use via the Online Survey
Tool to access the CPT Codes. We are also adding this procedure to Leapfrog’s Ambulatory Surgery
Center (ASC) Survey.
Norwood Procedure
Leapfrog is removing questions regarding the Society of Thoracic Surgeons (STS) Congenital Heart
Surgery Database (CHSD) Participant Postoperative Length of Stay and Participant Operative Mortality
measures but is continuing to ask about hospital participation in the STS CHSD. In 2023, hospitals that
perform the Norwood Procedure will be scored using three criteria: total hospital volume, incorporating
Leapfrog’s minimum annual surgeon volume standards into their process for privileging surgeons, and
participation in the STS CHSD. The points assigned to each criterion reflect the
Complex Surgery Expert
Panel's opinion on its importance to patient outcomes.
Section 3B: Surgical Appropriateness
Based on feedback from hospitals and in consultation with the Complex Surgery Expert Panel
, Leapfrog
is removing questions regarding surgical appropriateness for Open Aortic Procedures as these
procedures are performed as a part of life saving efforts.
There are no changes to the public reporting of Section 3B: Surgical Appropriateness for 2023. However,
the surgical appropriateness questions have been an important part of the Complex Surgery standards
for several years and remain an important area of interest for Leapfrog’s purchaser and employer
members. As such, Leapfrog plans to incorporate these questions into scoring and public reporting
beginning in 2024 for the following complex surgeries: Carotid Endarterectomy, Mitral Valve Repair and
Replacement, Bariatric Surgery for Weight Loss, Total Knee Replacement, Total Hip Replacement, Lung
Resection for Cancer, Esophageal Resection for Cancer, Pancreatic Resection for Cancer, and Rectal
Cancer Surgery.
Additionally, in 2024, Leapfrog plans to add a new question to Section 3B to assess the extent to which a
hospital’s appropriateness criteria are being utilized by asking hospitals to report on their findings from the
retrospective reviews completed in question #3 for the following procedures: Carotid Endarterectomy,
Mitral Valve Repair and Replacement, Bariatric Surgery for Weight Loss, Total Knee Replacement, and
Total Hip Replacement.
The updated scoring algorithm will be published with the Proposed Changes to the 2024 Leapfrog
Hospital Survey.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
95 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 3C: Safe Surgery Checklist for Adult and Pediatric Complex Surgery
Leapfrog is making two updates to Section 3C: Safe Surgery Checklist for Adult and Pediatric Complex
Surgery. First, Leapfrog is increasing the audit requirement from 15 sampled cases to 30 sampled cases
for hospitals only submitting Section 3 and not also submitting Section 9: Outpatient Procedures.
Hospitals that submit both Sections 3 and 9 should continue to audit 15 complex surgical cases and 15
outpatient surgical cases.
Second, for hospitals conducting the audit to assess compliance with the Safe Surgery Checklist,
Leapfrog is adding a question asking if the hospital performed an in-person observational audit, a
retrospective audit of medical records or EHR data, or both.
There are no changes to the scoring algorithm for Section 3C: Safe Surgery Checklist for Adult and
Pediatric Complex Surgery.
Change Summary Since Release
None. If substantive changes are made to this section of the Survey after release on April 1, 2023, they
will be documented in this Change Summary section.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
96 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Adult and Pediatric Complex Surgery Measure Specifications
Hospital and Surgeon Volume
Important Notes:
Note 1: Hospitals that share a CMS Certification Number are required to report by facility. Please carefully
review Leapfrog’s Multi-Campus Reporting Policy
.
Note 2: For each of the eleven high-risk procedures included in Section 3A: Hospital and Surgeon
Volume, Leapfrog has provided a set of ICD-10 procedure codes, and in some cases an additional set of
CPT codes (for Total Hip Replacement, Total Knee Replacement, and Bariatric Surgery for Weight Loss)
or ICD-10 diagnosis codes, for counting patient discharges. Please carefully review the measure
specifications for each individual procedure to ensure you have the complete list of codes needed. ICD-
10 codes are provided below and are also available in an Excel document on the
Survey Materials
webpage. See Note 3 below for instructions on downloading CPT codes.
Note 3: CPT Codes are provided in downloadable Excel files on the Survey Dashboard. To access the
files, click the CPT Code Workbook button next to Section 9. You will be required to complete the
American Medical Association’s Terms of Use before downloading the Excel file and using the individual
CPT codes to query your EHR or billing system. You are only required to complete the Terms of Use
once per Survey Cycle (April 1 – November 30).
The CPT Code Workbooks (Excel files) are labeled for Section 3A: Total Hip Replacement, Total Knee
Replacement, and Bariatric Surgery for Weight Loss and Section 9C: Volume of Procedures. Please note,
if you are part of a hospital system, each hospital will need to complete the Terms of Use. This is a
requirement of the American Medical Association.
Source: The Leapfrog Group
Reporting Period: 12 months or optionally 24 months (annual average)
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022 (12-month count) or 01/01/2021 – 12/31/2022 (24-month annual
average)
• Surveys (re)submitted on or after September 1:
o 07/01/2022 – 06/30/2023 (12-month count) or 07/01/2021 – 06/30/2023 (24-month annual
average)
Question #2: Check all procedures that your hospital performs as defined in the Adult and Pediatric
Complex Surgery Reference Information (listed below):
Carotid endarterectomy
Mitral valve repair and replacement
Open aortic procedures
Lung resection for cancer
Esophageal resection for cancer
Pancreatic resection for cancer
Rectal cancer surgery
Bariatric surgery for weight loss
Total knee replacement
Total hip replacement
Norwood procedure
Free-standing pediatric hospitals should only report on the Norwood procedure, if applicable.
Do not check the box for the procedure if:
• Your hospital does not perform the procedure or ONLY performs the procedure on an
emergency basis or when a patient is too unstable for safe transfer.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
97 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
• Your hospital has started to perform the procedure in the last 18 months. Leapfrog gives
hospitals an 18-month grace period before having to report on hospital volume and process for
privileging surgeons for new service lines.
Do check the box for the procedure if:
• Your hospital would perform the procedure but had zero cases during the reporting period.
Select the procedure and indicate a hospital volume of zero in question #3. Please note that
hospitals can elect to report on a 24-month annual average.
• Your hospital has reached the end of the 18-month grace period for a new service line. You will
now have to report on both hospital volume and your process for privileging surgeons for this
procedure.
Question #3: Total hospital volume for each selected procedure (from question #2) during the
reporting period:
Carotid endarterectomy
(Hospital Volume Standard: 20)
Mitral valve repair and replacement (Hospital Volume Standard: 40)
Open aortic procedures (Hospital Volume Standard: 10)
Lung resection for cancer (Hospital Volume Standard: 40)
Esophageal resection for cancer (Hospital Volume Standard: 20)
Pancreatic resection for cancer (Hospital Volume Standard: 20)
Rectal cancer surgery (Hospital Volume Standard: 16)
Bariatric surgery for weight loss (Hospital Volume Standard: 50)
Total knee replacement (Hospital Volume Standard: 50)
Total hip replacement (Hospital Volume Standard: 50)
Norwood procedure (Hospital Volume Standard: 8)
When calculating total hospital volume:
• Count the number of patients discharged from your hospital within the reporting period with
any one or more of the ICD-10 codes or CPT codes (for Total Hip Replacement, Total Knee
Replacement, and Bariatric Surgery for Weight Loss) specified for each procedure, subject to
the inclusion criteria below:
o Only the ICD-10 procedure, CPT, and diagnosis codes provided by Leapfrog should be
used to report on the questions in Section 3A: Hospital and Surgeon Volume. ICD-10
codes are provided below and are also available in an Excel document on the
Survey
Materials webpage. See Note 3 above for instructions on downloading CPT codes.
o For procedures that include TWO sets of codes (one set of procedure codes and one
set of diagnosis codes), both sets of codes must be used for counting patient
discharges (e.g., at least one procedure code AND one diagnosis code must be
present).
o Except for bariatric surgery for weight loss (where the ICD-10 diagnosis code must be
the primary diagnosis) and total knee replacement and total hip replacement (where
the procedure code must be the primary procedure), ICD-10 codes for the other
procedures can appear in ANY procedure field and ANY diagnosis field.
o Age restrictions apply – For the Norwood procedure only include discharges for
pediatric patients (ages 17 years and younger). For the remaining ten procedures, only
include discharges for adult patients (ages 18 years and older).
Question #4: Does the total hospital volume for total knee replacement, total hip replacement, and/or
bariatric surgery for weight loss reported on in question #3 above include procedures performed on an
inpatient basis, outpatient basis, or both?
• If your hospital only performs total knee replacement, total hip replacement, and/or bariatric
surgery for weight loss on an inpatient basis, select inpatient.
• If your hospital only performs total knee replacement, total hip replacement, and/or bariatric
surgery for weight loss on an outpatient basis, select outpatient.
• If your hospital performs total knee replacement, total hip replacement, and/or bariatric surgery
for weight loss on both an inpatient and outpatient basis, select both (inpatient and outpatient).

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
98 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Question #5: Does your hospital’s privileging process include the surgeon meeting or exceeding the
minimum annual surgeon volume standard listed below?
Carotid endarterectomy: 10
Mitral valve repair and replacement: 20
Open aortic procedures: 7
Lung resection for cancer: 15
Esophageal resection for cancer: 7
Pancreatic resection for cancer: 10
Rectal cancer surgery: 6
Bariatric surgery for weight loss: 20
Total knee replacement: 25
Total hip replacement: 25
Norwood procedure: 5
When determining whether surgeons have met or exceeded Leapfrog’s minimum annual surgeon
volume standards for the purposes of privileging, only refer to the ICD-10 procedure codes and CPT
codes (for Total Hip Replacement, Total Knee Replacement, and Bariatric Surgery for Weight Loss) –
diagnosis codes can be ignored.
Source: The Society of Thoracic Surgeons (STS)
Reporting Period: Latest 36-month report from Society of Thoracic Surgeons (STS) Adult Cardiac
Surgery Database (ACSD) for the Mitral Valve Repair/Replacement Composite Score.
Question #6: Does your hospital participate in the Society of Thoracic Surgeons (STS) Adult Cardiac
Surgery Database (ACSD) and did your hospital submit data for all applicable procedures during the
most recent 36-month period for which performance reports are available?
Select “yes” if your hospital participates in the Society of Thoracic Surgeons (STS)
Adult Cardiac
Surgery Database (ACSD) and has submitted data for all applicable procedures during the most recent
36-month period.
Question #7: What is the most recent 36-month reporting period for which STS performance reports
are available? Reporting period ending:
Select the most recent 36-month reporting period for which STS performance reports are available.
The reporting period is displayed when reviewing your “MVRR Composite Quality Ratings” at
https://publicreporting.sts.org/search/acsd. See screenshot provided for question #9 below
.
Question #8: Does your hospital choose to report data from the most recent performance report to this
Survey?
Select “yes” if your hospital elects to share the MVRR data from your most recent performance report
with Leapfrog.
Question #9: What is your hospital’s Mitral Valve Repair/Replacement Composite Score?
Report your Mitral Valve Repair/Replacement Overall Composite Score from the STS Public Reporting
website: https://publicreporting.sts.org/search/acsd
.
Click on “Search All Groups.” Then search for your hospital name/surgery group name in the
“Participant Surgery Group Name” field and click your hospital name/surgery group name. On the next
page find your “MVRR Composite Quality Rating” and your “Overall Composite Score.” Report the
number of stars displayed for “Overall Composite Score” represented in the screenshot below.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
99 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Source: The Society of Thoracic Surgeons (STS)
Question #10: Does your hospital participate in the Society of Thoracic Surgeons (STS) Congenital
Heart Surgery Database (CHSD) and did your hospital submit data for the Norwood procedure during
the most recent 48-month period for which performance reports are available?
Select “yes” if your hospital participates in the Society of Thoracic Surgeons (STS)
Congenital Heart
Surgery Database (CHSD) and has submitted data for the Norwood procedure during the most recent
48-month period.
Question #11: What is the most recent 48-month reporting period for which STS performance reports
are available? Reporting period ending:
Select the most recent 48-month reporting period for which STS performance reports are available.
The reporting period should appear at the top of your STS performance report.
See FAQs
for additional information about responding to the questions in this section.
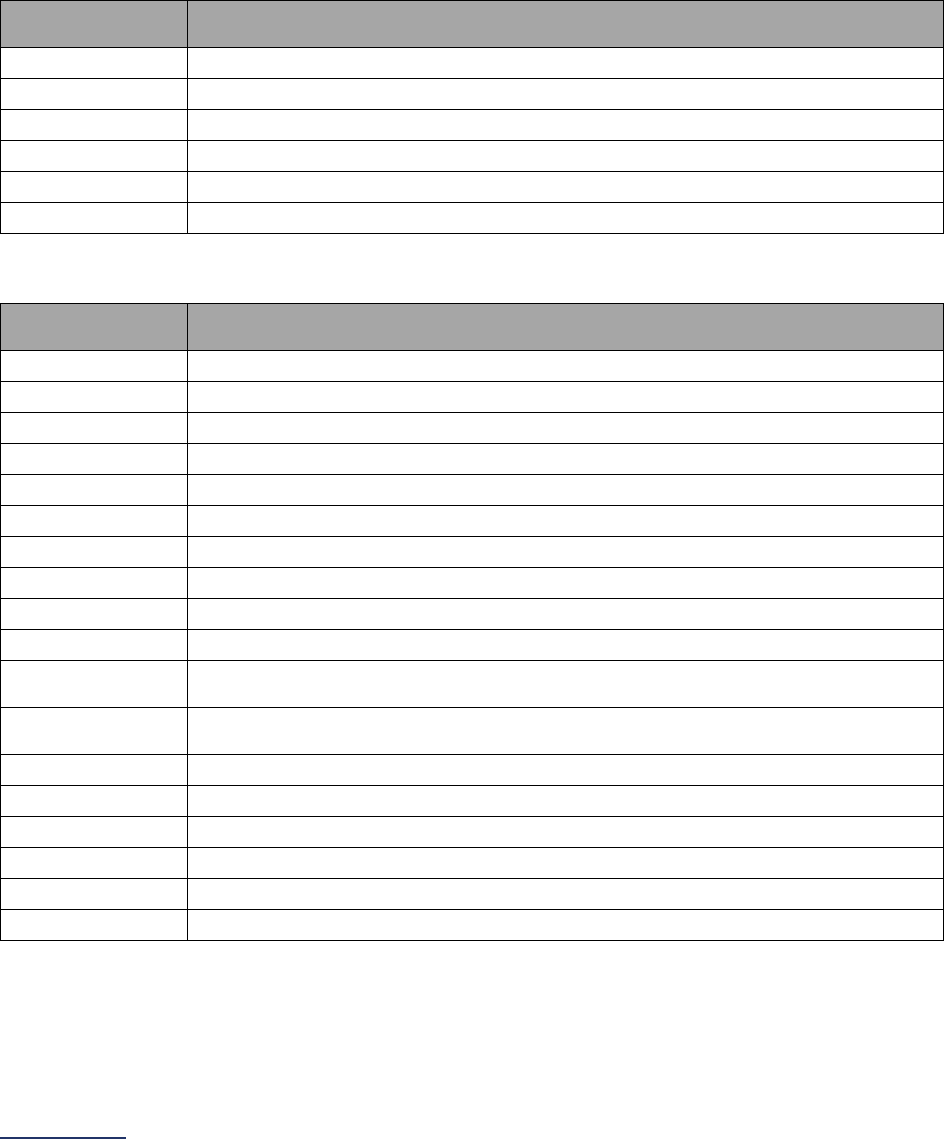
2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
100 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Carotid Endarterectomy Measure Specifications
For carotid endarterectomy, there are two sets of ICD-10 codes for counting patient discharges. The first
set of codes is to identify patients who have had the procedure. The second set of codes is to identify
patients with a specific diagnosis.
Count the number of patients, ages 18 years and older, discharged with the following ICD-10 codes in
any procedure field AND any of the following ICD-10 codes in any diagnosis field.
ICD-10 Carotid Endarterectomy Procedure Codes
ICD-10
Procedure Code
Code Description
03CH0ZZ Extirpation of Matter from Right Common Carotid Artery, Open Approach
03CJ0ZZ
Extirpation of Matter from Left Common Carotid Artery, Open Approach
03CK0ZZ Extirpation of Matter from Right Internal Carotid Artery, Open Approach
03CL0ZZ
Extirpation of Matter from Left Internal Carotid Artery, Open Approach
03CM0ZZ Extirpation of Matter from Right External Carotid Artery, Open Approach
03CN0ZZ
Extirpation of Matter from Left External Carotid Artery, Open Approach
ICD-10 Occlusion and Stenosis and Cerebral Infarction Diagnosis Codes
ICD-10 Diagnosis
Code
Code Description
I63.031
Cerebral infarction due to thrombosis of right carotid artery
I63.032 Cerebral infarction due to thrombosis of left carotid artery
I63.033
Cerebral infarction due to thrombosis of bilateral carotid arteries
I63.039 Cerebral infarction due to thrombosis of unspecified carotid artery
I63.131
Cerebral infarction due to embolism of right carotid artery
I63.132 Cerebral infarction due to embolism of left carotid artery
I63.133
Cerebral infarction due to embolism of bilateral carotid arteries
I63.139 Cerebral infarction due to embolism of unspecified carotid artery
I63.231
Cerebral infarction due to unspecified occlusion or stenosis of right carotid arteries
I63.232 Cerebral infarction due to unspecified occlusion or stenosis of left carotid arteries
I63.233
Cerebral infarction due to unspecified occlusion or stenosis of bilateral carotid
arteries
I63.239
Cerebral infarction due to unspecified occlusion or stenosis of unspecified carotid
arteries
I65.21
Occlusion and stenosis of right carotid artery
I65.22 Occlusion and stenosis of left carotid artery
I65.23
Occlusion and stenosis of bilateral carotid arteries
I65.29 Occlusion and stenosis of unspecified carotid artery
I65.8
Occlusion and stenosis of other precerebral arteries
I65.9 Occlusion and stenosis of unspecified precerebral artery

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
101 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Mitral Valve Repair and Replacement Measure Specifications
For mitral valve repair and replacement, there is only one set of ICD-10 codes for counting patient
discharges. The set of codes is to identify patients who have had the procedure.
Count the number of patients, ages 18 years and older, discharged with the following ICD-10 codes in
any procedure field.
ICD-10 Mitral Valve Repair and Replacement Procedure Codes
ICD-10
Procedure Code
Code Description
027G04Z
Dilation of Mitral Valve with Drug-eluting Intraluminal Device, Open Approach
027G0DZ Dilation of Mitral Valve with Intraluminal Device, Open Approach
027G0ZZ
Dilation of Mitral Valve, Open Approach
02CG0ZZ Extirpation of Matter from Mitral Valve, Open Approach
02NG0ZZ
Release Mitral Valve, Open Approach
02QG0ZE Repair Mitral Valve created from Left Atrioventricular Valve, Open Approach
02QG0ZZ
Repair Mitral Valve, Open Approach
02RG07Z Replacement of Mitral Valve with Autologous Tissue Substitute, Open Approach
02RG08Z
Replacement of Mitral Valve with Zooplastic Tissue, Open Approach
02RG0JZ Replacement of Mitral Valve with Synthetic Substitute, Open Approach
02RG0KZ
Replacement of Mitral Valve with Nonautologous Tissue Substitute, Open
Approach
02UG07E
Supplement Mitral Valve created from Left Atrioventricular Valve with Autologous
Tissue Substitute, Open Approach
02UG07Z
Supplement Mitral Valve with Autologous Tissue Substitute, Open Approach
02UG08E
Supplement Mitral Valve created from Left Atrioventricular Valve with Zooplastic
Tissue, Open Approach
02UG08Z
Supplement Mitral Valve with Zooplastic Tissue, Open Approach
02UG0JE
Supplement Mitral Valve created from Left Atrioventricular Valve with Synthetic
Substitute, Open Approach
02UG0JZ
Supplement Mitral Valve with Synthetic Substitute, Open Approach
02UG0KE
Supplement Mitral Valve created from Left Atrioventricular Valve with
Nonautologous Tissue Substitute, Open Approach
02UG0KZ
Supplement Mitral Valve with Nonautologous Tissue Substitute, Open Approach
02VG0ZZ Restriction of Mitral Valve, Open Approach
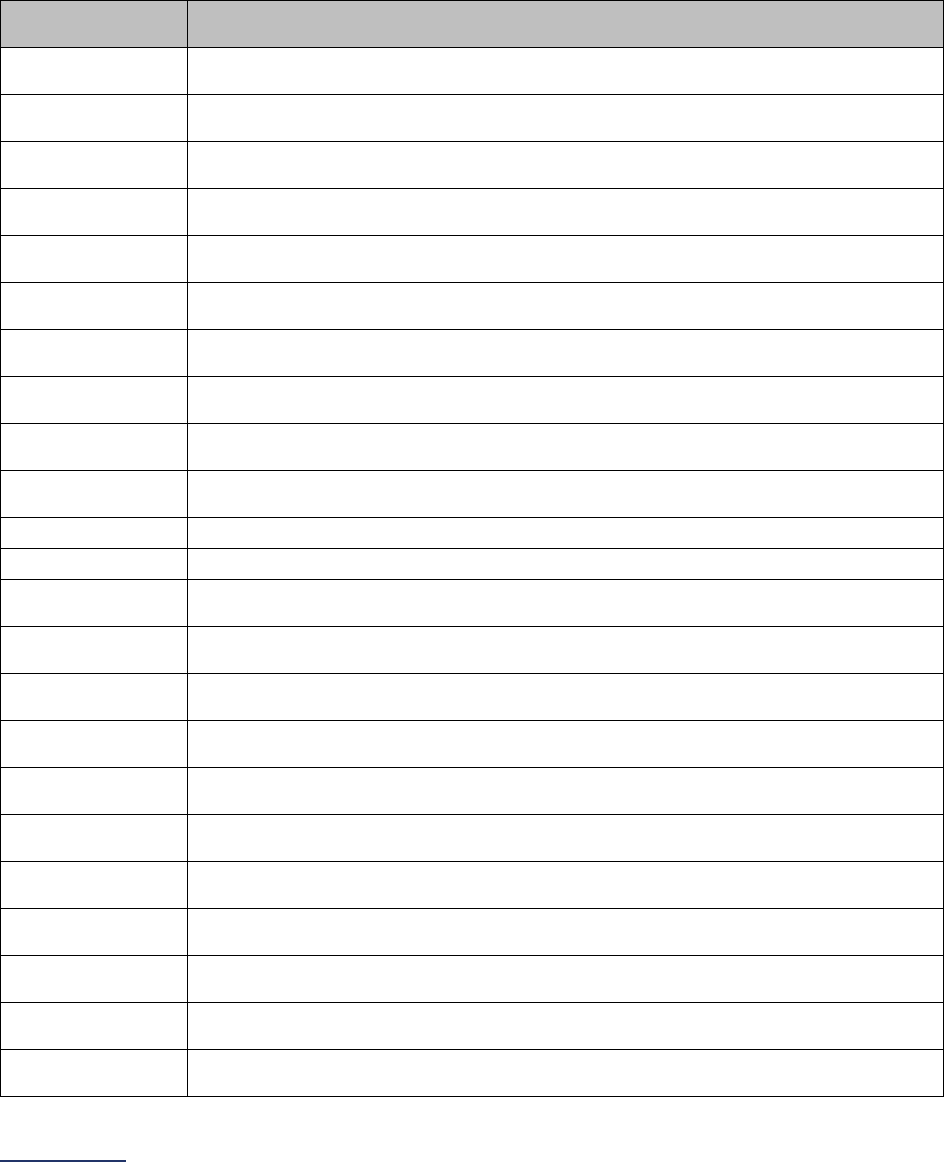
2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
102 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Open Aortic Procedures Measure Specifications
For open aortic procedures, there is only one set of ICD-10 codes for counting patient discharges. The
set of codes is to identify patients who have had the procedure.
Count the number of patients, ages 18 years and older, discharged with the following ICD-10 codes in
any procedure field.
ICD-10 Open Aortic Procedure Codes
ICD-10
Procedure Code
Code Description
0410090
Bypass Abdominal Aorta to Abdominal Aorta with Autologous Venous Tissue,
Open Approach
0410091
Bypass Abdominal Aorta to Celiac Artery with Autologous Venous Tissue, Open
Approach
0410092
Bypass Abdominal Aorta to Mesenteric Artery with Autologous Venous Tissue,
Open Approach
0410093
Bypass Abdominal Aorta to Right Renal Artery with Autologous Venous Tissue,
Open Approach
0410094
Bypass Abdominal Aorta to Left Renal Artery with Autologous Venous Tissue,
Open Approach
0410095
Bypass Abdominal Aorta to Bilateral Renal Artery with Autologous Venous Tissue,
Open Approach
0410096
Bypass Abdominal Aorta to Right Common Iliac Artery with Autologous Venous
Tissue, Open Approach
0410097
Bypass Abdominal Aorta to Left Common Iliac Artery with Autologous Venous
Tissue, Open Approach
0410098
Bypass Abdominal Aorta to Bilateral Common Iliac Arteries with Autologous
Venous Tissue, Open Approach
0410099
Bypass Abdominal Aorta to Right Internal Iliac Artery with Autologous Venous
Tissue, Open Approach
021K0ZW
Bypass Right Ventricle to Aorta, Open Approach
021L0ZW Bypass Left Ventricle to Aorta, Open Approach
021W08A
Bypass Thoracic Aorta, Descending to Innominate Artery with Zooplastic Tissue,
Open Approach
021W08B
Bypass Thoracic Aorta, Descending to Subclavian with Zooplastic Tissue, Open
Approach
021W08D
Bypass Thoracic Aorta, Descending to Carotid with Zooplastic Tissue, Open
Approach
021W08F
Bypass Thoracic Aorta, Descending to Abdominal Artery with Zooplastic Tissue,
Open Approach
021W08G
Bypass Thoracic Aorta, Descending to Axillary Artery with Zooplastic Tissue, Open
Approach
021W08H
Bypass Thoracic Aorta, Descending to Brachial Artery with Zooplastic Tissue,
Open Approach
021W08P
Bypass Thoracic Aorta, Descending to Pulmonary Trunk with Zooplastic Tissue,
Open Approach
021W08Q
Bypass Thoracic Aorta, Descending to Right Pulmonary Artery with Zooplastic
Tissue, Open Approach
021W08R
Bypass Thoracic Aorta, Descending to Left Pulmonary Artery with Zooplastic
Tissue, Open Approach
021W08V
Bypass Thoracic Aorta, Descending to Lower Extremity Artery with Zooplastic
Tissue, Open Approach
021W09B
Bypass Thoracic Aorta, Descending to Subclavian with Autologous Venous Tissue,
Open Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
103 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
021W09A
Bypass Thoracic Aorta, Descending to Innominate Artery with Autologous Venous
Tissue, Open Approach
021W09D
Bypass Thoracic Aorta, Descending to Carotid with Autologous Venous Tissue,
Open Approach
021W09F
Bypass Thoracic Aorta, Descending to Abdominal Artery with Autologous Venous
Tissue, Open Approach
021W09G
Bypass Thoracic Aorta, Descending to Axillary Artery with Autologous Venous
Tissue, Open Approach
021W09H
Bypass Thoracic Aorta, Descending to Brachial Artery with Autologous Venous
Tissue, Open Approach
021W09P
Bypass Thoracic Aorta, Descending to Pulmonary Trunk with Autologous Venous
Tissue, Open Approach
021W09Q
Bypass Thoracic Aorta, Descending to Right Pulmonary Artery with Autologous
Venous Tissue, Open Approach
021W09R
Bypass Thoracic Aorta, Descending to Left Pulmonary Artery with Autologous
Venous Tissue, Open Approach
021W09V
Bypass Thoracic Aorta, Descending to Lower Extremity Artery with Autologous
Venous Tissue, Open Approach
021W0AA
Bypass Thoracic Aorta, Descending to Innominate Artery with Autologous Arterial
Tissue, Open Approach
021W0AB
Bypass Thoracic Aorta, Descending to Subclavian with Autologous Arterial Tissue,
Open Approach
021W0AD
Bypass Thoracic Aorta, Descending to Carotid with Autologous Arterial Tissue,
Open Approach
021W0AF
Bypass Thoracic Aorta, Descending to Abdominal Artery with Autologous Arterial
Tissue, Open Approach
021W0AG
Bypass Thoracic Aorta, Descending to Axillary Artery with Autologous Arterial
Tissue, Open Approach
021W0AH
Bypass Thoracic Aorta, Descending to Brachial Artery with Autologous Arterial
Tissue, Open Approach
021W0AP
Bypass Thoracic Aorta, Descending to Pulmonary Trunk with Autologous Arterial
Tissue, Open Approach
021W0AQ
Bypass Thoracic Aorta, Descending to Right Pulmonary Artery with Autologous
Arterial Tissue, Open Approach
021W0AR
Bypass Thoracic Aorta, Descending to Left Pulmonary Artery with Autologous
Arterial Tissue, Open Approach
021W0AV
Bypass Thoracic Aorta, Descending to Lower Extremity Artery with Autologous
Arterial Tissue, Open Approach
021W0JA
Bypass Thoracic Aorta, Descending to Innominate Artery with Synthetic Substitute,
Open Approach
021W0JB
Bypass Thoracic Aorta, Descending to Subclavian with Synthetic Substitute, Open
Approach
021W0JD
Bypass Thoracic Aorta, Descending to Carotid with Synthetic Substitute, Open
Approach
021W0JF
Bypass Thoracic Aorta, Descending to Abdominal Artery with Synthetic Substitute,
Open Approach
021W0JG
Bypass Thoracic Aorta, Descending to Axillary Artery with Synthetic Substitute,
Open Approach
021W0JH
Bypass Thoracic Aorta, Descending to Brachial Artery with Synthetic Substitute,
Open Approach
021W0JP
Bypass Thoracic Aorta, Descending to Pulmonary Trunk with Synthetic Substitute,
Open Approach
021W0JQ
Bypass Thoracic Aorta, Descending to Right Pulmonary Artery with Synthetic
Substitute, Open Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
104 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
021W0JR
Bypass Thoracic Aorta, Descending to Left Pulmonary Artery with Synthetic
Substitute, Open Approach
021W0JV
Bypass Thoracic Aorta, Descending to Lower Extremity Artery with Synthetic
Substitute, Open Approach
021W0KA
Bypass Thoracic Aorta, Descending to Innominate Artery with Nonautologous
Tissue Substitute, Open Approach
021W0KB
Bypass Thoracic Aorta, Descending to Subclavian with Nonautologous Tissue
Substitute, Open Approach
021W0KD
Bypass Thoracic Aorta, Descending to Carotid with Nonautologous Tissue
Substitute, Open Approach
021W0KF
Bypass Thoracic Aorta, Descending to Abdominal Artery with Nonautologous
Tissue Substitute, Open Approach
021W0KG
Bypass Thoracic Aorta, Descending to Axillary Artery with Nonautologous Tissue
Substitute, Open Approach
021W0KH
Bypass Thoracic Aorta, Descending to Brachial Artery with Nonautologous Tissue
Substitute, Open Approach
021W0KP
Bypass Thoracic Aorta, Descending to Pulmonary Trunk with Nonautologous
Tissue Substitute, Open Approach
021W0KQ
Bypass Thoracic Aorta, Descending to Right Pulmonary Artery with Nonautologous
Tissue Substitute, Open Approach
021W0KR
Bypass Thoracic Aorta, Descending to Left Pulmonary Artery with Nonautologous
Tissue Substitute, Open Approach
021W0KV
Bypass Thoracic Aorta, Descending to Lower Extremity Artery with Nonautologous
Tissue Substitute, Open Approach
021W0ZA
Bypass Thoracic Aorta, Descending to Innominate Artery, Open Approach
021W0ZB Bypass Thoracic Aorta, Descending to Subclavian, Open Approach
021W0ZD
Bypass Thoracic Aorta, Descending to Carotid, Open Approach
021W0ZP Bypass Thoracic Aorta, Descending to Pulmonary Trunk, Open Approach
021W0ZQ
Bypass Thoracic Aorta, Descending to Right Pulmonary Artery, Open Approach
021W0ZR Bypass Thoracic Aorta, Descending to Left Pulmonary Artery, Open Approach
021X08A
Bypass Thoracic Aorta, Ascending/Arch to Innominate Artery with Zooplastic
Tissue, Open Approach
021X08B
Bypass Thoracic Aorta, Ascending/Arch to Subclavian with Zooplastic Tissue,
Open Approach
021X08D
Bypass Thoracic Aorta, Ascending/Arch to Carotid with Zooplastic Tissue, Open
Approach
021X08P
Bypass Thoracic Aorta, Ascending/Arch to Pulmonary Trunk with Zooplastic
Tissue, Open Approach
021X08Q
Bypass Thoracic Aorta, Ascending/Arch to Right Pulmonary Artery with Zooplastic
Tissue, Open Approach
021X08R
Bypass Thoracic Aorta, Ascending/Arch to Left Pulmonary Artery with Zooplastic
Tissue, Open Approach
021X09A
Bypass Thoracic Aorta, Ascending/Arch to Innominate Artery with Autologous
Venous Tissue, Open Approach
021X09B
Bypass Thoracic Aorta, Ascending/Arch to Subclavian with Autologous Venous
Tissue, Open Approach
021X09D
Bypass Thoracic Aorta, Ascending/Arch to Carotid with Autologous Venous
Tissue, Open Approach
021X09P
Bypass Thoracic Aorta, Ascending/Arch to Pulmonary Trunk with Autologous
Venous Tissue, Open Approach
021X09Q
Bypass Thoracic Aorta, Ascending/Arch to Right Pulmonary Artery with Autologous
Venous Tissue, Open Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
105 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
021X09R
Bypass Thoracic Aorta, Ascending/Arch to Left Pulmonary Artery with Autologous
Venous Tissue, Open Approach
021X0AA
Bypass Thoracic Aorta, Ascending/Arch to Innominate Artery with Autologous
Arterial Tissue, Open Approach
021X0AB
Bypass Thoracic Aorta, Ascending/Arch to Subclavian with Autologous Arterial
Tissue, Open Approach
021X0AD
Bypass Thoracic Aorta, Ascending/Arch to Carotid with Autologous Arterial Tissue,
Open Approach
021X0AP
Bypass Thoracic Aorta, Ascending/Arch to Pulmonary Trunk with Autologous
Arterial Tissue, Open Approach
021X0AQ
Bypass Thoracic Aorta, Ascending/Arch to Right Pulmonary Artery with Autologous
Arterial Tissue, Open Approach
021X0AR
Bypass Thoracic Aorta, Ascending/Arch to Left Pulmonary Artery with Autologous
Arterial Tissue, Open Approach
021X0JA
Bypass Thoracic Aorta, Ascending/Arch to Innominate Artery with Synthetic
Substitute, Open Approach
021X0JB
Bypass Thoracic Aorta, Ascending/Arch to Subclavian with Synthetic Substitute,
Open Approach
021X0JD
Bypass Thoracic Aorta, Ascending/Arch to Carotid with Synthetic Substitute, Open
Approach
021X0JP
Bypass Thoracic Aorta, Ascending/Arch to Pulmonary Trunk with Synthetic
Substitute, Open Approach
021X0JQ
Bypass Thoracic Aorta, Ascending/Arch to Right Pulmonary Artery with Synthetic
Substitute, Open Approach
021X0JR
Bypass Thoracic Aorta, Ascending/Arch to Left Pulmonary Artery with Synthetic
Substitute, Open Approach
021X0KA
Bypass Thoracic Aorta, Ascending/Arch to Innominate Artery with Nonautologous
Tissue Substitute, Open Approach
021X0KB
Bypass Thoracic Aorta, Ascending/Arch to Subclavian with Nonautologous Tissue
Substitute, Open Approach
021X0KD
Bypass Thoracic Aorta, Ascending/Arch to Carotid with Nonautologous Tissue
Substitute, Open Approach
021X0KP
Bypass Thoracic Aorta, Ascending/Arch to Pulmonary Trunk with Nonautologous
Tissue Substitute, Open Approach
021X0KQ
Bypass Thoracic Aorta, Ascending/Arch to Right Pulmonary Artery with
Nonautologous Tissue Substitute, Open Approach
021X0KR
Bypass Thoracic Aorta, Ascending/Arch to Left Pulmonary Artery with
Nonautologous Tissue Substitute, Open Approach
021X0ZA Bypass Thoracic Aorta, Ascending/Arch to Innominate Artery, Open Approach
021X0ZB
Bypass Thoracic Aorta, Ascending/Arch to Subclavian, Open Approach
021X0ZD Bypass Thoracic Aorta, Ascending/Arch to Carotid, Open Approach
021X0ZP
Bypass Thoracic Aorta, Ascending/Arch to Pulmonary Trunk, Open Approach
021X0ZQ
Bypass Thoracic Aorta, Ascending/Arch to Right Pulmonary Artery, Open
Approach
021X0ZR
Bypass Thoracic Aorta, Ascending/Arch to Left Pulmonary Artery, Open Approach
02BW0ZX Excision of Thoracic Aorta, Descending, Open Approach, Diagnostic
02BW0ZZ
Excision of Thoracic Aorta, Descending, Open Approach
02BX0ZX Excision of Thoracic Aorta, Ascending/Arch, Open Approach, Diagnostic
02BX0ZZ
Excision of Thoracic Aorta, Ascending/Arch, Open Approach
02QW0ZZ Repair Thoracic Aorta, Descending, Open Approach
02QX0ZZ
Repair Thoracic Aorta, Ascending/Arch, Open Approach
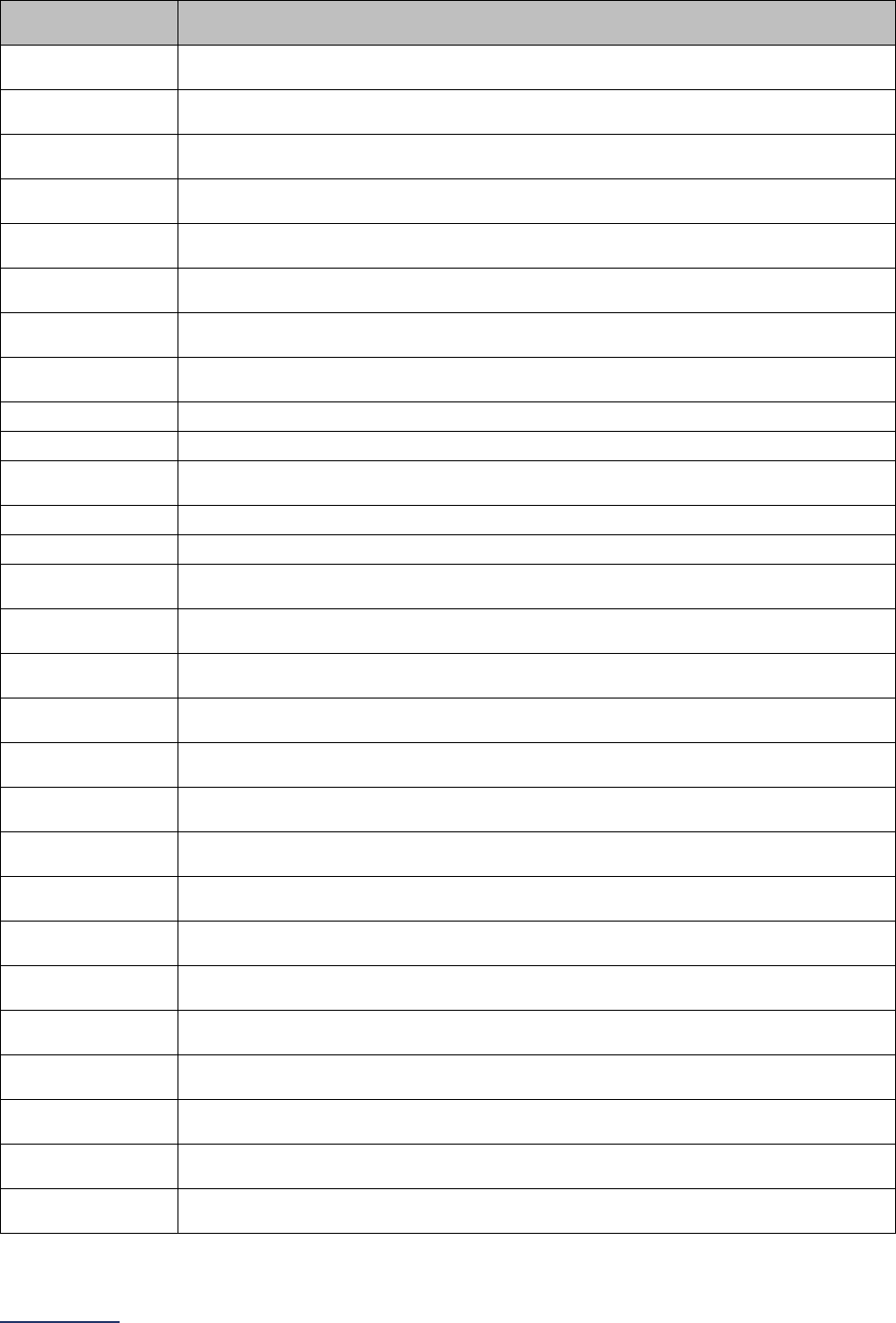
2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
106 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
02RW07Z
Replacement of Thoracic Aorta, Descending with Autologous Tissue Substitute,
Open Approach
02RW08Z
Replacement of Thoracic Aorta, Descending with Zooplastic Tissue, Open
Approach
02RW0JZ
Replacement of Thoracic Aorta, Descending with Synthetic Substitute, Open
Approach
02RW0KZ
Replacement of Thoracic Aorta, Descending with Nonautologous Tissue
Substitute, Open Approach
02RX07Z
Replacement of Thoracic Aorta, Ascending/Arch with Autologous Tissue
Substitute, Open Approach
02RX08Z
Replacement of Thoracic Aorta, Ascending/Arch with Zooplastic Tissue, Open
Approach
02RX0JZ
Replacement of Thoracic Aorta, Ascending/Arch with Synthetic Substitute, Open
Approach
02RX0KZ
Replacement of Thoracic Aorta, Ascending/Arch with Nonautologous Tissue
Substitute, Open Approach
02SW0ZZ
Reposition Thoracic Aorta, Descending, Open Approach
02SX0ZZ Reposition Thoracic Aorta, Ascending/Arch, Open Approach
02UW07Z
Supplement Thoracic Aorta, Descending with Autologous Tissue Substitute, Open
Approach
02UW08Z Supplement Thoracic Aorta, Descending with Zooplastic Tissue, Open Approach
02UW0JZ
Supplement Thoracic Aorta, Descending with Synthetic Substitute, Open Approach
02UW0KZ
Supplement Thoracic Aorta, Descending with Nonautologous Tissue Substitute,
Open Approach
02UX08Z
Supplement Thoracic Aorta, Ascending/Arch with Zooplastic Tissue, Open
Approach
041009B
Bypass Abdominal Aorta to Left Internal Iliac Artery with Autologous Venous
Tissue, Open Approach
041009C
Bypass Abdominal Aorta to Bilateral Internal Iliac Arteries with Autologous Venous
Tissue, Open Approach
041009D
Bypass Abdominal Aorta to Right External Iliac Artery with Autologous Venous
Tissue, Open Approach
041009F
Bypass Abdominal Aorta to Left External Iliac Artery with Autologous Venous
Tissue, Open Approach
041009G
Bypass Abdominal Aorta to Bilateral External Iliac Arteries with Autologous Venous
Tissue, Open Approach
041009H
Bypass Abdominal Aorta to Right Femoral Artery with Autologous Venous Tissue,
Open Approach
041009J
Bypass Abdominal Aorta to Left Femoral Artery with Autologous Venous Tissue,
Open Approach
041009K
Bypass Abdominal Aorta to Bilateral Femoral Arteries with Autologous Venous
Tissue, Open Approach
041009Q
Bypass Abdominal Aorta to Lower Extremity Artery with Autologous Venous
Tissue, Open Approach
041009R
Bypass Abdominal Aorta to Lower Artery with Autologous Venous Tissue, Open
Approach
04100A0
Bypass Abdominal Aorta to Abdominal Aorta with Autologous Arterial Tissue,
Open Approach
04100A1
Bypass Abdominal Aorta to Celiac Artery with Autologous Arterial Tissue, Open
Approach
04100A2
Bypass Abdominal Aorta to Mesenteric Artery with Autologous Arterial Tissue,
Open Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
107 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
04100A3
Bypass Abdominal Aorta to Right Renal Artery with Autologous Arterial Tissue,
Open Approach
04100A4
Bypass Abdominal Aorta to Left Renal Artery with Autologous Arterial Tissue,
Open Approach
04100A5
Bypass Abdominal Aorta to Bilateral Renal Artery with Autologous Arterial Tissue,
Open Approach
04100A6
Bypass Abdominal Aorta to Right Common Iliac Artery with Autologous Arterial
Tissue, Open Approach
04100A7
Bypass Abdominal Aorta to Left Common Iliac Artery with Autologous Arterial
Tissue, Open Approach
04100A8
Bypass Abdominal Aorta to Bilateral Common Iliac Arteries with Autologous
Arterial Tissue, Open Approach
04100A9
Bypass Abdominal Aorta to Right Internal Iliac Artery with Autologous Arterial
Tissue, Open Approach
04100AB
Bypass Abdominal Aorta to Left Internal Iliac Artery with Autologous Arterial
Tissue, Open Approach
04100AC
Bypass Abdominal Aorta to Bilateral Internal Iliac Arteries with Autologous Arterial
Tissue, Open Approach
04100AD
Bypass Abdominal Aorta to Right External Iliac Artery with Autologous Arterial
Tissue, Open Approach
04100AF
Bypass Abdominal Aorta to Left External Iliac Artery with Autologous Arterial
Tissue, Open Approach
04100AG
Bypass Abdominal Aorta to Bilateral External Iliac Arteries with Autologous Arterial
Tissue, Open Approach
04100AH
Bypass Abdominal Aorta to Right Femoral Artery with Autologous Arterial Tissue,
Open Approach
04100AJ
Bypass Abdominal Aorta to Left Femoral Artery with Autologous Arterial Tissue,
Open Approach
04100AK
Bypass Abdominal Aorta to Bilateral Femoral Arteries with Autologous Arterial
Tissue, Open Approach
04100AQ
Bypass Abdominal Aorta to Lower Extremity Artery with Autologous Arterial
Tissue, Open Approach
04100AR
Bypass Abdominal Aorta to Lower Artery with Autologous Arterial Tissue, Open
Approach
04100J0
Bypass Abdominal Aorta to Abdominal Aorta with Synthetic Substitute, Open
Approach
04100J1
Bypass Abdominal Aorta to Celiac Artery with Synthetic Substitute, Open
Approach
04100J2
Bypass Abdominal Aorta to Mesenteric Artery with Synthetic Substitute, Open
Approach
04100J3
Bypass Abdominal Aorta to Right Renal Artery with Synthetic Substitute, Open
Approach
04100J4
Bypass Abdominal Aorta to Left Renal Artery with Synthetic Substitute, Open
Approach
04100J5
Bypass Abdominal Aorta to Bilateral Renal Artery with Synthetic Substitute, Open
Approach
04100J6
Bypass Abdominal Aorta to Right Common Iliac Artery with Synthetic Substitute,
Open Approach
04100J7
Bypass Abdominal Aorta to Left Common Iliac Artery with Synthetic Substitute,
Open Approach
04100J8
Bypass Abdominal Aorta to Bilateral Common Iliac Arteries with Synthetic
Substitute, Open Approach
04100J9
Bypass Abdominal Aorta to Right Internal Iliac Artery with Synthetic Substitute,
Open Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
108 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
04100JB
Bypass Abdominal Aorta to Left Internal Iliac Artery with Synthetic Substitute,
Open Approach
04100JC
Bypass Abdominal Aorta to Bilateral Internal Iliac Arteries with Synthetic
Substitute, Open Approach
04100JD
Bypass Abdominal Aorta to Right External Iliac Artery with Synthetic Substitute,
Open Approach
04100JF
Bypass Abdominal Aorta to Left External Iliac Artery with Synthetic Substitute,
Open Approach
04100JG
Bypass Abdominal Aorta to Bilateral External Iliac Arteries with Synthetic
Substitute, Open Approach
04100JH
Bypass Abdominal Aorta to Right Femoral Artery with Synthetic Substitute, Open
Approach
04100JJ
Bypass Abdominal Aorta to Left Femoral Artery with Synthetic Substitute, Open
Approach
04100JK
Bypass Abdominal Aorta to Bilateral Femoral Arteries with Synthetic Substitute,
Open Approach
04100JQ
Bypass Abdominal Aorta to Lower Extremity Artery with Synthetic Substitute, Open
Approach
04100JR
Bypass Abdominal Aorta to Lower Artery with Synthetic Substitute, Open
Approach
04100K0
Bypass Abdominal Aorta to Abdominal Aorta with Nonautologous Tissue
Substitute, Open Approach
04100K1
Bypass Abdominal Aorta to Celiac Artery with Nonautologous Tissue Substitute,
Open Approach
04100K2
Bypass Abdominal Aorta to Mesenteric Artery with Nonautologous Tissue
Substitute, Open Approach
04100K3
Bypass Abdominal Aorta to Right Renal Artery with Nonautologous Tissue
Substitute, Open Approach
04100K4
Bypass Abdominal Aorta to Left Renal Artery with Nonautologous Tissue
Substitute, Open Approach
04100K5
Bypass Abdominal Aorta to Bilateral Renal Artery with Nonautologous Tissue
Substitute, Open Approach
04100K6
Bypass Abdominal Aorta to Right Common Iliac Artery with Nonautologous Tissue
Substitute, Open Approach
04100K7
Bypass Abdominal Aorta to Left Common Iliac Artery with Nonautologous Tissue
Substitute, Open Approach
04100K8
Bypass Abdominal Aorta to Bilateral Common Iliac Arteries with Nonautologous
Tissue Substitute, Open Approach
04100K9
Bypass Abdominal Aorta to Right Internal Iliac Artery with Nonautologous Tissue
Substitute, Open Approach
04100KB
Bypass Abdominal Aorta to Left Internal Iliac Artery with Nonautologous Tissue
Substitute, Open Approach
04100KC
Bypass Abdominal Aorta to Bilateral Internal Iliac Arteries with Nonautologous
Tissue Substitute, Open Approach
04100KD
Bypass Abdominal Aorta to Right External Iliac Artery with Nonautologous Tissue
Substitute, Open Approach
04100KF
Bypass Abdominal Aorta to Left External Iliac Artery with Nonautologous Tissue
Substitute, Open Approach
04100KG
Bypass Abdominal Aorta to Bilateral External Iliac Arteries with Nonautologous
Tissue Substitute, Open Approach
04100KH
Bypass Abdominal Aorta to Right Femoral Artery with Nonautologous Tissue
Substitute, Open Approach
04100KJ
Bypass Abdominal Aorta to Left Femoral Artery with Nonautologous Tissue
Substitute, Open Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
109 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
04100KK
Bypass Abdominal Aorta to Bilateral Femoral Arteries with Nonautologous Tissue
Substitute, Open Approach
04100KQ
Bypass Abdominal Aorta to Lower Extremity Artery with Nonautologous Tissue
Substitute, Open Approach
04100KR
Bypass Abdominal Aorta to Lower Artery with Nonautologous Tissue Substitute,
Open Approach
04100Z0 Bypass Abdominal Aorta to Abdominal Aorta, Open Approach
04100Z1
Bypass Abdominal Aorta to Celiac Artery, Open Approach
04100Z2 Bypass Abdominal Aorta to Mesenteric Artery, Open Approach
04100Z3
Bypass Abdominal Aorta to Right Renal Artery, Open Approach
04100Z4 Bypass Abdominal Aorta to Left Renal Artery, Open Approach
04100Z5
Bypass Abdominal Aorta to Bilateral Renal Artery, Open Approach
04100Z6 Bypass Abdominal Aorta to Right Common Iliac Artery, Open Approach
04100Z7
Bypass Abdominal Aorta to Left Common Iliac Artery, Open Approach
04100Z8 Bypass Abdominal Aorta to Bilateral Common Iliac Arteries, Open Approach
04100Z9
Bypass Abdominal Aorta to Right Internal Iliac Artery, Open Approach
04100ZB Bypass Abdominal Aorta to Left Internal Iliac Artery, Open Approach
04100ZC
Bypass Abdominal Aorta to Bilateral Internal Iliac Arteries, Open Approach
04100ZD Bypass Abdominal Aorta to Right External Iliac Artery, Open Approach
04100ZF
Bypass Abdominal Aorta to Left External Iliac Artery, Open Approach
04100ZG Bypass Abdominal Aorta to Bilateral External Iliac Arteries, Open Approach
04100ZH
Bypass Abdominal Aorta to Right Femoral Artery, Open Approach
04100ZJ Bypass Abdominal Aorta to Left Femoral Artery, Open Approach
04100ZK
Bypass Abdominal Aorta to Bilateral Femoral Arteries, Open Approach
04100ZQ Bypass Abdominal Aorta to Lower Extremity Artery, Open Approach
04100ZR
Bypass Abdominal Aorta to Lower Artery, Open Approach
041C090
Bypass Right Common Iliac Artery to Abdominal Aorta with Autologous Venous
Tissue, Open Approach
041C0A0
Bypass Right Common Iliac Artery to Abdominal Aorta with Autologous Arterial
Tissue, Open Approach
041C0J0
Bypass Right Common Iliac Artery to Abdominal Aorta with Synthetic Substitute,
Open Approach
041C0K0
Bypass Right Common Iliac Artery to Abdominal Aorta with Nonautologous Tissue
Substitute, Open Approach
041C0Z0 Bypass Right Common Iliac Artery to Abdominal Aorta, Open Approach
041D090
Bypass Left Common Iliac Artery to Abdominal Aorta with Autologous Venous
Tissue, Open Approach
041D0A0
Bypass Left Common Iliac Artery to Abdominal Aorta with Autologous Arterial
Tissue, Open Approach
041D0J0
Bypass Left Common Iliac Artery to Abdominal Aorta with Synthetic Substitute,
Open Approach
041D0K0
Bypass Left Common Iliac Artery to Abdominal Aorta with Nonautologous Tissue
Substitute, Open Approach
041D0Z0
Bypass Left Common Iliac Artery to Abdominal Aorta, Open Approach
04B00ZX Excision of Abdominal Aorta, Open Approach, Diagnostic
04B00ZZ
Excision of Abdominal Aorta, Open Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
110 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
04Q00ZZ
Repair Abdominal Aorta, Open Approach
04R007Z
Replacement of Abdominal Aorta with Autologous Tissue Substitute, Open
Approach
04R00JZ
Replacement of Abdominal Aorta with Synthetic Substitute, Open Approach
04R00KZ
Replacement of Abdominal Aorta with Nonautologous Tissue Substitute, Open
Approach
04S00ZZ
Reposition Abdominal Aorta, Open Approach
04U007Z Supplement Abdominal Aorta with Autologous Tissue Substitute, Open Approach
04U00JZ
Supplement Abdominal Aorta with Synthetic Substitute, Open Approach
04U00KZ
Supplement Abdominal Aorta with Nonautologous Tissue Substitute, Open
Approach
X2RX0N7
Replacement of Thoracic Aorta, Arch using Branched Synthetic Substitute with
Intraluminal Device, Open Approach, New Technology Group 7

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
111 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Lung Resection for Cancer Measure Specifications
For lung resection for cancer, there are two sets of ICD-10 codes for counting patient discharges. The
first set of codes is to identify patients who have had the procedure. The second set of codes is to identify
patients with a specific diagnosis.
Count the number of patients, ages 18 years and older, discharged with the following ICD-10 codes in
any procedure field AND any of the following ICD-10 codes in any diagnosis field.
ICD-10 Lung Resection for Cancer Procedure Codes
ICD-10
Procedure Code
Code Description
0BBC0ZZ Excision of Right Upper Lung Lobe, Open Approach
0BBC3ZZ
Excision of Right Upper Lung Lobe, Percutaneous Approach
0BBC4ZZ Excision of Right Upper Lung Lobe, Percutaneous Endoscopic Approach
0BBD0ZZ
Excision of Right Middle Lung Lobe, Open Approach
0BBD3ZZ Excision of Right Middle Lung Lobe, Percutaneous Approach
0BBD4ZZ
Excision of Right Middle Lung Lobe, Percutaneous Endoscopic Approach
0BBF0ZZ Excision of Right Lower Lung Lobe, Open Approach
0BBF3ZZ
Excision of Right Lower Lung Lobe, Percutaneous Approach
0BBF4ZZ Excision of Right Lower Lung Lobe, Percutaneous Endoscopic Approach
0BBG0ZZ
Excision of Left Upper Lung Lobe, Open Approach
0BBG3ZZ Excision of Left Upper Lung Lobe, Percutaneous Approach
0BBG4ZZ
Excision of Left Upper Lung Lobe, Percutaneous Endoscopic Approach
0BBH0ZZ Excision of Lung Lingula, Open Approach
0BBH3ZZ
Excision of Lung Lingula, Percutaneous Approach
0BBH4ZZ Excision of Lung Lingula, Percutaneous Endoscopic Approach
0BBJ0ZZ
Excision of Left Lower Lung Lobe, Open Approach
0BBJ3ZZ Excision of Left Lower Lung Lobe, Percutaneous Approach
0BBJ4ZZ
Excision of Left Lower Lung Lobe, Percutaneous Endoscopic Approach
0BBK0ZZ Excision of Right Lung, Open Approach
0BBK3ZZ
Excision of Right Lung, Percutaneous Approach
0BBK4ZZ Excision of Right Lung, Percutaneous Endoscopic Approach
0BBL0ZZ
Excision of Left Lung, Open Approach
0BBL3ZZ Excision of Left Lung, Percutaneous Approach
0BBL4ZZ
Excision of Left Lung, Percutaneous Endoscopic Approach
0BBL7ZZ Excision of Left Lung, Via Natural or Artificial Opening
0BTC0ZZ
Resection of Right Upper Lung Lobe, Open Approach
0BTC4ZZ Resection of Right Upper Lung Lobe, Percutaneous Endoscopic Approach
0BTD0ZZ
Resection of Right Middle Lung Lobe, Open Approach
0BTD4ZZ Resection of Right Middle Lung Lobe, Percutaneous Endoscopic Approach
0BTF0ZZ
Resection of Right Lower Lung Lobe, Open Approach
0BTF4ZZ Resection of Right Lower Lung Lobe, Percutaneous Endoscopic Approach
0BTG0ZZ
Resection of Left Upper Lung Lobe, Open Approach
0BTG4ZZ Resection of Left Upper Lung Lobe, Percutaneous Endoscopic Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
112 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
0BTH0ZZ
Resection of Lung Lingula, Open Approach
0BTH4ZZ Resection of Lung Lingula, Percutaneous Endoscopic Approach
0BTJ0ZZ
Resection of Left Lower Lung Lobe, Open Approach
0BTJ4ZZ Resection of Left Lower Lung Lobe, Percutaneous Endoscopic Approach
0BTK0ZZ
Resection of Right Lung, Open Approach
0BTK4ZZ Resection of Right Lung, Percutaneous Endoscopic Approach
0BTL0ZZ
Resection of Left Lung, Open Approach
0BTL4ZZ Resection of Left Lung, Percutaneous Endoscopic Approach
ICD-10 Malignant Tumor and Cancer in Situ Diagnosis Codes
ICD-10 Diagnosis
Code
Code Description
C7A.090 Malignant carcinoid tumor of the bronchus and lung
C7A.1
Malignant poorly differentiated neuroendocrine tumors
C7A.8 Other malignant neuroendocrine tumors
C34.00
Malignant neoplasm of main bronchus
C34.01 Malignant neoplasm of right main bronchus
C34.02
Malignant neoplasm of left main bronchus
C34.10 Malignant neoplasm of upper lobe, unspecified bronchus or lung
C34.11
Malignant neoplasm of upper lobe, right bronchus or lung
C34.12 Malignant neoplasm of upper lobe, left bronchus or lung
C34.2
Malignant neoplasm of middle lobe, bronchus or lung
C34.30 Malignant neoplasm of lower lobe, unspecified bronchus or lung
C34.31
Malignant neoplasm of lower lobe, right bronchus or lung
C34.32 Malignant neoplasm of lower lobe, left bronchus or lung
C34.80
Malignant neoplasm of overlapping sites of unspecified bronchus and lung
C34.81 Malignant neoplasm of overlapping sites of right bronchus and lung
C34.82
Malignant neoplasm of overlapping sites of left bronchus and lung
C34.90 Malignant neoplasm of unspecified part of unspecified bronchus or lung
C34.91
Malignant neoplasm of unspecified part of right bronchus or lung
C34.92 Malignant neoplasm of unspecified part of left bronchus or lung
D02.20
Carcinoma in situ of unspecified bronchus and lung
D02.21 Carcinoma in situ of right bronchus and lung
D02.22
Carcinoma in situ of left bronchus and lung

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
113 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Esophageal Resection for Cancer Measure Specifications
For esophageal resection for cancer, there are two sets of ICD-10 codes for counting patient discharges.
The first set of codes is to identify patients who have had the procedure. The second set of codes is to
identify patients with a specific diagnosis.
Count the number of patients, ages 18 years and older, discharged with the following ICD-10 codes in
any procedure field AND any of the following ICD-10 codes in any diagnosis field.
ICD-10 Esophageal Resection for Cancer Procedure Codes
ICD-10
Procedure Code
Code Description
0D11074
Bypass Upper Esophagus to Cutaneous with Autologous Tissue Substitute, Open
Approach
0D11076
Bypass Upper Esophagus to Stomach with Autologous Tissue Substitute, Open
Approach
0D11079
Bypass Upper Esophagus to Duodenum with Autologous Tissue Substitute, Open
Approach
0D1107A
Bypass Upper Esophagus to Jejunum with Autologous Tissue Substitute, Open
Approach
0D1107B
Bypass Upper Esophagus to Ileum with Autologous Tissue Substitute, Open
Approach
0D110J4
Bypass Upper Esophagus to Cutaneous with Synthetic Substitute, Open Approach
0D110J6 Bypass Upper Esophagus to Stomach with Synthetic Substitute, Open Approach
0D110J9
Bypass Upper Esophagus to Duodenum with Synthetic Substitute, Open Approach
0D110JA Bypass Upper Esophagus to Jejunum with Synthetic Substitute, Open Approach
0D110JB
Bypass Upper Esophagus to Ileum with Synthetic Substitute, Open Approach
0D110K4
Bypass Upper Esophagus to Cutaneous with Nonautologous Tissue Substitute,
Open Approach
0D110K6
Bypass Upper Esophagus to Stomach with Nonautologous Tissue Substitute,
Open Approach
0D110K9
Bypass Upper Esophagus to Duodenum with Nonautologous Tissue Substitute,
Open Approach
0D110KA
Bypass Upper Esophagus to Jejunum with Nonautologous Tissue Substitute,
Open Approach
0D110KB
Bypass Upper Esophagus to Ileum with Nonautologous Tissue Substitute, Open
Approach
0D110Z4
Bypass Upper Esophagus to Cutaneous, Open Approach
0D110Z6 Bypass Upper Esophagus to Stomach, Open Approach
0D110Z9
Bypass Upper Esophagus to Duodenum, Open Approach
0D110ZA Bypass Upper Esophagus to Jejunum, Open Approach
0D110ZB
Bypass Upper Esophagus to Ileum, Open Approach
0D113J4
Bypass Upper Esophagus to Cutaneous with Synthetic Substitute, Percutaneous
Approach
0D11474
Bypass Upper Esophagus to Cutaneous with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D11476
Bypass Upper Esophagus to Stomach with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D11479
Bypass Upper Esophagus to Duodenum with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D1147A
Bypass Upper Esophagus to Jejunum with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
114 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
0D1147B
Bypass Upper Esophagus to Ileum with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D114J4
Bypass Upper Esophagus to Cutaneous with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D114J6
Bypass Upper Esophagus to Stomach with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D114J9
Bypass Upper Esophagus to Duodenum with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D114JA
Bypass Upper Esophagus to Jejunum with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D114JB
Bypass Upper Esophagus to Ileum with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D114K4
Bypass Upper Esophagus to Cutaneous with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D114K6
Bypass Upper Esophagus to Stomach with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D114K9
Bypass Upper Esophagus to Duodenum with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D114KA
Bypass Upper Esophagus to Jejunum with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D114KB
Bypass Upper Esophagus to Ileum with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D114Z4 Bypass Upper Esophagus to Cutaneous, Percutaneous Endoscopic Approach
0D114Z6
Bypass Upper Esophagus to Stomach, Percutaneous Endoscopic Approach
0D114Z9 Bypass Upper Esophagus to Duodenum, Percutaneous Endoscopic Approach
0D114ZA
Bypass Upper Esophagus to Jejunum, Percutaneous Endoscopic Approach
0D114ZB Bypass Upper Esophagus to Ileum, Percutaneous Endoscopic Approach
0D11874
Bypass Upper Esophagus to Cutaneous with Autologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D11876
Bypass Upper Esophagus to Stomach with Autologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D11879
Bypass Upper Esophagus to Duodenum with Autologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D1187A
Bypass Upper Esophagus to Jejunum with Autologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D1187B
Bypass Upper Esophagus to Ileum with Autologous Tissue Substitute, Via Natural
or Artificial Opening Endoscopic
0D118J4
Bypass Upper Esophagus to Cutaneous with Synthetic Substitute, Via Natural or
Artificial Opening Endoscopic
0D118J6
Bypass Upper Esophagus to Stomach with Synthetic Substitute, Via Natural or
Artificial Opening Endoscopic
0D118J9
Bypass Upper Esophagus to Duodenum with Synthetic Substitute, Via Natural or
Artificial Opening Endoscopic
0D118JA
Bypass Upper Esophagus to Jejunum with Synthetic Substitute, Via Natural or
Artificial Opening Endoscopic
0D118JB
Bypass Upper Esophagus to Ileum with Synthetic Substitute, Via Natural or
Artificial Opening Endoscopic
0D118K4
Bypass Upper Esophagus to Cutaneous with Nonautologous Tissue Substitute,
Via Natural or Artificial Opening Endoscopic
0D118K6
Bypass Upper Esophagus to Stomach with Nonautologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
115 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
0D118K9
Bypass Upper Esophagus to Duodenum with Nonautologous Tissue Substitute,
Via Natural or Artificial Opening Endoscopic
0D118KA
Bypass Upper Esophagus to Jejunum with Nonautologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D118KB
Bypass Upper Esophagus to Ileum with Nonautologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D118Z4
Bypass Upper Esophagus to Cutaneous, Via Natural or Artificial Opening
Endoscopic
0D118Z6
Bypass Upper Esophagus to Stomach, Via Natural or Artificial Opening
Endoscopic
0D118Z9
Bypass Upper Esophagus to Duodenum, Via Natural or Artificial Opening
Endoscopic
0D118ZA
Bypass Upper Esophagus to Jejunum, Via Natural or Artificial Opening Endoscopic
0D118ZB Bypass Upper Esophagus to Ileum, Via Natural or Artificial Opening Endoscopic
0D12074
Bypass Middle Esophagus to Cutaneous with Autologous Tissue Substitute, Open
Approach
0D12076
Bypass Middle Esophagus to Stomach with Autologous Tissue Substitute, Open
Approach
0D12079
Bypass Middle Esophagus to Duodenum with Autologous Tissue Substitute, Open
Approach
0D1207A
Bypass Middle Esophagus to Jejunum with Autologous Tissue Substitute, Open
Approach
0D1207B
Bypass Middle Esophagus to Ileum with Autologous Tissue Substitute, Open
Approach
0D120J4
Bypass Middle Esophagus to Cutaneous with Synthetic Substitute, Open
Approach
0D120J6
Bypass Middle Esophagus to Stomach with Synthetic Substitute, Open Approach
0D120J9
Bypass Middle Esophagus to Duodenum with Synthetic Substitute, Open
Approach
0D120JA
Bypass Middle Esophagus to Jejunum with Synthetic Substitute, Open Approach
0D120JB Bypass Middle Esophagus to Ileum with Synthetic Substitute, Open Approach
0D120K4
Bypass Middle Esophagus to Cutaneous with Nonautologous Tissue Substitute,
Open Approach
0D120K6
Bypass Middle Esophagus to Stomach with Nonautologous Tissue Substitute,
Open Approach
0D120K9
Bypass Middle Esophagus to Duodenum with Nonautologous Tissue Substitute,
Open Approach
0D120KA
Bypass Middle Esophagus to Jejunum with Nonautologous Tissue Substitute,
Open Approach
0D120KB
Bypass Middle Esophagus to Ileum with Nonautologous Tissue Substitute, Open
Approach
0D120Z4 Bypass Middle Esophagus to Cutaneous, Open Approach
0D120Z6
Bypass Middle Esophagus to Stomach, Open Approach
0D120Z9 Bypass Middle Esophagus to Duodenum, Open Approach
0D120ZA
Bypass Middle Esophagus to Jejunum, Open Approach
0D120ZB Bypass Middle Esophagus to Ileum, Open Approach
0D123J4
Bypass Middle Esophagus to Cutaneous with Synthetic Substitute, Percutaneous
Approach
0D12474
Bypass Middle Esophagus to Cutaneous with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
116 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
0D12476
Bypass Middle Esophagus to Stomach with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D12479
Bypass Middle Esophagus to Duodenum with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D1247A
Bypass Middle Esophagus to Jejunum with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D1247B
Bypass Middle Esophagus to Ileum with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D124J4
Bypass Middle Esophagus to Cutaneous with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D124J6
Bypass Middle Esophagus to Stomach with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D124J9
Bypass Middle Esophagus to Duodenum with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D124JA
Bypass Middle Esophagus to Jejunum with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D124JB
Bypass Middle Esophagus to Ileum with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D124K4
Bypass Middle Esophagus to Cutaneous with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D124K6
Bypass Middle Esophagus to Stomach with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D124K9
Bypass Middle Esophagus to Duodenum with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D124KA
Bypass Middle Esophagus to Jejunum with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D124KB
Bypass Middle Esophagus to Ileum with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D124Z4
Bypass Middle Esophagus to Cutaneous, Percutaneous Endoscopic Approach
0D124Z6 Bypass Middle Esophagus to Stomach, Percutaneous Endoscopic Approach
0D124Z9
Bypass Middle Esophagus to Duodenum, Percutaneous Endoscopic Approach
0D124ZA Bypass Middle Esophagus to Jejunum, Percutaneous Endoscopic Approach
0D124ZB
Bypass Middle Esophagus to Ileum, Percutaneous Endoscopic Approach
0D12874
Bypass Middle Esophagus to Cutaneous with Autologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D12876
Bypass Middle Esophagus to Stomach with Autologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D12879
Bypass Middle Esophagus to Duodenum with Autologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D1287A
Bypass Middle Esophagus to Jejunum with Autologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D1287B
Bypass Middle Esophagus to Ileum with Autologous Tissue Substitute, Via Natural
or Artificial Opening Endoscopic
0D128J4
Bypass Middle Esophagus to Cutaneous with Synthetic Substitute, Via Natural or
Artificial Opening Endoscopic
0D128J6
Bypass Middle Esophagus to Stomach with Synthetic Substitute, Via Natural or
Artificial Opening Endoscopic
0D128J9
Bypass Middle Esophagus to Duodenum with Synthetic Substitute, Via Natural or
Artificial Opening Endoscopic
0D128JA
Bypass Middle Esophagus to Jejunum with Synthetic Substitute, Via Natural or
Artificial Opening Endoscopic

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
117 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
0D128JB
Bypass Middle Esophagus to Ileum with Synthetic Substitute, Via Natural or
Artificial Opening Endoscopic
0D128K4
Bypass Middle Esophagus to Cutaneous with Nonautologous Tissue Substitute,
Via Natural or Artificial Opening Endoscopic
0D128K6
Bypass Middle Esophagus to Stomach with Nonautologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D128K9
Bypass Middle Esophagus to Duodenum with Nonautologous Tissue Substitute,
Via Natural or Artificial Opening Endoscopic
0D128KA
Bypass Middle Esophagus to Jejunum with Nonautologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D128KB
Bypass Middle Esophagus to Ileum with Nonautologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D128Z4
Bypass Middle Esophagus to Cutaneous, Via Natural or Artificial Opening
Endoscopic
0D128Z6
Bypass Middle Esophagus to Stomach, Via Natural or Artificial Opening
Endoscopic
0D128Z9
Bypass Middle Esophagus to Duodenum, Via Natural or Artificial Opening
Endoscopic
0D128ZA
Bypass Middle Esophagus to Jejunum, Via Natural or Artificial Opening
Endoscopic
0D128ZB
Bypass Middle Esophagus to Ileum, Via Natural or Artificial Opening Endoscopic
0D13074
Bypass Lower Esophagus to Cutaneous with Autologous Tissue Substitute, Open
Approach
0D13076
Bypass Lower Esophagus to Stomach with Autologous Tissue Substitute, Open
Approach
0D13079
Bypass Lower Esophagus to Duodenum with Autologous Tissue Substitute, Open
Approach
0D1307A
Bypass Lower Esophagus to Jejunum with Autologous Tissue Substitute, Open
Approach
0D1307B
Bypass Lower Esophagus to Ileum with Autologous Tissue Substitute, Open
Approach
0D130J4
Bypass Lower Esophagus to Cutaneous with Synthetic Substitute, Open Approach
0D130J6 Bypass Lower Esophagus to Stomach with Synthetic Substitute, Open Approach
0D130J9
Bypass Lower Esophagus to Duodenum with Synthetic Substitute, Open Approach
0D130JA Bypass Lower Esophagus to Jejunum with Synthetic Substitute, Open Approach
0D130JB
Bypass Lower Esophagus to Ileum with Synthetic Substitute, Open Approach
0D130K4
Bypass Lower Esophagus to Cutaneous with Nonautologous Tissue Substitute,
Open Approach
0D130K6
Bypass Lower Esophagus to Stomach with Nonautologous Tissue Substitute,
Open Approach
0D130K9
Bypass Lower Esophagus to Duodenum with Nonautologous Tissue Substitute,
Open Approach
0D130KA
Bypass Lower Esophagus to Jejunum with Nonautologous Tissue Substitute,
Open Approach
0D130KB
Bypass Lower Esophagus to Ileum with Nonautologous Tissue Substitute, Open
Approach
0D130Z4
Bypass Lower Esophagus to Cutaneous, Open Approach
0D130Z6 Bypass Lower Esophagus to Stomach, Open Approach
0D130Z9
Bypass Lower Esophagus to Duodenum, Open Approach
0D130ZA Bypass Lower Esophagus to Jejunum, Open Approach
0D130ZB
Bypass Lower Esophagus to Ileum, Open Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
118 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
0D133J4
Bypass Lower Esophagus to Cutaneous with Synthetic Substitute, Percutaneous
Approach
0D13474
Bypass Lower Esophagus to Cutaneous with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D13476
Bypass Lower Esophagus to Stomach with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D13479
Bypass Lower Esophagus to Duodenum with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D1347A
Bypass Lower Esophagus to Jejunum with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D1347B
Bypass Lower Esophagus to Ileum with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D134J4
Bypass Lower Esophagus to Cutaneous with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D134J6
Bypass Lower Esophagus to Stomach with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D134J9
Bypass Lower Esophagus to Duodenum with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D134JA
Bypass Lower Esophagus to Jejunum with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D134JB
Bypass Lower Esophagus to Ileum with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D134K4
Bypass Lower Esophagus to Cutaneous with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D134K6
Bypass Lower Esophagus to Stomach with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D134K9
Bypass Lower Esophagus to Duodenum with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D134KA
Bypass Lower Esophagus to Jejunum with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D134KB
Bypass Lower Esophagus to Ileum with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D134Z4
Bypass Lower Esophagus to Cutaneous, Percutaneous Endoscopic Approach
0D134Z6 Bypass Lower Esophagus to Stomach, Percutaneous Endoscopic Approach
0D134Z9
Bypass Lower Esophagus to Duodenum, Percutaneous Endoscopic Approach
0D134ZA Bypass Lower Esophagus to Jejunum, Percutaneous Endoscopic Approach
0D134ZB
Bypass Lower Esophagus to Ileum, Percutaneous Endoscopic Approach
0D13874
Bypass Lower Esophagus to Cutaneous with Autologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D13876
Bypass Lower Esophagus to Stomach with Autologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D13879
Bypass Lower Esophagus to Duodenum with Autologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D1387A
Bypass Lower Esophagus to Jejunum with Autologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D1387B
Bypass Lower Esophagus to Ileum with Autologous Tissue Substitute, Via Natural
or Artificial Opening Endoscopic
0D138J4
Bypass Lower Esophagus to Cutaneous with Synthetic Substitute, Via Natural or
Artificial Opening Endoscopic
0D138J6
Bypass Lower Esophagus to Stomach with Synthetic Substitute, Via Natural or
Artificial Opening Endoscopic

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
119 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
0D138J9
Bypass Lower Esophagus to Duodenum with Synthetic Substitute, Via Natural or
Artificial Opening Endoscopic
0D138JA
Bypass Lower Esophagus to Jejunum with Synthetic Substitute, Via Natural or
Artificial Opening Endoscopic
0D138JB
Bypass Lower Esophagus to Ileum with Synthetic Substitute, Via Natural or
Artificial Opening Endoscopic
0D138K4
Bypass Lower Esophagus to Cutaneous with Nonautologous Tissue Substitute,
Via Natural or Artificial Opening Endoscopic
0D138K6
Bypass Lower Esophagus to Stomach with Nonautologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D138K9
Bypass Lower Esophagus to Duodenum with Nonautologous Tissue Substitute,
Via Natural or Artificial Opening Endoscopic
0D138KA
Bypass Lower Esophagus to Jejunum with Nonautologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D138KB
Bypass Lower Esophagus to Ileum with Nonautologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D138Z4
Bypass Lower Esophagus to Cutaneous, Via Natural or Artificial Opening
Endoscopic
0D138Z6
Bypass Lower Esophagus to Stomach, Via Natural or Artificial Opening
Endoscopic
0D138Z9
Bypass Lower Esophagus to Duodenum, Via Natural or Artificial Opening
Endoscopic
0D138ZA Bypass Lower Esophagus to Jejunum, Via Natural or Artificial Opening Endoscopic
0D138ZB
Bypass Lower Esophagus to Ileum, Via Natural or Artificial Opening Endoscopic
0D15074
Bypass Esophagus to Cutaneous with Autologous Tissue Substitute, Open
Approach
0D15076
Bypass Esophagus to Stomach with Autologous Tissue Substitute, Open
Approach
0D15079
Bypass Esophagus to Duodenum with Autologous Tissue Substitute, Open
Approach
0D1507A
Bypass Esophagus to Jejunum with Autologous Tissue Substitute, Open Approach
0D1507B Bypass Esophagus to Ileum with Autologous Tissue Substitute, Open Approach
0D150J4
Bypass Esophagus to Cutaneous with Synthetic Substitute, Open Approach
0D150J6 Bypass Esophagus to Stomach with Synthetic Substitute, Open Approach
0D150J9
Bypass Esophagus to Duodenum with Synthetic Substitute, Open Approach
0D150JA Bypass Esophagus to Jejunum with Synthetic Substitute, Open Approach
0D150JB
Bypass Esophagus to Ileum with Synthetic Substitute, Open Approach
0D150K4
Bypass Esophagus to Cutaneous with Nonautologous Tissue Substitute, Open
Approach
0D150K6
Bypass Esophagus to Stomach with Nonautologous Tissue Substitute, Open
Approach
0D150K9
Bypass Esophagus to Duodenum with Nonautologous Tissue Substitute, Open
Approach
0D150KA
Bypass Esophagus to Jejunum with Nonautologous Tissue Substitute, Open
Approach
0D150KB
Bypass Esophagus to Ileum with Nonautologous Tissue Substitute, Open
Approach
0D150Z4
Bypass Esophagus to Cutaneous, Open Approach
0D150Z6 Bypass Esophagus to Stomach, Open Approach
0D150Z9
Bypass Esophagus to Duodenum, Open Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
120 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
0D150ZA
Bypass Esophagus to Jejunum, Open Approach
0D150ZB Bypass Esophagus to Ileum, Open Approach
0D153J4
Bypass Esophagus to Cutaneous with Synthetic Substitute, Percutaneous
Approach
0D15474
Bypass Esophagus to Cutaneous with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D15476
Bypass Esophagus to Stomach with Autologous Tissue Substitute, Percutaneous
Endoscopic Approach
0D15479
Bypass Esophagus to Duodenum with Autologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D1547A
Bypass Esophagus to Jejunum with Autologous Tissue Substitute, Percutaneous
Endoscopic Approach
0D1547B
Bypass Esophagus to Ileum with Autologous Tissue Substitute, Percutaneous
Endoscopic Approach
0D154J4
Bypass Esophagus to Cutaneous with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D154J6
Bypass Esophagus to Stomach with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D154J9
Bypass Esophagus to Duodenum with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D154JA
Bypass Esophagus to Jejunum with Synthetic Substitute, Percutaneous
Endoscopic Approach
0D154JB
Bypass Esophagus to Ileum with Synthetic Substitute, Percutaneous Endoscopic
Approach
0D154K4
Bypass Esophagus to Cutaneous with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D154K6
Bypass Esophagus to Stomach with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D154K9
Bypass Esophagus to Duodenum with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D154KA
Bypass Esophagus to Jejunum with Nonautologous Tissue Substitute,
Percutaneous Endoscopic Approach
0D154KB
Bypass Esophagus to Ileum with Nonautologous Tissue Substitute, Percutaneous
Endoscopic Approach
0D154Z4
Bypass Esophagus to Cutaneous, Percutaneous Endoscopic Approach
0D154Z6 Bypass Esophagus to Stomach, Percutaneous Endoscopic Approach
0D154Z9
Bypass Esophagus to Duodenum, Percutaneous Endoscopic Approach
0D154ZA Bypass Esophagus to Jejunum, Percutaneous Endoscopic Approach
0D154ZB
Bypass Esophagus to Ileum, Percutaneous Endoscopic Approach
0D15874
Bypass Esophagus to Cutaneous with Autologous Tissue Substitute, Via Natural
or Artificial Opening Endoscopic
0D15876
Bypass Esophagus to Stomach with Autologous Tissue Substitute, Via Natural or
Artificial Opening Endoscopic
0D15879
Bypass Esophagus to Duodenum with Autologous Tissue Substitute, Via Natural
or Artificial Opening Endoscopic
0D1587A
Bypass Esophagus to Jejunum with Autologous Tissue Substitute, Via Natural or
Artificial Opening Endoscopic
0D1587B
Bypass Esophagus to Ileum with Autologous Tissue Substitute, Via Natural or
Artificial Opening Endoscopic
0D158J4
Bypass Esophagus to Cutaneous with Synthetic Substitute, Via Natural or Artificial
Opening Endoscopic
2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
121 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
0D158J6
Bypass Esophagus to Stomach with Synthetic Substitute, Via Natural or Artificial
Opening Endoscopic
0D158J9
Bypass Esophagus to Duodenum with Synthetic Substitute, Via Natural or Artificial
Opening Endoscopic
0D158JA
Bypass Esophagus to Jejunum with Synthetic Substitute, Via Natural or Artificial
Opening Endoscopic
0D158JB
Bypass Esophagus to Ileum with Synthetic Substitute, Via Natural or Artificial
Opening Endoscopic
0D158K4
Bypass Esophagus to Cutaneous with Nonautologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D158K6
Bypass Esophagus to Stomach with Nonautologous Tissue Substitute, Via Natural
or Artificial Opening Endoscopic
0D158K9
Bypass Esophagus to Duodenum with Nonautologous Tissue Substitute, Via
Natural or Artificial Opening Endoscopic
0D158KA
Bypass Esophagus to Jejunum with Nonautologous Tissue Substitute, Via Natural
or Artificial Opening Endoscopic
0D158KB
Bypass Esophagus to Ileum with Nonautologous Tissue Substitute, Via Natural or
Artificial Opening Endoscopic
0D158Z4 Bypass Esophagus to Cutaneous, Via Natural or Artificial Opening Endoscopic
0D158Z6
Bypass Esophagus to Stomach, Via Natural or Artificial Opening Endoscopic
0D158Z9 Bypass Esophagus to Duodenum, Via Natural or Artificial Opening Endoscopic
0D158ZA
Bypass Esophagus to Jejunum, Via Natural or Artificial Opening Endoscopic
0D158ZB Bypass Esophagus to Ileum, Via Natural or Artificial Opening Endoscopic
0DB10ZZ
Excision of Upper Esophagus, Open Approach
0DB13ZZ Excision of Upper Esophagus, Percutaneous Approach
0DB14ZZ
Excision of Upper Esophagus, Percutaneous Endoscopic Approach
0DB17ZZ Excision of Upper Esophagus, Via Natural or Artificial Opening
0DB18ZZ
Excision of Upper Esophagus, Via Natural or Artificial Opening Endoscopic
0DB20ZZ Excision of Middle Esophagus, Open Approach
0DB23ZZ
Excision of Middle Esophagus, Percutaneous Approach
0DB24ZZ Excision of Middle Esophagus, Percutaneous Endoscopic Approach
0DB27ZZ
Excision of Middle Esophagus, Via Natural or Artificial Opening
0DB28ZZ Excision of Middle Esophagus, Via Natural or Artificial Opening Endoscopic
0DB30ZZ
Excision of Lower Esophagus, Open Approach
0DB33ZZ Excision of Lower Esophagus, Percutaneous Approach
0DB34ZZ
Excision of Lower Esophagus, Percutaneous Endoscopic Approach
0DB37ZZ Excision of Lower Esophagus, Via Natural or Artificial Opening
0DB38ZZ
Excision of Lower Esophagus, Via Natural or Artificial Opening Endoscopic
0DB50ZZ Excision of Esophagus, Open Approach
0DB53ZZ
Excision of Esophagus, Percutaneous Approach
0DB54ZZ Excision of Esophagus, Percutaneous Endoscopic Approach
0DB57ZZ
Excision of Esophagus, Via Natural or Artificial Opening
0DB58ZZ Excision of Esophagus, Via Natural or Artificial Opening Endoscopic
0DT10ZZ
Resection of Upper Esophagus, Open Approach
0DT14ZZ Resection of Upper Esophagus, Percutaneous Endoscopic Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
122 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
0DT17ZZ
Resection of Upper Esophagus, Via Natural or Artificial Opening
0DT18ZZ Resection of Upper Esophagus, Via Natural or Artificial Opening Endoscopic
0DT20ZZ
Resection of Middle Esophagus, Open Approach
0DT24ZZ Resection of Middle Esophagus, Percutaneous Endoscopic Approach
0DT27ZZ
Resection of Middle Esophagus, Via Natural or Artificial Opening
0DT28ZZ Resection of Middle Esophagus, Via Natural or Artificial Opening Endoscopic
0DT30ZZ
Resection of Lower Esophagus, Open Approach
0DT34ZZ Resection of Lower Esophagus, Percutaneous Endoscopic Approach
0DT37ZZ
Resection of Lower Esophagus, Via Natural or Artificial Opening
0DT38ZZ Resection of Lower Esophagus, Via Natural or Artificial Opening Endoscopic
0DT44ZZ
Resection of Esophagogastric Junction, Percutaneous Endoscopic Approach
0DT50ZZ Resection of Esophagus, Open Approach
0DT54ZZ
Resection of Esophagus, Percutaneous Endoscopic Approach
0DT57ZZ Resection of Esophagus, Via Natural or Artificial Opening
0DT58ZZ
Resection of Esophagus, Via Natural or Artificial Opening Endoscopic
0DT60ZZ Resection of Stomach, Open Approach
0DT64ZZ
Resection of Stomach, Percutaneous Endoscopic Approach
0DT67ZZ Resection of Stomach, Via Natural or Artificial Opening
0DT68ZZ
Resection of Stomach, Via Natural or Artificial Opening Endoscopic
0DX60Z5 Transfer Stomach to Esophagus, Open Approach
0DX64Z5
Transfer Stomach to Esophagus, Percutaneous Endoscopic Approach
0DX80Z5 Transfer Small Intestine to Esophagus, Open Approach
0DX84Z5
Transfer Small Intestine to Esophagus, Percutaneous Endoscopic Approach
0DXE0Z5 Transfer Large Intestine to Esophagus, Open Approach
0DXE4Z5
Transfer Large Intestine to Esophagus, Percutaneous End
ICD-10 Malignant Tumor and Carcinoma in Situ Diagnosis Codes
ICD-10 Diagnosis
Code
Code Description
C15.3
Malignant neoplasm of upper third of esophagus
C15.4 Malignant neoplasm of middle third of esophagus
C15.5
Malignant neoplasm of lower third of esophagus
C15.8 Malignant neoplasm of overlapping sites of esophagus
C15.9
Malignant neoplasm of esophagus, unspecified
C16.0 Malignancy of the cardio-esophageal junction
D00.1
Carcinoma in situ of esophagus

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
123 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Pancreatic Resection for Cancer Measure Specifications
For pancreatic resection for cancer, there are two sets of ICD-10 codes for counting patient discharges.
The first set of codes is to identify patients who have had the procedure. The second set of codes is to
identify patients with a specific diagnosis.
Count the number of patients, ages 18 years and older, discharged with the following ICD-10 codes in
any procedure field AND any of the following ICD-10 codes in any diagnosis field.
ICD-10 Pancreatic Resection for Cancer Procedure Codes
ICD-10
Procedure Code
Code Description
0DB90ZZ Excision of Duodenum, Open Approach
0DB93ZZ
Excision of Duodenum, Percutaneous Approach
0DB94ZZ Excision of Duodenum, Percutaneous Endoscopic Approach
0DB97ZZ
Excision of Duodenum, Via Natural or Artificial Opening
0DB98ZZ Excision of Duodenum, Via Natural or Artificial Opening Endoscopic
0DT90ZZ
Resection of Duodenum, Open Approach
0DT94ZZ Resection of Duodenum, Percutaneous Endoscopic Approach
0DT97ZZ
Resection of Duodenum, Via Natural or Artificial Opening
0DT98ZZ Resection of Duodenum, Via Natural or Artificial Opening Endoscopic
0FBG0ZZ
Excision of Pancreas, Open Approach
0FBG3ZZ Excision of Pancreas, Percutaneous Approach
0FBG4ZZ
Excision of Pancreas, Percutaneous Endoscopic Approach
0FBG8ZZ Excision of Pancreas, Via Natural or Artificial Opening Endoscopic
0FTG0ZZ
Resection of Pancreas, Open Approach
0FTG4ZZ Resection of Pancreas, Percutaneous Endoscopic Approach
ICD-10 Malignant Tumor Diagnosis Codes
ICD-10 Diagnosis
Code
Code Description
C7A.1
Malignant poorly differentiated neuroendocrine tumors
C7A.8 Other malignant neuroendocrine tumors
C17.0
Malignant neoplasm of duodenum
C24.0 Malignant neoplasm of extrahepatic bile duct
C24.1
Malignant neoplasm of ampulla of Vater
C24.8 Malignant neoplasm of overlapping sites of biliary tract
C24.9
Malignant neoplasm of biliary tract, unspecified
C25.0 Malignant neoplasm of head of pancreas
C25.1
Malignant neoplasm of body of pancreas
C25.2 Malignant neoplasm of tail of pancreas
C25.3
Malignant neoplasm of pancreatic duct
C25.4 Malignant neoplasm of endocrine pancreas
C25.7
Malignant neoplasm of other parts of pancreas
C25.8 Malignant neoplasm of overlapping sites of pancreas
C25.9
Malignant neoplasm of pancreas, unspecified

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
124 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Rectal Cancer Surgery Measure Specifications
For rectal cancer surgery, there are two sets of ICD-10 codes for counting patient discharges. The first set
of codes is to identify patients who have had the procedure. The second set of codes is to identify
patients with a specific diagnosis.
Count the number of patients, ages 18 years and older, discharged with the following ICD-10 codes in
any procedure field AND any of the following ICD-10 codes in any diagnosis field.
ICD-10 Rectal Cancer Surgery Procedure Codes
ICD-10
Procedure Code
Code Description
0DBP0ZZ Excision of Rectum, Open Approach
0DBP3ZZ
Excision of Rectum, Percutaneous Approach
0DBP4ZZ Excision of Rectum, Percutaneous Endoscopic Approach
0DBP7ZZ
Excision of Rectum, Via Natural or Artificial Opening
0DBP8ZZ Excision of rectum, via natural or artificial opening endoscopic
0DTP0ZZ
Resection of Rectum, Open Approach
0DTP4ZZ Resection of Rectum, Percutaneous Endoscopic Approach
0DTP7ZZ
Resection of Rectum, Via Natural or Artificial Opening
0DTP8ZZ Resection of Rectum, Via Natural or Artificial Opening Endoscopic
ICD-10 Malignant Tumor and Carcinoma in Situ Diagnosis Codes
ICD-10 Diagnosis
Code
Code Description
C19 Malignant neoplasm of rectosigmoid junction
C20
Malignant neoplasm of rectum
C21.0 Malignant neoplasm of anus, unspecified
C21.1
Malignant neoplasm of anal canal
C21.2 Malignant neoplasm of cloacogenic zone
C21.8
Malignant neoplasm of overlapping sites of rectum, anus and anal canal
C78.5 Secondary malignant neoplasm of large intestine and rectum
D01.1
Carcinoma in situ of rectosigmoid junction
D01.2 Carcinoma in situ of rectum
D01.3
Carcinoma in situ of anus and anal canal

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
125 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Bariatric Surgery for Weight Loss Measure Specifications
For bariatric surgery for weight loss, there are three sets of codes. One set of ICD-10 codes for counting
inpatient discharges and one set of CPT codes for counting outpatient discharges. The first two sets of
codes are to identify patients who have had the procedure. The third set of codes (ICD-10 diagnosis
codes) is to identify patients with a specific diagnosis.
To determine the number of patients, ages 18 years and older, discharged for this procedure, hospitals
have two options:
1. Count the number of patients, ages 18 years and older, discharged with the following ICD-10
codes or the CPT Codes in any procedure field AND any of the following ICD-10 codes in the
primary diagnosis field. This method does not require chart review.
2. Count the number of patients, ages 18 years and older, discharged with the following ICD-10
codes or the CPT Codes in any procedure field AND any of the following ICD-10 codes in any
diagnosis field. In addition, the procedure must have been done explicitly for weight loss
purposes (i.e., presence of one of the diagnosis codes is necessary, but not sufficient for
inclusion). This method requires chart review to ensure the procedure was performed explicitly for
weight loss purposes.
ICD-10 Bariatric Surgery Procedure Codes
ICD-10
Procedure Code
Code Description
0D16079 Bypass Stomach to Duodenum with Autologous Tissue Substitute, Open Approach
0D1607A
Bypass Stomach to Jejunum with Autologous Tissue Substitute, Open Approach
0D1607B Bypass Stomach to Ileum with Autologous Tissue Substitute, Open Approach
0D160Z9
Bypass Stomach to Duodenum, Open Approach
0D160ZA Bypass Stomach to Jejunum, Open Approach
0D160ZB
Bypass Stomach to Ileum, Open Approach
0D16479
Bypass Stomach to Duodenum with Autologous Tissue Substitute, Percutaneous
Endoscopic Approach
0D1647A
Bypass Stomach to Jejunum with Autologous Tissue Substitute, Percutaneous
Endoscopic Approach
0D1647B
Bypass Stomach to Ileum with Autologous Tissue Substitute, Percutaneous
Endoscopic Approach
0D164Z9
Bypass Stomach to Duodenum, Percutaneous Endoscopic Approach
0D164ZA Bypass Stomach to Jejunum, Percutaneous Endoscopic Approach
0D164ZB
Bypass Stomach to Ileum, Percutaneous Endoscopic Approach
0DB60Z3 Excision of Stomach, Open Approach, Vertical
0DB60ZZ
Excision of Stomach, Open Approach
0DB63Z3 Excision of Stomach, Percutaneous Approach, Vertical
0DB63ZZ
Excision of Stomach, Percutaneous Approach
0DB64Z3 Excision of Stomach, Percutaneous Endoscopic Approach, Vertical
0DB64ZZ
Excision of Stomach, Percutaneous Endoscopic Approach
ICD-10 Morbid Obesity Diagnosis Codes
ICD-10 Diagnosis
Code
Code Description
E66.01
Morbid (severe) obesity due to excess calories
E66.09 Other obesity due to excess calories

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
126 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10 Diagnosis
Code
Code Description
E66.8
Other obesity
Z68.35 Body mass index (BMI) 35.0-35.9, adult
Z68.36
Body mass index (BMI) 36.0-36.9, adult
Z68.37 Body mass index (BMI) 37.0-37.9, adult
Z68.38
Body mass index (BMI) 38.0-38.9, adult
Z68.39 Body mass index (BMI) 39.0-39.9, adult
Z68.41
Body mass index (BMI) 40.0-44.9, adult
Z68.42 Body mass index (BMI) 45.0-49.9, adult
Z68.43
Body mass index (BMI) 50-59.9, adult
Z68.44 Body mass index (BMI) 60.0-69.9, adult
Z68.45
Body mass index (BMI) 70 or greater, adult
CPT Bariatric Surgery for Weight Loss Procedure Codes
Leapfrog has provided a set of CPT codes for counting patients discharged from your hospital who have
undergone the procedure during the reporting period on an outpatient basis.
CPT Codes are provided in downloadable Excel files on the Survey Dashboard. To access the files, click
the CPT Code Workbook button next to Section 9. You will be required to complete the American Medical
Association’s Terms of Use before downloading the Excel file and using the individual CPT codes to
query your EHR or billing system. You are only required to complete the Terms of Use once per Survey
Cycle (April 1 – November 30).
The CPT Code Workbooks (Excel files) are labeled for Section 3A: Total Hip Replacement, Total Knee
Replacement, and Bariatric Surgery for Weight Loss and Section 9C: Volume of Procedures. Please note,
if you are part of a hospital system, each hospital will need to complete the Terms of Use. This is a
requirement of the American Medical Association.
Using the CPT codes found for Bariatric Surgery for Weight Loss in the “Instructions” sheet, count the
total number of adult (18 years of age or older) patients discharged for the procedures with the CPT
codes listed AND any of the above ICD-10 diagnosis codes in the primary diagnosis field. The CPT
codes can be in any procedure field.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
127 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Total Knee Replacement Measure Specifications
For total knee replacement, there are two sets of codes. One set of ICD-10 codes for counting inpatient
discharges and one CPT code for counting outpatient discharges. The two sets of codes are to identify
patients who have had the procedure.
To determine the number of patients, ages 18 years and older, discharged for this procedure, hospitals
have two options:
• Count the number of patients, ages 18 years and older, discharged with any of the following ICD-
10 codes in the primary procedure field or the CPT Code in any procedure field. This method
does not require chart review. Hospitals should report on total knee replacement procedures
performed in the outpatient locations included in Section 9: Outpatient Procedures.
• Count the number of patients, ages 18 years and older, discharged with any of the following ICD-
10 codes or CPT Code in any procedure field. For inpatient cases using ICD-10 codes,
exclude any patients where the procedure was done as a revision (i.e., the presence of one
of the procedure codes is necessary, but not sufficient for inclusion). This method requires chart
review of inpatient cases to ensure that revisions are not included. Chart review is not required for
outpatient cases using the specified CPT Code. Hospitals should report on total knee
replacement procedures performed in the outpatient locations included in Section 9: Outpatient
Procedures.
ICD-10 Total Knee Replacement Procedure Codes
ICD10
Procedure
Code
Code Description
0SRC0J9
Replacement of Right Knee Joint with Synthetic Substitute, Cemented, Open
Approach
0SRC0JA
Replacement of Right Knee Joint with Synthetic Substitute, Uncemented, Open
Approach
0SRC0JZ Replacement of Right Knee Joint with Synthetic Substitute, Open Approach
0SRD0J9
Replacement of Left Knee Joint with Synthetic Substitute, Cemented, Open
Approach
0SRD0JA
Replacement of Left Knee Joint with Synthetic Substitute, Uncemented, Open
Approach
0SRD0JZ
Replacement of Left Knee Joint with Synthetic Substitute, Open Approach
0SRC069
Replacement of Right Knee Joint with Oxidized Zirconium on Polyethylene
Synthetic Substitute, Cemented, Open Approach
0SRC06A
Replacement of Right Knee Joint with Oxidized Zirconium on Polyethylene
Synthetic Substitute, Uncemented, Open Approach
0SRC06Z
Replacement of Right Knee Joint with Oxidized Zirconium on Polyethylene
Synthetic Substitute, Open Approach
0SRD069
Replacement of Left Knee Joint with Oxidized Zirconium on Polyethylene Synthetic
Substitute, Cemented, Open Approach
0SRD06A
Replacement of Left Knee Joint with Oxidized Zirconium on Polyethylene Synthetic
Substitute, Uncemented, Open Approach
0SRD06Z
Replacement of Left Knee Joint with Oxidized Zirconium on Polyethylene Synthetic
Substitute, Open Approach
CPT Total Knee Replacement Procedure Code
Leapfrog has provided a CPT code for counting patients discharged from your hospital who have
undergone the procedure during the reporting period on an outpatient basis.
CPT Codes are provided in downloadable Excel files on the Survey Dashboard. To access the files, click
the CPT Code Workbook button next to Section 9. You will be required to complete the American Medical

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
128 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Association’s Terms of Use before downloading the Excel file and using the individual CPT codes to
query your EHR or billing system. You are only required to complete the Terms of Use once per Survey
Cycle (April 1 – November 30).
The CPT Code Workbooks (Excel files) are labeled for Section 3A: Total Hip Replacement, Total Knee
Replacement, and Bariatric Surgery for Weight Loss and Section 9C: Volume of Procedures. Please note,
if you are part of a hospital system, each hospital will need to complete the Terms of Use. This is a
requirement of the American Medical Association.
Using the CPT code found for Total Knee Replacement in the “Instructions” sheet, count the total number
of adult (18 years of age or older) patients discharged for the procedure with the CPT code listed. The
CPT code can be in any procedure field.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
129 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Total Hip Replacement Measure Specifications
For total hip replacement, there are two sets of codes. One set of ICD-10 codes for counting inpatient
discharges and one CPT code for counting outpatient discharges. The two sets of codes are to identify
patients who have had the procedure.
To determine the number of patients, ages 18 years and older, discharged for this procedure, hospitals
have two options:
• Count the number of patients, ages 18 years and older, discharged with any of the following ICD-
10 codes in the primary procedure field or the CPT code in any procedure field. This method
does not require chart review. Hospitals should report on total hip replacement procedures
performed in the outpatient locations included in Section 9: Outpatient Procedures.
• Count the number of patients, ages 18 years and older, discharged with any of the following ICD-
10 codes or the CPT code in any procedure field. For inpatient cases using ICD-10 codes,
exclude any patients where the procedure was done as a revision (i.e., the presence of one
of the procedure codes is necessary, but not sufficient for inclusion). This method requires chart
review of inpatient cases to ensure that revisions are not included. Chart review is not required for
outpatient cases using the specified CPT Code. Hospitals should report on total hip replacement
procedures performed in the outpatient locations included in Section 9: Outpatient Procedures.
ICD-10 Total Hip Replacement Procedure Codes
ICD10 Procedure
Code
Code Description
0SR9019
Replacement of Right Hip Joint with Metal Synthetic Substitute, Cemented, Open
Approach
0SR901A
Replacement of Right Hip Joint with Metal Synthetic Substitute, Uncemented,
Open Approach
0SR901Z Replacement of Right Hip Joint with Metal Synthetic Substitute, Open Approach
0SR9029
Replacement of Right Hip Joint with Metal on Polyethylene Synthetic Substitute,
Cemented, Open Approach
0SR902A
Replacement of Right Hip Joint with Metal on Polyethylene Synthetic Substitute,
Uncemented, Open Approach
0SR902Z
Replacement of Right Hip Joint with Metal on Polyethylene Synthetic Substitute,
Open Approach
0SR9039
Replacement of Right Hip Joint with Ceramic Synthetic Substitute, Cemented,
Open Approach
0SR903A
Replacement of Right Hip Joint with Ceramic Synthetic Substitute, Uncemented,
Open Approach
0SR903Z Replacement of Right Hip Joint with Ceramic Synthetic Substitute, Open Approach
0SR9049
Replacement of Right Hip Joint with Ceramic on Polyethylene Synthetic Substitute,
Cemented, Open Approach
0SR904A
Replacement of Right Hip Joint with Ceramic on Polyethylene Synthetic Substitute,
Uncemented, Open Approach
0SR904Z
Replacement of Right Hip Joint with Ceramic on Polyethylene Synthetic Substitute,
Open Approach
0SR90J9
Replacement of Right Hip Joint with Synthetic Substitute, Cemented, Open
Approach
0SR90JA
Replacement of Right Hip Joint with Synthetic Substitute, Uncemented, Open
Approach
0SR90JZ Replacement of Right Hip Joint with Synthetic Substitute, Open Approach
0SRA009
Replacement of Right Hip Joint, Acetabular Surface with Polyethylene Synthetic
Substitute, Cemented, Open Approach
0SRA00A
Replacement of Right Hip Joint, Acetabular Surface with Polyethylene Synthetic
Substitute, Uncemented, Open Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
130 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
0SRA00Z
Replacement of Right Hip Joint, Acetabular Surface with Polyethylene Synthetic
Substitute, Open Approach
0SRA019
Replacement of Right Hip Joint, Acetabular Surface with Metal Synthetic
Substitute, Cemented, Open Approach
0SRA01A
Replacement of Right Hip Joint, Acetabular Surface with Metal Synthetic
Substitute, Uncemented, Open Approach
0SRA01Z
Replacement of Right Hip Joint, Acetabular Surface with Metal Synthetic
Substitute, Open Approach
0SRA039
Replacement of Right Hip Joint, Acetabular Surface with Ceramic Synthetic
Substitute, Cemented, Open Approach
0SRA03A
Replacement of Right Hip Joint, Acetabular Surface with Ceramic Synthetic
Substitute, Uncemented, Open Approach
0SRA03Z
Replacement of Right Hip Joint, Acetabular Surface with Ceramic Synthetic
Substitute, Open Approach
0SRA0J9
Replacement of Right Hip Joint, Acetabular Surface with Synthetic Substitute,
Cemented, Open Approach
0SRA0JA
Replacement of Right Hip Joint, Acetabular Surface with Synthetic Substitute,
Uncemented, Open Approach
0SRA0JZ
Replacement of Right Hip Joint, Acetabular Surface with Synthetic Substitute,
Open Approach
0SRB019
Replacement of Left Hip Joint with Metal Synthetic Substitute, Cemented, Open
Approach
0SRB01A
Replacement of Left Hip Joint with Metal Synthetic Substitute, Uncemented, Open
Approach
0SRB01Z Replacement of Left Hip Joint with Metal Synthetic Substitute, Open Approach
0SRB029
Replacement of Left Hip Joint with Metal on Polyethylene Synthetic Substitute,
Cemented, Open Approach
0SRB02A
Replacement of Left Hip Joint with Metal on Polyethylene Synthetic Substitute,
Uncemented, Open Approach
0SRB02Z
Replacement of Left Hip Joint with Metal on Polyethylene Synthetic Substitute,
Open Approach
0SRB039
Replacement of Left Hip Joint with Ceramic Synthetic Substitute, Cemented, Open
Approach
0SRB03A
Replacement of Left Hip Joint with Ceramic Synthetic Substitute, Uncemented,
Open Approach
0SRB03Z Replacement of Left Hip Joint with Ceramic Synthetic Substitute, Open Approach
0SRB049
Replacement of Left Hip Joint with Ceramic on Polyethylene Synthetic Substitute,
Cemented, Open Approach
0SRB04A
Replacement of Left Hip Joint with Ceramic on Polyethylene Synthetic Substitute,
Uncemented, Open Approach
0SRB04Z
Replacement of Left Hip Joint with Ceramic on Polyethylene Synthetic Substitute,
Open Approach
0SRB0J9
Replacement of Left Hip Joint with Synthetic Substitute, Cemented, Open
Approach
0SRB0JA
Replacement of Left Hip Joint with Synthetic Substitute, Uncemented, Open
Approach
0SRB0JZ Replacement of Left Hip Joint with Synthetic Substitute, Open Approach
0SRE009
Replacement of Left Hip Joint, Acetabular Surface with Polyethylene Synthetic
Substitute, Cemented, Open Approach
0SRE00A
Replacement of Left Hip Joint, Acetabular Surface with Polyethylene Synthetic
Substitute, Uncemented, Open Approach
0SRE00Z
Replacement of Left Hip Joint, Acetabular Surface with Polyethylene Synthetic
Substitute, Open Approach
0SRE019
Replacement of Left Hip Joint, Acetabular Surface with Metal Synthetic Substitute,
Cemented, Open Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
131 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
0SRE01A
Replacement of Left Hip Joint, Acetabular Surface with Metal Synthetic Substitute,
Uncemented, Open Approach
0SRE01Z
Replacement of Left Hip Joint, Acetabular Surface with Metal Synthetic Substitute,
Open Approach
0SRE039
Replacement of Left Hip Joint, Acetabular Surface with Ceramic Synthetic
Substitute, Cemented, Open Approach
0SRE03A
Replacement of Left Hip Joint, Acetabular Surface with Ceramic Synthetic
Substitute, Uncemented, Open Approach
0SRE03Z
Replacement of Left Hip Joint, Acetabular Surface with Ceramic Synthetic
Substitute, Open Approach
0SRE0J9
Replacement of Left Hip Joint, Acetabular Surface with Synthetic Substitute,
Cemented, Open Approach
0SRE0JA
Replacement of Left Hip Joint, Acetabular Surface with Synthetic Substitute,
Uncemented, Open Approach
0SRE0JZ
Replacement of Left Hip Joint, Acetabular Surface with Synthetic Substitute, Open
Approach
0SR9069
Replacement of Right Hip Joint with Oxidized Zirconium on Polyethylene Synthetic
Substitute, Cemented, Open Approach
0SR906A
Replacement of Right Hip Joint with Oxidized Zirconium on Polyethylene Synthetic
Substitute, Uncemented, Open Approach
0SR906Z
Replacement of Right Hip Joint with Oxidized Zirconium on Polyethylene Synthetic
Substitute, Open Approach
0SRB069
Replacement of Left Hip Joint with Oxidized Zirconium on Polyethylene Synthetic
Substitute, Cemented, Open Approach
0SRB06A
Replacement of Left Hip Joint with Oxidized Zirconium on Polyethylene Synthetic
Substitute, Uncemented, Open Approach
0SRB06Z
Replacement of Left Hip Joint with Oxidized Zirconium on Polyethylene Synthetic
Substitute, Open Approach
CPT Total Hip Replacement Procedure Code
Leapfrog has provided a CPT code for counting patients discharged from your hospital who have
undergone the procedure during the reporting period on an outpatient basis.
CPT Codes are provided in downloadable Excel files on the Survey Dashboard. To access the files, click
the CPT Code Workbook button next to Section 9. You will be required to complete the American Medical
Association’s Terms of Use before downloading the Excel file and using the individual CPT codes to
query your EHR or billing system. You are only required to complete the Terms of Use once per Survey
Cycle (April 1 – November 30).
The CPT Code Workbooks (Excel files) are labeled for Section 3A: Total Hip Replacement, Total Knee
Replacement, and Bariatric Surgery for Weight Loss and Section 9C: Volume of Procedures. Please note,
if you are part of a hospital system, each hospital will need to complete the Terms of Use. This is a
requirement of the American Medical Association.
Using the CPT code found for Total Hip Replacement in the “Instructions” sheet, count the total number of
adult (18 years of age or older) patients discharged for the procedure with the CPT code listed. The CPT
code can be in any procedure field.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
132 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Norwood Procedure Measure Specifications
For Norwood procedure, there are two sets of ICD-10 procedure codes for counting patient discharges.
For a patient to be counted, they must have at least one ICD-10 procedure code from each list.
The first set of codes is to identify patients who have had an arch repair. The second set of codes is to
identify patients who have had a shunt.
Count the number of patients, ages 17 years and younger, discharged with at least one of the following
ICD-10 procedure codes from each list in any procedure field (i.e., each patient discharge will need to
have at least one ICD-10 procedure code from the arch repair set AND at least one ICD-10 procedure
code from the shunt set). Both procedures would need to be done as part of the same operative session.
ICD-10 Arch Repair Procedure Codes
ICD-10
Procedure Code
Code Description
02UX07Z
Supplement Thoracic Aorta, Ascending/Arch with Autologous Tissue Substitute,
Open Approach
02UX0JZ
Supplement Thoracic Aorta, Ascending/Arch with Synthetic Substitute, Open
Approach
02UX0KZ
Supplement Thoracic Aorta, Ascending/Arch with Nonautologous Tissue
Substitute, Open Approach
ICD-10 Shunt Procedure Codes
ICD-10
Procedure Code
Code Description
021K08P
Bypass Right Ventricle to Pulmonary Trunk with Zooplastic Tissue Substitute,
Open Approach
021K08Q
Bypass Right Ventricle to Right Pulmonary Artery with Zooplastic Tissue
Substitute, Open Approach
021K08R
Bypass Right Ventricle to Left Pulmonary Artery with Zooplastic Tissue Substitute,
Open Approach
021K09P
Bypass Right Ventricle to Pulmonary Trunk with Autologous Venous Tissue, Open
Approach
021K09Q
Bypass Right Ventricle to Right Pulmonary Artery with Autologous Venous Tissue,
Open Approach
021K09R
Bypass Right Ventricle to Right Pulmonary Artery with Autologous Venous Tissue,
Open Approach
021K0AP
Bypass Right Ventricle to Pulmonary Trunk with Autologous Arterial Tissue, Open
Approach
021K0AQ
Bypass Right Ventricle to Right Pulmonary Artery with Autologous Arterial Tissue,
Open Approach
021K0AR
Bypass Right Ventricle to Right Pulmonary Artery with Autologous Arterial Tissue,
Open Approach
021K0JP
Bypass Right Ventricle to Pulmonary Trunk with Synthetic Substitute, Open
Approach
021K0JQ
Bypass Right Ventricle to Right Pulmonary Artery with Synthetic Substitute, Open
Approach
021K0JR
Bypass Right Ventricle to Left Pulmonary Artery with Synthetic Substitute, Open
Approach
021K0KP
Bypass Right Ventricle to Pulmonary Trunk with Nonautologous Tissue Substitute,
Open Approach
021K0KQ
Bypass Right Ventricle to Right Pulmonary Artery with Nonautologous Tissue
Substitute, Open Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
133 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICD-10
Procedure Code
Code Description
021K0KR
Bypass Right Ventricle to Right Pulmonary Artery with Nonautologous Tissue
Substitute, Open Approach
021Q08A
Bypass Right Pulmonary Artery from Innominate Artery with Zooplastic Tissue,
Open Approach
021Q08B
Bypass Right Pulmonary Artery from Subclavian with Zooplastic Tissue, Open
Approach
021Q08D
Bypass Right Pulmonary Artery from Carotid with Zooplastic Tissue, Open
Approach
021Q09A
Bypass Right Pulmonary Artery from Innominate Artery with Autologous Venous
Tissue, Open Approach
021Q09B
Bypass Right Pulmonary Artery from Subclavian with Autologous Venous Tissue,
Open Approach
021Q09D
Bypass Right Pulmonary Artery from Carotid with Autologous Venous Tissue,
Open Approach
021Q0AA
Bypass Right Pulmonary Artery from Innominate Artery with Autologous Arterial
Tissue, Open Approach
021Q0AB
Bypass Right Pulmonary Artery from Subclavian with Autologous Arterial Tissue,
Open Approach
021Q0AD
Bypass Right Pulmonary Artery from Carotid with Autologous Arterial Tissue, Open
Approach
021Q0JA
Bypass Right Pulmonary Artery from Innominate with Synthetic Substitute, Open
Approach
021Q0JB
Bypass Right Pulmonary Artery from Subclavian with Synthetic Substitute, Open
Approach
021Q0JD
Bypass Right Pulmonary Artery from Carotid with Synthetic Substitute, Open
Approach
021Q0KA
Bypass Right Pulmonary Artery from Innominate Artery with Nonautologous Tissue
Substitute, Open Approach
021Q0KB
Bypass Right Pulmonary Artery from Subclavian with Nonautologous Tissue
Substitute, Open Approach
021Q0KD
Bypass Right Pulmonary Artery from Carotid with Nonautologous Tissue
Substitute, Open Approach
021V0ZP
Bypass Superior Vena Cava to Pulmonary Trunk, Open Approach
021V0ZQ Bypass Superior Vena Cava to Right Pulmonary Artery, Open Approach
021V0ZR
Bypass Superior Vena Cava to Left Pulmonary Artery, Open Approach

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
134 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Safe Surgery Checklist for Adult and Pediatric Complex Surgery Measure
Specifications
Important Note: Hospitals that share a CMS Certification Number are required to report by facility.
Please carefully review Leapfrog’s Multi-Campus Reporting Policy
.
Source
: The Leapfrog Group, WHO Surgical Safety Checklist, AHRQ Endoscopy Checklist
Reporting Period
: Latest 6-month period prior to submission of this section of the Survey
Safe Surgery Checklist Workbook (Excel)
To complete the data collection and respond to questions #6-8, hospitals should download the Safe
Surgery Checklist Workbook (Excel). This workbook includes five tabs: Instructions, Section 3C
(Complex Surgery), Section 3C – Data Entry, Section 9D (Outpatient Procedures), Section 9D – Data
Entry. The Section 3C tabs can be used to complete the safe surgery checklist audits for this
subsection and to calculate the response to select in the Online Survey Tool for question #8.
The workbook is available on the Survey Materials Webpage
.
Sampling:
Hospitals that perform procedures in both Section 3A and Section 9C, and submit both Section 3 and
9, can randomly sample 15 patients (who had one of the procedures included in Section 3A) and
measure and report adherence to the safe surgery checklist based on that sample.
Hospitals that ONLY perform the procedures in Section 3A, or that ONLY submit Section 3, must
randomly sample 30 patients (who had one of the procedures included in Section 3A) and measure
and report adherence to the safe surgery checklist based on that sample.
When sampling from a larger population of cases, hospitals that perform multiple procedures
(e.g., bariatric surgery for weight loss, total hip replacement, total knee replacement, etc.) included in
Section 3A and hospitals that perform procedures for both adult and pediatric patients (e.g., the
Norwood Procedures) should obtain a representative sample (i.e., include patients who underwent
different procedures and include both adult and pediatric patients, if applicable).
Free-standing pediatric hospitals that perform the Norwood Procedure and hospitals that reported a
combined total hospital volume of less than the sufficient sample for all the procedures in Section 3A
can sample any patients that had a procedure performed under general anesthesia.
Question #6: Did your hospital perform an audit (either in-person or via the medical record or
other EHR data) on a sufficient sample of patients who underwent a procedure included in
Section 3A and measure adherence to the safe surgery checklist?
To respond “yes” to question #6, hospitals must measure and document whether all the elements in
questions #3, #4, and #5 were verbalized in the presence of the appropriate personnel for each
sampled case. Hospitals that completed the audit should respond “yes” to this question regardless of
whether the adherence to the checklist was 100%. Hospitals will report on adherence to the checklist in
question #8.
Question #7: What method was used to perform the audit on a sufficient sample in question
#6?
Hospitals can only perform a retrospective audit by reviewing medical records or EHR data if
adherence to the safe surgery checklist is clearly documented in the medical record or EHR and the
documentation clearly demonstrates that each element was verbalized in the presence of the
appropriate personnel. If hospitals cannot clearly determine whether (a) the checklist element was
verbalized and (b) that the appropriate personnel were present via a retrospective audit, an in-person
observational audit must be completed.
Hospitals can perform the in-person audits up to six months prior to and during the reporting period.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
135 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Question #8: Based on your hospital’s audit (either in-person or via the medical record or other
EHR data) on a sufficient sample of patients who underwent a procedure included in Section
3A, what was your hospitals documented rate of adherence to the safe surgery checklist (i.e.,
what percentage of the sampled cases had all elements in questions #3, #4, and #5 completed)?
Based on the audit completed for question #6, determine the total number of patient audits where all
elements of the safe surgery checklist (included in questions #3, #4, and #5) were read aloud in the
presence of the appropriate personnel 1) before the induction of anesthesia, 2) before the skin incision
and/or the procedure began, and 3) before the patient left the operating and/or procedure room.
Included cases:
• The patient audit shows that all elements of the safe surgery checklist, at all three time points,
were read aloud in the presence of the appropriate personnel.
Excluded cases:
• The patient audit does not demonstrate that all elements of the safe surgery checklist were
read aloud in the presence of the appropriate personnel (i.e., one or more elements of the
checklist were not read aloud or the appropriate personnel were not present during the
checklist).
• T
he patient audit does not demonstrate that the safe surgery checklist was read aloud in the
presence of the appropriate personnel at all three time points (i.e., before anesthesia, before
skin incision, and before the patient leaves).
Important Note: If a Safe Surgery Checklist element includes the qualifier “if applicable,” hospitals
should determine if that element of the checklist was applicable to the patient and procedure being
performed. If the element was determined to be “not applicable” to the patient and procedure, it does
not count against the total adherence. For example, if your hospital performed a bariatric surgery for
weight loss, the availability of a device on-site would not be applicable. Therefore, when completing the
Safe Surgery Checklist Workbook for this patient, indicate N/A for that element (device on-site) and
your hospital would be able to count that case as adherent.
See FAQs
for additional information about responding to the questions in this section.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
136 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Adult and Pediatric Complex Surgery Frequently Asked Questions
(FAQs)
General Questions
1. Does this section apply to critical access hospitals?
Yes. In general, critical access hospitals do not perform many of the procedures that are included
in this section, but if the critical access hospital does perform the procedure, the standards still
apply.
2. Should we select a procedure from the list in Section 3A question #2 if we only perform
that procedure on an outpatient basis?
Hospitals that only perform total hip replacements, total knee replacements, and/or bariatric
surgery for weight loss on an outpatient basis should select and report on those procedures in
Section 3A.
Otherwise, hospitals should not select and report on the remaining eight procedures if the
procedures are always scheduled with the intention of the patient being released on the same
day.
If some of the procedures are scheduled with the intention of the patient being admitted and
staying in the hospital overnight, the hospital should report on performing the procedure in
question #2 and then ONLY report on the total number of patients discharged following an
inpatient stay.
3. My hospital has a tumor center that only performs total hip replacements and total knee
replacements as they come up during a tumor removal surgery, should we select these
procedures from the list in Section 3A question #2?
If your hospital has a tumor center where orthopedic tumor surgeries are performed and the
O
NLY total hip replacement and/or total knee replacement procedures ever done at that hospital
are for the treatment of the tumor, you should not select and report on total hip replacements or
total knee replacements in Section 3A.
Hospital Volume
4. How should hospitals calculate volume using a 24-month annual average?
To report on a 24-month annual average, calculate the total volume over the past 24 months, and
then divide by 2 (i.e., the volume of year one plus the volume of year 2 divided by two equals the
24-month annual average).
5. Can we count patients who have had one of the procedures listed in Section 3A in
combination with another procedure not listed in Section 3A?
Yes, hospitals should count all patients that meet the criteria specified in the
Hospital and
Surgeon Volume Measure Specifications. For example, if a patient has a CABG and a mitral
valve repair done at the same time, the hospital should count the patient when calculating total
volume for mitral valve repair and replacement.
6. When counting patients, should we only include those patients who had the procedure
performed on a scheduled basis?
No. Once a hospital selects a procedure from the list in question #2, they should use the measure
specifications for each procedure to count all patients that meet the criteria. This includes patients
who had the procedure performed urgently.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
137 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
7. If a hospital elects to begin a new service line of procedures, how should the hospital
report its volume and surgeon volumes while establishing the new line?
Leapfrog gives hospitals an 18-month grace period before having to report on hospital and
surgeon volume for a new procedure. From the day that the hospital performs the procedure for
the first time, the hospital and its surgeons will have 18 months to reach the annual volume
standard. During this period, the hospital does not have to report its procedure volumes for the
hospital or surgeons. However, once the hospital reaches the end of the 18-month grace period,
it must report its hospital and surgeon procedure volume.
8. How should we deal with a temporary drop in volume due to losing a surgeon’s service?
To accommodate fluctuations in hospital volumes, hospitals have the option of reporting on their
average case volumes over a 24-month period.
9. Can we count minimally invasive and/or robotic Mitral Valve Repair and Replacement
procedures towards our hospital’s volume?
Yes, the ICD-10 codes Leapfrog has provided for this procedure will capture minimally invasive
and/or robotic MVRR procedures in the hospital’s count as these are the codes used for those
cases.
10. Our hospital has a free-standing outpatient surgery center that shares our license and/or
CMS Certification Number (CCN). Many of our same day total hip replacements, total knee
replacements, and bariatric surgery for weight loss procedures are performed there. Can
we include these procedures in our total hospital volume when responding to question #3
in Section 3A?
Yes, hospitals may count patient discharges with the appropriate CPT procedure code during the
reporting period from hospital outpatient departments that share your hospital’s license and/or
CMS Certification Number (CCN), including, but not limited to free-standing surgery centers and
hospital outpatient departments.
Surgeon Volume
11. Does the specific procedure and minimum annual surgeon volume standard listed need to
be included in our process for privileging surgeons?
Yes. Hospitals must ensure that the specific procedure (as defined using the ICD-10 and CPT
procedure codes) and minimum annual surgeon volume standard are included in your process for
privileging surgeons. Diagnosis codes can be ignored.
12. Does our privileging process for surgeons have to include the annual surgeon volume
standard for initial privileging only or ongoing/renewal of privileging as well?
Both. Leapfrog’s minimum annual surgeon volume standards should be fully integrated into your
hospital’s process for privileging surgeons, including both initial and ongoing privileging. There
are two exceptions:
a. Surgeons who have just finished training: see FAQ #14
below.
b. Surgeons who were not active for the entire reporting period: see FAQ #15 below.
13. When counting annual surgeon volume for the purposes of privileging, should we
consider procedures performed by the surgeon at other hospitals?
Yes. When determining whether a surgeon has met or has exceeded Leapfrog's minimum annual
surgeon volume standard, we expect that hospitals will consider total experience in the privileging
process – this would include procedures performed within the reporting period at different
facilities. It is our understanding that through the OPPE process
, hospitals have access to total
surgeon volume, including the number of procedures performed at other hospitals.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
138 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
14. For determining surgeon volume for the purposes of privileging, how should we count
procedures that involve surgeons who have just finished training and are building up their
experience?
Surgeons who have just finished their training should receive a 24-month grace period to build up
their experience. After that point, the surgeon’s volume should be tracked and included in
privileging decisions. The procedures performed by this surgeon during the reporting period
should still be counted towards the hospital’s volume total, as the broader staff still had the
experience with the procedure.
15. If a surgeon was not “active” during the entire reporting period (e.g., just hired, sabbatical,
illness, etc.), should they be included when responding to question #5?
If a surgeon was absent for an extended time during the reporting period, the procedures
performed by this surgeon during the reporting period should still be counted towards the
hospital’s procedure total (question #3). However, the surgeon would not need to be considered
when responding to question #5 regarding whether your hospital’s process for privileging includes
the surgeon having to meet Leapfrog’s minimum annual surgeon volume standards until they
have been active again for an entire reporting period (likely the next year).
16. When counting surgeon volume for the purposes of privileging, if a procedure is
completed by two surgeons (i.e., an assistant or co-surgeon) would they both be able to
count the case?
If a surgeon assists another surgeon or is a co-surgeon during a procedure, the procedure should
NOT count for both surgeons’ procedure totals. The case should be applied to a single surgeon.
17. When determining which surgeons should be required to meet Leapfrog’s minimum
annual surgeon volume standards for the process of privileging, should surgeons who
only ever perform a procedure in emergency circumstances and never perform the
procedure on a scheduled basis be included?
For the purposes of reporting on question #5 in Section 3A, hospitals can exclude surgeons who
will only ever perform a procedure in emergency circumstances if they never perform the
procedure on a scheduled basis.
However, for the purposes of reporting on question #3 in Section 3A, hospitals should count all
patients discharged with the relevant procedure or diagnosis for those procedures indicated in
question #2 in Section 3A. See FAQ #6.
Surgical Appropriateness
18. What documents can be used as supporting evidence of a hospital’s implementation of
surgical appropriateness?
Hospitals may use meeting minutes, centers of excellence criteria, retrospective reviews of
surgical cases, or any other materials that show that there are surgical appropriateness standards
of care.
19. How often do we need to perform “regular retrospective reviews” as described in question
#3 in Section 3B? How many should we perform?
Hospitals should perform regular retrospective reviews more than once per year. Some hospitals
may decide to perform the reviews quarterly or even monthly, depending on the size of the
service.
Hospitals should review a sample of at least 30 cases per year total across all procedures they
reported implementing appropriateness criteria for in Section 3B question #3. For example,
hospitals implementing appropriateness criteria for all five procedures in question #3 should
review 6 cases per procedure each year. Hospitals reporting a combined total of less than 30
cases across all procedures indicated in Section 3B question #3 should review all cases.

2023 Leapfrog Hospital Survey – Hard Copy Section 3: Adult and Pediatric Complex Surgery
139 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
20. What is a multidisciplinary tumor board?
A multidisciplinary tumor board is a board that may include radiologists, pathologists, surgeons,
medical oncologists, radiation oncologists, nurses, social workers, psychiatrists or psychologists,
palliative care staff, nutritional services, and physical/occupational therapy, that prospectively
reviews cancer cases to ensure surgical appropriateness.
21. Our hospital’s multidisciplinary tumor board reviews some, but not all cancer cases. Can
we check the box for each cancer procedure in Section 3B, question #6?
In 2023, hospitals can receive credit for any cancer procedure where at least some cases are
presented to their multidisciplinary tumor board for prospective review to ensure surgical
appropriateness.
Safe Surgery Checklist for Adult and Pediatric Complex Surgery
22. Can different elements of the “Before the induction of anesthesia” checklist (question #3)
be performed in different places?
Yes, hospitals may perform some elements of the pre-anesthesia checklist outside of the
operating room. Hospitals may read aloud the following elements in a pre-procedure (preop)
area: Patient ID, Confirmation of procedure, Patient consent, Site marked, if applicable, and
Allergies assessed.
Hospitals should not remove conversation prompts as it is harmful to the purpose of the checklist
and should continue debriefing and requiring participation of the surgical team members in key
moments.
23. Do the Safe Surgery Checklist components included in questions #3, #4, and #5 need to be
in one document that would align with the WHO example
or can they be in different
documents (i.e., pre-anesthesia notes, surgeon H&P, and pre-surgical checklists, etc.)?
It is possible to have separate checklists for each phase of the surgical procedure (i.e., before the
induction of anesthesia, before the skin incision and/or before the procedure begins, and before
the patient leaves the operating or procedure room). However, each individual component
included in questions #3, #4, and #5 should be listed on the checklist(s) and hospitals should
document whether the components were read aloud at the appropriate time with the appropriate
members of the surgical team present.
24. How is the “whole surgical team” defined?
“Whole surgical team” is comprised of the surgeons, anesthesia professionals, nurses,
technicians, and other operating room personnel involved in surgery. (Based off the
World
Alliance for Patient Safety Implementation Manual Surgical Safety Checklist (First Edition).

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
141 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
SECTION 4: MATERNITY CARE
This section includes questions and reference information for Section 4: Maternity Care. Please carefully
review the questions, endnotes, and reference information (e.g., measure specifications, notes, and
frequently asked questions) before you begin. Failure to review the reference information could result in
inaccurate responses.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
142 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 4: 2023 Maternity Care
Maternity Care Fact Sheet and Bibliography:
https://ratings.leapfroggroup.org/measure/hospital/2023/maternity-care
Hospitals that did not deliver newborns during the reporting period or did not have an open labor
and delivery unit during the entire reporting period should select “no” in question #2 and move on
to the Affirmation of Accuracy. Your hospital’s results will be displayed as “Does Not Apply” on
Leapfrog’s public reporting website.
Section 4 includes questions about elective delivery, cesarean birth, episiotomy, newborn bilirubin
screening, and DVT prophylaxis for women undergoing cesarean delivery. The section also includes
questions about high-risk deliveries, including volume and outcomes. In 2023, the subsection on
maternity care volume (4A) includes new questions about maternity care services that will be publicly
reported. The subsection on cesarean birth (4C) includes new optional fact-finding questions about
cesarean births stratified by racial and ethnic categories that will not be scored or publicly reported.
Each hospital achieving the standard for Elective Deliveries:
Meets or is better than the 5.0% target for performance on the nationally endorsed outcome measure.
Each hospital achieving the standard for Cesarean Birth:
Meets or is better than the 23.6% target for performance on the nationally endorsed outcome measure.
Each hospital achieving the standard for Episiotomy:
Meets or is better than the 5.0% target for performance on the nationally endorsed outcome measure.
Each hospital achieving the standard for Newborn Bilirubin Screening Prior to Discharge:
Meets or exceeds the 90% target for the process measure of care.
Each hospital achieving the standard for Appropriate DVT Prophylaxis in Women Undergoing
Cesarean Delivery:
Meets or exceeds the 90% target for the process measure of care.
Each hospital achieving the standard for High-Risk Deliveries:
Achieves favorable hospital volume characteristics for high-risk deliveries by admitting 50 or more very
low birth weight newborns/year to its neonatal ICU,
OR
Achieves favorable outcomes for high-risk deliveries as measured by the Vermont Oxford Network.
Download the 2023 Leapfrog Hospital Survey Scoring Algorithm on the
Scoring and Results
webpage.
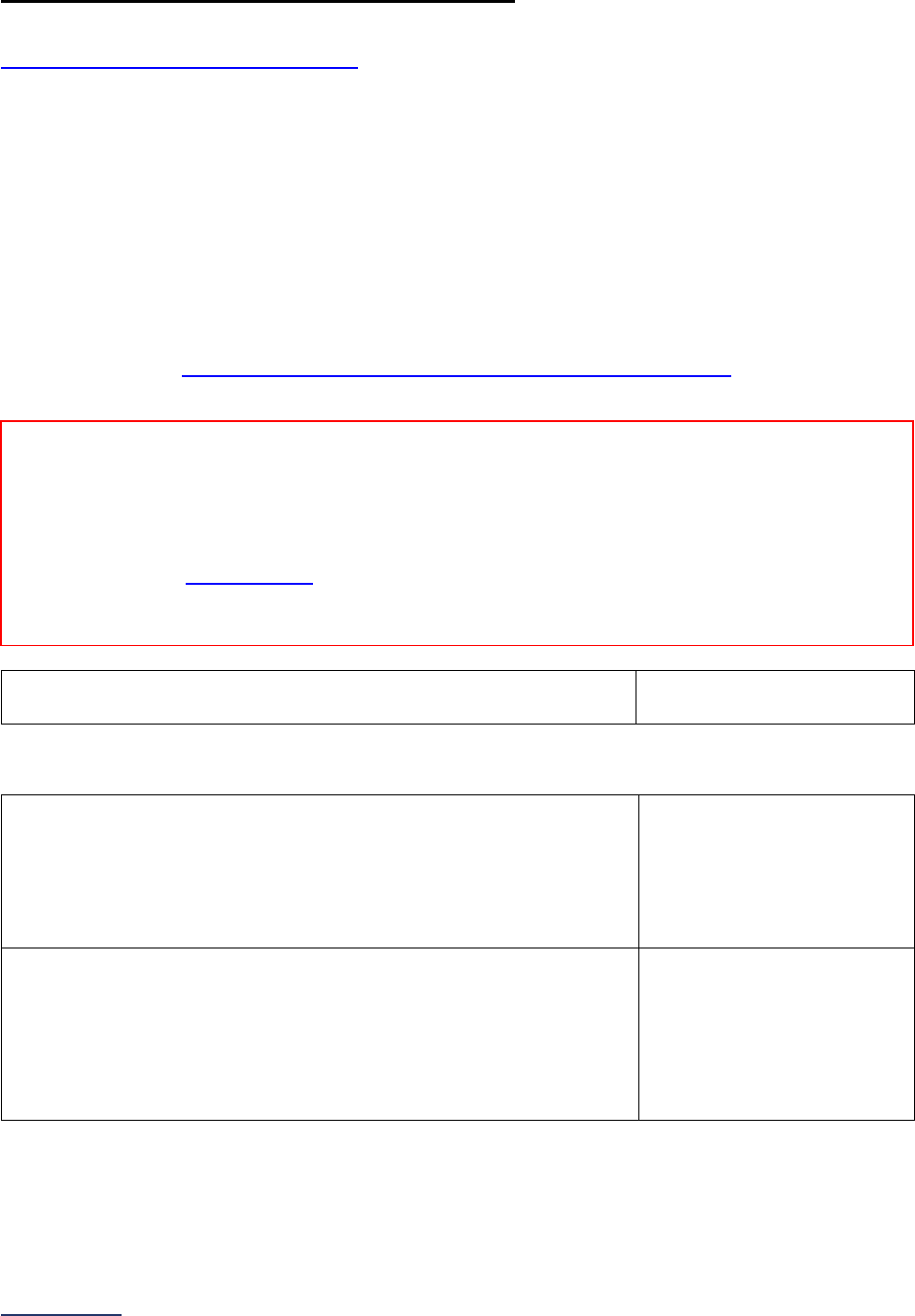
2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
143 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
4A: Maternity Care Volume and Services
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Notes:
Note 1: This section is only applicable to hospitals that deliver newborn babies. Hospitals that do not
deliver newborn babies should select “no” in question #2 in 4A and move on to the Affirmation of
Accuracy. Your hospital’s results will be displayed a “Does Not Apply” on Leapfrog’s public reporting
website.
Note 2: Hospitals must adhere to the reporting period selected below and use the same 12-month
reporting period for all subsections in Section 4: Maternity Care.
Note 3: Maternity Care Services (questions #4-9) will not be scored but will be used in public reporting.
Specifications: See Maternity Care Volume and Services Measure Specifications
in the Reference
Information beginning on page 156.
1) 12-month reporting period used:
01/01/2022 – 12/31/2022
07/01/2022 – 06/30/2023
Maternity Care Volume
2) Did the hospital deliver newborn babies during the reporting period?
If “no” or “yes, but unit is now closed or wasn’t open for the entire
reporting period,” skip the remaining questions in Section 4,
including all subsections, and go to the Affirmation of Accuracy. The
hospital will be scored as “Does Not Apply.”
Yes
No
Yes, but unit is now closed or
wasn’t open for the entire
reporting period
3) Total number of live births (i.e., liveborn infants) at this hospital
location for the reporting period.
If fewer than 10 cases, skip the remaining questions in Section 4,
including all subsections, and go to the Affirmation of Accuracy. The
hospital will be scored as “Unable to Calculate Score” or “Does Not
Apply.”
______
Reporting Period: 12 months
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o 07/01/2022 – 06/30/2023
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted Surveys
only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission and
Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or publicly
reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
144 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Maternity Care Services
4) Does your hospital have certified nurse-midwives and/or certified
midwives deliver newborns?
Yes
No
5) Does your hospital use doulas for labor and delivery?
Select all that apply.
Yes, the hospital employs
or contracts with doulas
Yes, the hospital allows
patients to bring their own
doulas
No
6) Does your hospital offer breastfeeding/lactation consultants?
Select all that apply.
Yes, in the hospital
Yes, in the outpatient
setting
Yes, at home after
discharge
No
7) Is your hospital designated as a Baby-Friendly hospital based on the
World Health Organization/UNICEF Baby-Friendly Hospital Initiative
(i.e., they adhere to the Ten Steps to Successful Breastfeeding
)?
Yes
No
8) Does your hospital routinely offer vaginal birth after cesarean
section (VBAC)?
Yes
No
9) Does your hospital offer postpartum tubal ligation during the labor
and delivery admission?
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
145 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
4B: Elective Deliveries
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Note: Hospitals must adhere to the reporting period selected below and use the same 12-
month reporting period for all subsections in Section 4: Maternity Care.
Specifications: See Elective Deliveries Measure Specifications
in the Reference Information
beginning on page 157.
Sufficient Sample: See Elective Deliveries Measure Specifications
for instructions for identifying a
sufficient sample for questions #2 and #3.
1) 12-month reporting period used:
01/01/2022 – 12/31/2022
07/01/2022 – 06/30/2023
2) Total number of mothers (or sufficient sample of them) that
delivered newborns with >=37 weeks of gestation completed
and <39 weeks of gestation completed, with Excluded
Populations removed:
If fewer than 10 cases met the criteria for the denominator,
skip questions #3-5 and continue to the next subsection. The
hospital will be scored as “Unable to Calculate Score.”
______
3) Total number of mothers indicated in question #2 that had
their newborn delivered electively (not spontaneously):
______
4) Do the responses in questions #2 and #3 above represent a
sample of cases?
Yes
No
5) If “yes” to question #4, did your hospital sample using The
Joint Commission’s sampling algorithm or Leapfrog’s
sampling instructions, as provided in the Maternity Care
Reference Information?
The Joint Commission
The Leapfrog Group
Reporting Period: 12 months
Answer questions #1-5 based on all cases (or a sufficient sample of them)
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o 07/01/2022 – 06/30/2023
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
146 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
4C: Cesarean Birth
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Note: Hospitals must adhere to the reporting period selected below and use the same 12-
month reporting period for all subsections in Section 4: Maternity Care.
Specifications: See Cesarean Birth
Measure Specifications in the Reference Information beginning
on page 159.
Sufficient Sample: See Cesarean Birth Measure Specifications
for instructions for identifying a
sufficient sample for questions #2 and #3.
1) 12-month reporting period used:
01/01/2022 – 12/31/2022
07/01/2022 – 06/30/2023
2) Total number of nulliparous mothers (or sufficient sample of them)
that delivered a live term singleton newborn in the vertex
presentation with >=37 weeks of gestation completed, with
Excluded Populations removed:
If fewer than 10 cases met the criteria for the denominator, skip
questions #3-7 and continue to the next subsection. The hospital will
be scored as “Unable to Calculate Score.”
______
3) Total number of mothers indicated in question #2 that had their
newborn delivered via cesarean section:
______
4) Do the responses in questions #2 and #3 above represent a sample
of cases?
Yes
No
5) If “yes” to question #4, did your hospital sample using The Joint
Commission’s sampling algorithm or Leapfrog’s sampling
instructions, as provided in the Maternity Care Reference
Information?
The Joint Commission
The Leapfrog Group
Reporting Period: 12 months
Answer questions #1-5 based on all cases (or a sufficient sample of them for questions #2-3)
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o 07/01/2022 – 06/30/2023
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted Surveys
only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission and
Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or publicly
reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
147 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Additional Questions (Optional – Fact Finding Only)
6) Did your hospital stratify NTSV cesarean births by the racial and
ethnic categories below for the reporting period and do you choose
to report those data to this Survey?
If “no” to question #6, skip question #7 and continue to the next
subsection.
Yes
No
7) Enter your hospital’s responses below by racial and ethnic category:
If the number of cases for a racial and ethnic category is less than 10 (in column a), skip column b
and then move to the next category. If zero, enter “0” in column a.
Racial and Ethnic Category
a) Total number of nulliparous
mothers (or sufficient sample of
them) that delivered a live term
singleton newborn in the vertex
presentation with >= 37 weeks of
gestation completed, with
Excluded Populations removed
(denominator)
b) Total number of mothers
indicated in question #7a that had
their newborn delivered via
cesarean section (numerator)
Non-Hispanic White ______ ______
Non-Hispanic Black ______ ______
Non-Hispanic American Indian
or Alaska Native
______ ______
Non-Hispanic Asian or Pacific
Islander
______ ______
Hispanic ______ ______
Non-Hispanic Other (including
two or more races)
______ ______
Unknown ______ ______

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
148 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
4D: Episiotomy
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Note: Hospitals must adhere to the reporting period selected below and use the same 12-
month reporting period for all subsections in Section 4: Maternity Care.
Specifications: See Episiotomy Measure Specifications
in the Reference Information beginning on
page 163.
1) 12-month reporting period used:
01/01/2022 – 12/31/2022
07/01/2022 – 06/30/2023
2) Total number of vaginal deliveries, with Excluded Populations
removed:
If fewer than 10 cases met the criteria for the denominator, skip
question #3 and continue to the next subsection. The hospital will be
scored as “Unable to Calculate Score.”
______
3) Total number of mothers indicated in question #2 that had an
episiotomy procedure performed:
______
Reporting Period: 12 months
Answer questions #1-3 based on all cases.
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o 07/01/2022 – 06/30/2023
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted Surveys
only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission and
Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or publicly
reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
149 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
4E: Process Measures of Quality
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Note: Hospitals must adhere to the reporting period selected below and use the same 12-
month reporting period for all subsections in Section 4: Maternity Care.
Specifications: See Process Measures of Quality Measure Specifications
in the Reference
Information beginning on page 164.
Sufficient Sample: See Process Measures of Quality Measure Specifications
for instructions for
identifying a sufficient sample for questions #3-4 and #7-8.
1) 12-month reporting period used:
01/01/2022 – 12/31/2022
07/01/2022 – 06/30/2023
Newborn Bilirubin Screening Prior to Discharge
2) Did your hospital perform a medical record audit on all cases (or a
sufficient sample of them) and measure adherence to the newborn
bilirubin screening prior to discharge clinical guideline?
If “no” to question #2, skip questions #3-5 and continue to question
#6. The hospital will be scored as “Limited Achievement.”
If “yes, but fewer than 10 cases met the inclusion criteria for the
denominator,” skip questions #3-5 and continue to question #6. The
hospital will be scored as “Unable to Calculate Score.”
Yes
No
Yes, but fewer than 10 cases
met the inclusion criteria for
the denominator
3) Number of cases measured against the guideline, either all cases or
a sufficient sample of them (denominator):
______
4) Number of cases in question #3 that adhere to the clinical process
guideline (numerator):
______
5) Do the responses in questions #3 and #4 represent a sample of
cases?
Yes
No
Reporting Period: 12 months
Answer questions #1-9 based on all cases (or a sufficient sample of them).
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o 07/01/2022 – 06/30/2023
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted Surveys
only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission and
Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or publicly
reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
150 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Appropriate DVT Prophylaxis in Women Undergoing Cesarean Delivery
6) Did your hospital perform a medical record audit on all cases (or a
sufficient sample of them) and measure adherence to the
appropriate DVT prophylaxis in women undergoing cesarean
delivery clinical guideline?
If “no” to question #6, skip questions #7-9 and continue to the next
subsection. The hospital will be scored as “Limited Achievement.”
If “yes, but fewer than 10 cases met the inclusion criteria for the
denominator,” skip questions #7-9 and continue to the next
subsection. The hospital will be scored as “Unable to Calculate
Score.”
Yes
No
Yes, but fewer than 10 cases
met the inclusion criteria for
the denominator
7) Number of cases measured against the guideline, either all cases or
a sufficient sample of them (denominator):
______
8) Number of cases in question #7 that adhere to the clinical process
guideline (numerator):
______
9) Do the responses in questions #7 and #8 represent a sample of
cases?
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
151 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
4F: High-Risk Deliveries
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
1) Does your hospital electively admit high-risk deliveries
29
?
If “no” to question #1, skip the remaining questions in Section 4F, and go
to the Affirmation of Accuracy.
Yes
No
2) Does your hospital operate a neonatal ICU (NICU), or is it co-located
30
with a hospital that operates a NICU, that admits or accepts transfers of
very low birth weight babies
31
?
If “no” to question #2, skip the remaining questions in Section 4F, and go
to the Affirmation of Accuracy. The hospital will be scored as “Limited
Achievement.”
If the NICU is co-located in another hospital and your hospital
immediately transfers all complicated newborns there, answer question
#3 and either questions #4-5 or #6-10 based on information pertaining to
the co-located hospital’s NICU.
Yes
No
3) Hospitals that participate in the Vermont Oxford Network (VON) and
have a recent 12-month report available may elect to report their
hospital’s Volume (questions #4-5)
OR
the VON’s Death or Morbidity Measure
32
(questions #6-10).
Hospitals that do not participate in the Vermont Oxford Network should
report their hospital’s Volume (questions #4-5).
Please indicate which measure the hospital will report on:
If you elect to report on Volume, answer questions #4-5 and skip
questions #6-10.
If you elect to report on the VON National Performance Measure, skip
questions #4-5. Please ensure that you have entered an accurate VON
Transfer Code in the Hospital Profile and submitted the Data Share
Authorization letter to VON. Otherwise, you will be scored as “Declined
to Respond.”
Volume
VON National
Performance Measure

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
152 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Neonatal Intensive Care Unit(s) – Volume
Important Note: Hospitals must adhere to the reporting period selected below and use the same 12-
month reporting period for all subsections in Section 4: Maternity Care.
Specifications: See Neonatal Intensive Care Unit(s) – Volume Measure Specifications
in the
Reference Information beginning on page 166.
4) 12-month reporting period used:
01/01/2022 – 12/31/2022
07/01/2022 – 06/30/2023
5) For the reporting period, how many very low birth weight babies were
admitted to your hospital’s neonatal intensive care unit(s)?
_______
Neonatal Intensive Care Unit(s) – National Performance Measurement
Important Note: Hospitals participating in the Vermont Oxford Network (VON) may opt to report on the
VON National Performance Measure. In order for Leapfrog to obtain the data from VON, the VON data
must be finalized, and hospitals must complete the following steps:
1. Provide an accurate VON Transfer Code
in the Hospital Profile,
2. Complete a Data Sharing Authorization letter and submit it to VON by June 15. Hospitals that
submitted their Data Sharing Authorization letter to VON in previous Leapfrog Hospital Survey
Cycles will not have to re-submit a new letter in 2023.
3. Select “VON National Performance Measure” in question #3, and
4. Submit the 2023 Leapfrog Hospital Survey by the June 30 Submission Deadline.
Hospitals that select “VON National Performance Measure” in question #3, but do not adhere to the other
steps will be scored and publicly reported as “Declined to Respond” for the High-Risk Deliveries measure.
Specifications: See Neonatal Intensive Care Unit(s) – National Performance Measurement Measure
Specifications in the Reference Information beginning on page 166.
6) Most recent 12-month reporting period for which VON performance
results are available:
No response required
here. Determined
automatically based on
VON data.
Reporting Period: 12 months
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o 07/01/2022 – 06/30/2023
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.
Reporting Period:
12 months
Responses are based on the latest 12-month report received from the Vermont Oxford Network
(VON) for the Death or Morbidity Measure.
• 2021 VON data
• 2022 VON data (usually available after August 31)
Leapfrog will update the VON data three times per Survey Cycle for all hospitals that have completed the steps noted above.
See deadlines and reporting periods in the
Neonatal Intensive Care Unit(s) - National Performance Measurement Measure
Specifications.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
153 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
7) Hospital’s volume based on VON report:
No response required
here. Determined
automatically based on
VON data.
8) Hospital’s SMR 95% lower bound based on VON report:
No response required
here. Determined
automatically based on
VON data.
9) Hospital’s observed to expected ratio of morbidity or mortality (SMR
shrunken) based on VON report:
No response required
here. Determined
automatically based on
VON data.
10) Hospital’s SMR 95% upper bound based on VON report:
No response required
here. Determined
automatically based on
VON data.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
154 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Affirmation of Accuracy
As the hospital CEO or as an employee of the hospital to whom the hospital CEO has delegated
responsibility, I have reviewed this information pertaining to the Maternity Care Section at our hospital,
and I hereby certify that this information is true, accurate, and reflects the current, normal operating
circumstances at our hospital. I am authorized to make this certification on behalf of our hospital.
The hospital and I understand that The Leapfrog Group, its members, the public and entities and persons
who contract or have other business dealings with The Leapfrog Group are relying on the truth and
accuracy of this information. The hospital and I also understand that The Leapfrog Group will make this
information and/or analyses of this information public through the Survey Results public reporting website,
The Leapfrog Group’s Hospital Safety Grade, and/or other Leapfrog Group products and services. This
information and/or analyses and all intellectual property rights therein shall be and remain the sole and
exclusive property of The Leapfrog Group in which The Leapfrog Group retains exclusive ownership. This
information does not infringe upon any third-party intellectual property rights or any other third-party rights
whatsoever and is free and clear of all encumbrances and liens of any kind. The hospital and I
acknowledge that The Leapfrog Group may use this information in a commercial manner for profit. The
hospital shall be liable for and shall hold harmless and indemnify The Leapfrog Group from any and all
damages, demands, costs, or causes of action resulting from any inaccuracies in the information or any
misrepresentations in this Affirmation of Accuracy. The Leapfrog Group and its members and entities and
persons who contract or have other business dealings with The Leapfrog Group reserve the right to omit
or disclaim information that is not current, accurate or truthful.
Affirmed by _____________________, the hospital’s ___________________________,
(first name and last name) (title)
on _______________________.
(date)

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
155 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 4: 2023 Maternity Care Reference Information
What’s New in the 2023 Survey
Leapfrog is updating the measure specifications from The Joint Commission (TJC) for PC-01 Elective
Deliveries (Section 4B) and PC-02 Cesarean Birth (Section 4C) for those hospitals that do not already
submit data to TJC and therefore need to retrospectively collect data. Hospitals measuring these quality
indicators and reporting results to TJC should continue to use the data reported to TJC when responding
to these subsections of the Survey. Hospitals participating in the California Maternal Quality Care
Collaborative (CMQCC) Maternal Data Center may continue to use the data provided in their CMQCC
reports when responding to subsections 4B: Elective Deliveries, 4C: Cesarean Birth, 4D: Episiotomy, and
4E: Process Measures of Quality. Hospitals participating in the Michigan Obstetrics Initiative (OBI) may
also continue to use the data provided in their OBI reports to report on subsection 4C: Cesarean Birth.
Section 4A: Maternity Care Volume and Services
Leapfrog is renaming Section 4A: Maternity Care Volume to Section 4A: Maternity Care Volume and
Services and adding new questions regarding service offerings that will be used for public reporting only.
The new questions focus on the availability of midwives and doulas, breastfeeding support, vaginal
delivery after cesarean section, and postpartum tubal ligation. The questions will not be scored but are
required and will be used in public reporting.
Section 4C: Cesarean Birth
Under the guidance of Leapfrog’s Maternity Care Expert Panel
, Leapfrog is adding a set of optional fact-
finding questions to this subsection to collect cesarean birth rates stratified by racial and ethnic category.
Hospitals are asked to provide numerators and denominators for the cesarean birth measure for each of
the following racial and ethnic categories: Non-Hispanic White, Non-Hispanic Black, Non-Hispanic
American Indian or Alaska Native, Non-Hispanic Asian or Pacific Islander, Hispanic, and Non-Hispanic
Other (including two or more races). These fact-finding questions are optional and will not be used in
scoring or public reporting in 2023.
There are no changes to the scoring algorithm for Section 4C: Cesarean Birth.
Section 4F: High-Risk Deliveries – Neonatal Intensive Care Unit(s) – National
Performance Measurement
Leapfrog is continuing to obtain data directly from the Vermont Oxford Network (VON) for those hospitals
that electively admit high-risk deliveries and opt to use VON’s Death or Morbidity Outcome Measure when
reporting on Section 4F: High-Risk Deliveries. Hospitals still need to complete the following steps:
1. Complete a Data Sharing Authorization letter and submit it to VON by the appropriate dates
(Hospitals that successfully submitted a Data Sharing Authorization letter in previous Leapfrog
Hospital Survey Cycles will not be required to submit a new letter in 2023),
2. Select “VON National Performance Measure” in Section 4F: High-Risk Deliveries question #3,
3. Provide an accurate VON Transfer Code in the Hospital Profile (this will be pre-populated if
previously provided), and
4. Submit the Leapfrog Hospital Survey by the appropriate date.
Hospitals that select “VON National Performance Measure” in question #3 of Section 4F: High-Risk
Deliveries, but do not complete all the steps listed above will be scored and publicly reported as “Declined
to Respond” for the High-Risk Deliveries measure.
Change Summary Since Release
June 13, 2023 – Section 4D Episiotomy and Section 4E Process Measures of Quality: Added APR-DRG
and Tricare DRG codes used to identify vaginal deliveries and cesarean deliveries for reporting on

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
156 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Episiotomy in Section 4D and DVT Prophylaxis in Section 4E. Please review the updated measure
specifications on pages 163 and 165 of the Survey.
Maternity Care Measure Specifications
Maternity Care Volume and Services
Important Note: Hospitals that share a CMS Certification Number are required to report by facility.
Please carefully review Leapfrog’s Multi-Campus Reporting Policy
.
Reporting Period: 12 months
• Surveys submitted prior to September 1:
• 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
• 07/01/2022 – 06/30/2023
Question #3: The number of live births (i.e., infants) at this hospital location (inborn cases only), reported
to your state during the reporting period.
Alternatively, the below list of Z codes can be used to identify live births, with the caution that these codes
are coded for the newborn, not the mother; likely to be found in your hospital’s birth CIS/medical record
system; but often not in claims data since normal newborn care may be included in the mother’s claim
without baby’s diagnosis coding.
Z38.00 – Z38.01: Single liveborn infant, born in hospital
Z38.30 – Z38.31: Twin liveborn infant, born in hospital
Z38.61 – Z38.69: Other multiple liveborn infant, born in hospital
Note: This data point is simply used to qualify a hospital for further reporting of the normal delivery
measures.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
157 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Elective Deliveries
Important Notes:
Note 1: Hospitals that share a CMS Certification Number are required to report by facility. Please carefully
review Leapfrog’s Multi-Campus Reporting Policy
.
Note 2: Leapfrog uses the specifications created by The Joint Commission (TJC) for the Elective
Deliveries measure. As such, Leapfrog will update its instructions annually, and more frequently if
appropriate, to maintain alignment with TJC. Hospitals can access TJC’s measure specifications directly
using the links in the table below.
Note 3: Elective Deliveries can be reported based on all eligible cases OR a sufficient sample of cases as
outlined in the denominator specifications.
Source: Joint Commission PC-01 (version 2022B2)
Reporting Period: 12 months
• Surveys submitted prior to September 1:
• 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
• 07/01/2022 – 06/30/2023
If you measured this quality indicator, reported the results to The Joint Commission (TJC), and
continue to submit these data to The Joint Commission, use those data when responding to this
subsection of the Survey.
Hospitals participating in the California Maternal Quality Care Collaborative (CMQCC) Maternal Data
Center may use the data provided in CMQCC reports when responding to this subsection of the
Survey. Download instructions for using the CMQCC reports on the
Survey and CPOE Materials
webpage.
Otherwise, use TJC’s PC-01 Elective Deliveries measure specifications (version 2022B2
) to
retrospectively collect and report data for this measure. The PC-01 measure specifications are outlined
below. To access the measure specifications directly on The Joint Commission’s website visit:
https://manual.jointcommission.org/releases/TJC2022B2/MIF0166.html.
Note: Leapfrog will also accept data collected for ePC-01 using TJC’s electronic clinical quality
measure (eCQM) specifications for the appropriate reporting period. eCQM specifications can be found
here
.
Sampling Cases: Hospitals that report the Perinatal Care Measure Set to TJC may use the sampling
methodology used by the TJC to report on these questions.
Otherwise, hospitals opting to identify a sufficient sample of mothers for this measure, in lieu of full
case reporting, should follow the instructions below. Hospitals may refer to the TJC Measure Algorithm
flow chart at the bottom of the TJC specifications linked above, for an example of how to identify cases.
• Review your hospital’s first delivery as of April 15, 2022 if submitting a Survey prior to
September 1 (or optionally, July 15, 2022 if (re)submitting a Survey on or after September 1,
2023 and sampling from 07/01/2022 – 06/30/2023 discharges).
• Evaluate this case against the inclusion criteria; retain the case for the sample if it meets the
inclusion criteria.
• Evaluate this case against the exclusion criteria; retain the case for the sample if it does not
meet any of the listed exclusions.
• Move to the next delivery and evaluate for inclusion/exclusion applicability.
• Continue through cases in sequential order until a sample of at least 30 cases is reached, or
all cases discharged during the reporting period are reviewed, whichever comes first.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
158 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Question #2 (denominator): Patients delivering newborns with >= 37 and < 39 weeks of gestation
completed with Excluded Populations removed.
Note: The denominator should include both mothers that had their newborn delivered
electively and mothers that delivered spontaneously at the specified weeks of gestation.
Included Populations:
• ICD-10-PCS Principal Procedure Code or ICD-10-PCS Other Procedure Codes for delivery as
defined in Appendix A, Table 11.01.1.
• ICD-10-CM Principal Diagnosis Code or ICD-10-CM Other Diagnosis Codes for planned
cesarean birth in labor as defined in Appendix A, Table 11.06.1.
Excluded Populations:
ICD-10-CM Principal Diagnosis Code or ICD-10-CM Other Diagnosis Codes for conditions possibly
justifying elective delivery prior to 39 weeks gestation as defined in Appendix A, Table 11.07
• History of prior stillbirth
• Less than 8 years of age
• Greater than or equal to 65 years of age
• Length of stay > 120 days
• Gestational Age
< 37 or >= 39 weeks or UTD
Data Elements: Visit https://manual.jointcommission.org/releases/TJC2022B2/MIF0166.html
.
If fewer than 10 cases during the reporting period, skip the next question.
Question #3 (numerator): Patients with elective deliveries included in the denominator.
Included Populations:
ICD-10-PCS Principal Procedure Code or ICD-10-PCS Other Procedure Codes for one or more of the
following:
• Medical induction of labor as defined in Appendix A, Table 11.05 while not in Labor prior to the
procedure
• Cesarean birth as defined in Appendix A, Table 11.06 and all the following:
o not in Labor
o no history of a Prior Uterine Surgery
Excluded Populations: None
Data Elements: Visit https://manual.jointcommission.org/releases/TJC2022B2/MIF0166.html.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
159 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Cesarean Birth
Important Notes:
Note 1: Hospitals that share a CMS Certification Number are required to report by facility. Please carefully
review Leapfrog’s Multi-Campus Reporting Policy
.
Note 2: Leapfrog uses the specifications created by The Joint Commission (TJC) for the Cesarean Births
measure. As such, Leapfrog will update its instructions annually, and more frequently if appropriate, to
maintain alignment with TJC. Hospitals can access the TJC’s measure specifications directly using the
links in the table below.
Note 3: Cesarean Births can be reported based on all eligible cases OR a sufficient sample of cases as
outlined in the denominator specifications.
Source: Joint Commission PC-02 (version 2022B2
)
Reporting Period: 12 months
• Surveys submitted prior to September 1:
• 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
• 07/01/2022 – 06/30/2023
If you measured this quality indicator, reported the results to The Joint Commission (TJC), and continue
to submit these data to The Joint Commission, use those data when responding to this subsection of
the Survey.
Hospitals participating in the California Maternal Quality Care Collaborative (CMQCC) Maternal Data
Center may use the data provided in CMQCC reports when responding to this subsection of the Survey.
Download instructions for using the CMQCC reports on the Survey and CPOE Materials webpage
.
Hospitals participating in the Michigan Obstetrics Initiative (OBI) may use the data provided in OBI reports
when responding to this subsection of the Survey.
Otherwise, use TJC’s PC-02 Cesarean Birth measure specifications (version 2022B2
) to retrospectively
collect and report data for this measure. The PC-02 measure specifications are outlined below. To access
the measure specifications directly on The Joint Commission’s website, visit
https://manual.jointcommission.org/releases/TJC2022B2/MIF0167.html.
Note: Leapfrog will also accept data collected for ePC-02 using TJC’s electronic clinical quality measure
(eCQM) specifications for the appropriate reporting period. eCQM specifications can be found here.
Sampling Cases: Hospitals that report the Perinatal Care Measure Set to TJC may use the sampling
methodology used by the TJC to report on questions #2-3.
Otherwise, hospitals opting to identify a sufficient sample of mothers for this measure, in lieu of full case
reporting, should follow the instructions below. Hospitals may refer to the TJC Measure Algorithm flow
chart at the bottom of each set of specifications linked above, for an example of how to identify cases.
• Review your hospital’s first delivery as of April 15, 2022 if submitting a Survey prior to September
1 (or optionally, July 15, 2022 if (re)submitting a Survey on or after September 1, 2023 and
sampling from 07/01/2022 – 06/30/2023 discharges).
• Evaluate this case against the inclusion criteria; retain the case for the sample if it meets the
inclusion criteria.
• Evaluate this case against the exclusion criteria; retain the case for the sample if it does not meet
any of the listed exclusions.
• Move to the next delivery and evaluate for inclusion/exclusion applicability.
• Continue through cases in sequential order until a sample of at least 30 cases is reached, or all
cases discharged during the reporting period are reviewed, whichever comes first.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
160 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Question #2 (denominator): Nulliparous patients delivered of a live term singleton newborn in vertex
presentation with Excluded populations removed.
Note: The denominator should include both nulliparous mothers with a live term singleton newborn in
vertex presentation that had their newborn delivered via cesarean section and nulliparous mothers that
delivered vaginally.
Included Populations:
• ICD-10-PCS Principal Procedure Code or ICD-10-PCS Other Procedure Codes for delivery as
defined in Appendix A, Table 11.01.1
.
• Nulliparous patients with ICD-10-CM Principal Diagnosis Code or ICD-10-CM Other Diagnosis
Codes for outcome of delivery as defined in Appendix A, Table 11.08
, and with a delivery of a
newborn with 37 weeks or more of gestation completed.
Excluded Populations:
• ICD-10-CM Principal Diagnosis Code or ICD-10-CM Other Diagnosis Codes for multiple
gestations and other presentations as defined in Appendix A, Table 11.09
• Less than 8 years of age
• Greater than or equal to 65 years of age
• Length of stay >120 days
• Gestational Age
< 37 weeks or UTD
Data Elements: Visit https://manual.jointcommission.org/releases/TJC2022B2/MIF0167.html
.
If fewer than 10 cases during the reporting period, skip the next question.
Question #3 (numerator): Patients in the denominator with cesarean births.
Included Populations:
ICD-10-PCS Principal Procedure Code or ICD-10-PCS Other Procedure Codes for cesarean birth as
defined in Appendix A, Table 11.06
Excluded Populations: None
Data Elements: Visit https://manual.jointcommission.org/releases/TJC2022B2/MIF0167.html.
Additional Questions (Optional – Fact Finding Only)
Source: Joint Commission PC-02 (version 2022B2)
Reporting Period: 12 months
• Surveys submitted prior to September 1:
• 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
• 07/01/2022 – 06/30/2023
Hospitals participating in the California Maternal Quality Care Collaborative (CMQCC) Maternal Data
Center may use the data provided in CMQCC reports when responding to these optional, fact-finding
questions. Download instructions for using the CMQCC reports on the
Survey and CPOE Materials
webpage.
Hospitals reporting to the U.S. News & World Report Maternity Services Survey may use the data
provided to U.S. News & World Report when responding to these optional, fact-finding questions.
Otherwise, hospitals should use TJC’s PC-02 Cesarean Birth measure specifications (version 2022B2
) to
retrospectively review all cases and then stratify those cases by the racial/ethnic categories provided.
The PC-02 measure specifications are outlined below. To access the measure specifications directly on

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
161 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
The Joint Commission’s website, visit
https://manual.jointcommission.org/releases/TJC2022B2/MIF0167.html.
Question #7a (denominator): Total number of nulliparous patients delivered of a live term singleton
newborn in vertex presentation with Excluded populations removed by the following racial/ethnic
categories:
• Non-Hispanic White
• Non-Hispanic Black
• Non-Hispanic American Indian or Alaska Native
• Non-Hispanic Asian or Pacific Islander
• Hispanic
• Non-Hispanic Other (including two or more races)
• Unknown
Each patient should only be counted once and included in the appropriate racial/ethnic category based on
known racial/ethnic information. If race or ethnicity is unknown, include the case in the “Unknown”
category. Hispanic patients should be included in the “Hispanic” category regardless of race. If a
racial/ethnic category is not used by your hospital, include the case in the “Unknown” category, i.e., if
patients with more than one race are listed as “multiracial” regardless of their ethnicity, include these
cases in the “Unknown” category.
Hospitals reporting to U.S. News can use the same racial/ethnic categories when reporting to Leapfrog.
Hospital using CMQCC can use the below crosswalk or information provided directly in their Leapfrog
CMQCC report:
CMQCC Race/Ethnicity
U.S. News Race/Ethnicity
Leapfrog Race/Ethnicity
Hispanic-US Born
Hispanic
Hispanic
Hispanic Non-US Born
White
Non-Hispanic White
Non-Hispanic White
Black
Non-Hispanic Black
Non-Hispanic Black
Asian
Non-Hispanic Asian or
Pacific Islander
Non-Hispanic Asian or
Pacific Islander
Pacific Islander
Native American
Non-Hispanic Other
(including two or more
races)
Non-Hispanic Other
(including two or more
races)
Other
Multiracial
Unknown
Unknown
Note: The denominator should include both nulliparous mothers with a live term singleton newborn in
vertex presentation that had their newborn delivered via cesarean section and nulliparous mothers that
delivered vaginally.
Included Populations:
• ICD-10-PCS Principal Procedure Code or ICD-10-PCS Other Procedure Codes for delivery as
defined in Appendix A, Table 11.01.1
.
• Nulliparous patients with ICD-10-CM Principal Diagnosis Code or ICD-10-CM Other Diagnosis
Codes for outcome of delivery as defined in Appendix A, Table 11.08
, and with a delivery of a
newborn with 37 weeks or more of gestation completed.
Excluded Populations:
• ICD-10-CM Principal Diagnosis Code or ICD-10-CM Other Diagnosis Codes for multiple
gestations and other presentations as defined in Appendix A, Table 11.09
• Less than 8 years of age
• Greater than or equal to 65 years of age
• Length of stay >120 days
• Gestational Age < 37 weeks or UTD

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
162 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Data Elements: Visit https://manual.jointcommission.org/releases/TJC2022B2/MIF0167.html.
If fewer than 10 cases during the reporting period for any racial/ethnic category, skip the next
question.
Question #7b (numerator): Patients in the denominator with cesarean births by the following
racial/ethnic categories:
• Non-Hispanic White
• Non-Hispanic Black
• Non-Hispanic American Indian or Alaska Native
• Non-Hispanic Asian or Pacific Islander
• Hispanic
• Non-Hispanic Other (including two or more races)
• Unknown
Included Populations:
ICD-10-PCS Principal Procedure Code or ICD-10-PCS Other Procedure Codes for cesarean birth as
defined in Appendix A, Table 11.06
Excluded Populations: None
Data Elements: Visit https://manual.jointcommission.org/releases/TJC2022B2/MIF0167.html.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
163 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Episiotomy
Important Note: Hospitals that share a CMS Certification Number are required to report by facility.
Please carefully review Leapfrog’s Multi-Campus Reporting Policy
.
Source: National Quality Forum #0470
Reporting Period: 12 months
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o
07/01/2022 – 06/30/2023
Hospitals participating in the California Maternal Quality Care Collaborative (CMQCC) Maternal Data
Center may use the data provided in CMQCC reports when responding to this subsection of the Survey.
Download instructions for using the CMQCC reports on the Survey and CPOE Materials webpage
.
Question #2 (denominator): Total number of vaginal deliveries during the reporting period, with
Excluded Populations removed.
For the purposes of this measure, use the following MS-DRGs to identify a vaginal delivery:
• 768: Vaginal delivery with O.R. procedure except sterilization and/or D&C
• 796: Vaginal delivery with sterilization/D&C with MCC
• 797: Vaginal delivery with sterilization/D&C with CC
• 798: Vaginal delivery with sterilization/D&C without CC/MCC
• 805: Vaginal delivery without sterilization/D&C with MCC
• 806: Vaginal delivery without sterilization/D&C with CC
• 807: Vaginal delivery without sterilization/D&C without CC/MCC
The following APR-DRGs should also be used to identify a vaginal delivery if your hospital uses APR-
DRG coding:
• 541: Vaginal delivery with sterilization and/or D&C
• 542: Vaginal delivery with complicating procedures excluding sterilization and/or D&C
• 560: Vaginal delivery
The following Tricare DRGs should also be used to identify a vaginal delivery if your hospitals uses
Tricare DRG coding:
• 762 Vaginal delivery with sterilization or D&C with MCC
• 763 Vaginal delivery with sterilization or D&C with CC
• 764 Vaginal delivery with sterilization or D&C without CC/MCC
• 768 Vaginal delivery with O.R. procedure except sterilization and/or D&C
• 805 Vaginal delivery without sterilization or D&C with MCC
• 806 Vaginal delivery without sterilization or D&C with CC
• 807 Vaginal delivery without sterilization or D&C without CC/MCC
Excluded Populations:
Exclude any cases with the following ICD-10-CM diagnostic code in any field:
• O66.0: Obstructed labor due to shoulder dystocia
Question #3 (numerator): Total number of mothers included in question #2 (the denominator) that had
an episiotomy procedure performed.
For the purposes of this measure, the following ICD-10-PCS procedure codes should be used for
identifying an episiotomy:
• 0W8NXZZ: Division of female perineum, external approach

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
164 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Process Measures of Quality
Important Notes:
Note 1: Hospitals that share a CMS Certification Number are required to report by facility. Please carefully
review Leapfrog’s Multi-Campus Reporting Policy
.
Note 2: For Maternity Care Process Measures, hospitals with a sufficient sample size (as defined below),
can randomly sample for the denominator of each indicator, and measure and report adherence based on
that sample. Most likely, the numerator criteria for these two measures will require medical chart review if
these specific data are not already extracted or coded consistently for other purposes.
Newborn Bilirubin Screening Prior to Discharge
Source: Providence Health
Reporting Period: 12 months
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o
07/01/2022 – 06/30/2023
Hospitals participating in the California Maternal Quality Care Collaborative (CMQCC) Maternal Data
Center may use the data provided in CMQCC reports when responding to this subsection of the Survey.
Download instructions for using the CMQCC reports on the Survey and CPOE Materials webpage
.
Sampling: If you have fewer than 30 cases that meet the criteria for inclusion in the denominator of the
process measure during the time period of the medical record audit, include ALL of these cases in
measuring adherence to the process guidelines. You need NOT use more than 12 months of historical
data to increase the eligible cases beyond 30; just measure and report on ALL eligible cases that you
have in that reporting period.
If you have more than 30 cases that meet the criteria for inclusion in the denominator of the process
measure during the time period of the medical record audit, you may randomly sample at least 30 of them
for the denominator of each guideline, and measure and report adherence based on that sample.
Question #3 (denominator): Eligible cases include all normal newborns born at or beyond 35 completed
weeks gestation that were delivered in the hospital during the reporting period (all inborns) with Excluded
Populations removed.
Excluded Populations:
• admitted to a neonatal ICU, either at your hospital or another hospital; or
• with parental refusal to test; or
• prenatal documentation of severe congenital anomalies in the newborn and documentation that
the newborn will receive comfort care measures only; or
• newborn died prior to discharge
Question #4 (numerator): Number of eligible cases included in the denominator who have a serum or
transcutaneous bilirubin screen prior to discharge to identify risk of hyperbilirubinemia.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
165 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Appropriate DVT Prophylaxis in Women Undergoing Cesarean Delivery
Source: National Quality Forum #0473
Reporting Period: 12 months
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o
07/01/2022 – 06/30/2023
Hospitals participating in the California Maternal Quality Care Collaborative (CMQCC) Maternal Data
Center may use the data provided in CMQCC reports when responding to this subsection of the Survey.
Download instructions for using the CMQCC reports on the Survey and CPOE Materials webpage
.
Sampling: If you have fewer than 30 cases that meet the criteria for inclusion in the denominator of the
process measure during the time period of the medical record audit, include ALL of these cases in
measuring adherence to the process guidelines. You need NOT use more than 12 months of historical
data to increase the eligible cases beyond 30; just measure and report on ALL eligible cases that you
have in that reporting period.
If you have more than 30 cases that meet the criteria for inclusion in the denominator of the process
measure during the time period of the medical record audit, you may randomly sample at least 30 of them
for the denominator of each guideline, and measure and report adherence based on that sample.
Question #7 (denominator): Eligible cases include all women undergoing cesarean delivery during the
reporting period.
Include cases with one of the following MS-DRG codes:
• 783: Cesarean section with sterilization with MCC
• 784: Cesarean section with sterilization with CC
• 785: Cesarean section with sterilization without CC/MCC
• 786: Cesarean section without sterilization with MCC
• 787: Cesarean section without sterilization with CC
• 788: Cesarean section without sterilization without CC/MCC
The following APR-DRGs should also be used to identify a cesarean delivery if your hospital uses APR-
DRG coding:
• 539: Cesarean section with sterilization
• 540: Cesarean section without sterilization
The following Tricare DRGs should also be used to identify a cesarean delivery if your hospitals uses
Tricare DRG coding:
• 771 Cesarean section without sterilization with MCC
• 772 Cesarean section without sterilization with CC
• 773 Cesarean section without sterilization without CC/MCC
• 783 Cesarean section with sterilization with MCC
• 784 Cesarean section with sterilization with CC
• 785 Cesarean section with sterilization without CC/MCC
Excluded Populations: None.
Question #8 (numerator) Number of eligible cases included in the denominator who received either
fractionated or unfractionated heparin or heparinoid, or pneumatic compression devices prior to surgery.
Note: Use of a pneumatic compression device may be documented in the OR log but must be placed pre-
operatively to qualify for inclusion in the numerator.
For a list of approved pneumatic compression devices, see the devices listed under “Intermittent
Pneumatic Compression Device (IPC)” in Table 2.1 VTE Prophylaxis Inclusion Table
.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
166 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
High-Risk Deliveries
Important Notes:
Note 1: Hospitals that share a CMS Certification Number are required to report by facility. Please carefully
review Leapfrog’s Multi-Campus Reporting Policy
.
Note 2: Hospitals should report on the selection from Section 4F question #3 (either Volume OR the
National Performance Measure (VON)).
Neonatal Intensive Care Unit(s) – Volume
Hospitals opting to report on Volume should only use ICD-10-CM codes as indicated in the specifications
below. When calculating hospital volume, count the number of patients with any one or more of the
specified diagnosis codes for high-risk deliveries, subject to the other inclusion/exclusion criteria below.
The diagnosis codes may be in any field. The count can include inborn as well as transfer cases.
Source: The Leapfrog Group
Reporting Period: 12 months
• Surveys submitted prior to September 1:
• 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
• 07/01/2022 – 06/30/2023
Question #5 (Instructions for Volume Reporting)
Included Populations:
Number of newborns admitted to the neonatal ICU with the following ICD-10-CM codes:
ICD-10-CM Code Description
P05.02 Newborn light for gestational age, 500-749 grams
P05.03 Newborn light for gestational age, 750-999 grams
P05.04 Newborn light for gestational age, 1000-1249 grams
P05.05 Newborn light for gestational age, 1250-1499 grams
P05.12 Newborn small for gestational age, 500-749 grams
P05.13 Newborn small for gestational age, 750-999 grams
P05.14 Newborn small for gestational age, 1000-1249 grams
P05.15 Newborn small for gestational age, 1250-1499 grams
P05.2 Newborn affected by fetal malnutrition not light or small for gestational age
P05.9 Newborn affected by slow intrauterine growth, unspecified
P07.02 Extremely low birth weight newborn, 500-749 grams
P07.03 Extremely low birth weight newborn, 750-999 grams
P07.14 Other low birth weight newborn, 1000-1249 grams
P07.15 Other low birth weight newborn, 1250-1499 grams
Excluded Populations: Newborns admitted to the neonatal ICU weighing 1500 grams or more.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
167 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Neonatal Intensive Care Unit(s) – National Performance Measurement
Hospitals participating in the Vermont Oxford Network (VON) may opt to report on the VON National
Performance Measure. In order for Leapfrog to obtain the data from VON, the VON data must be
finalized, and hospitals must complete the following steps:
1. Provide an accurate VON Transfer Code
in the Hospital Profile,
2. Complete a Data Sharing Authorization letter and submit it to VON by June 15 (Hospitals that
submitted their Data Sharing Authorization letter to VON in in previous Leapfrog Hospital
Survey Cycles will not have to re-submit a new letter in 2023),
3. Select “VON National Performance Measure” in question #3, and
4. Submit the 2023 Leapfrog Hospital Survey by the June 30 Submission Deadline.
Hospitals that select “VON National Performance Measure” in question #3, but do not adhere to the other
steps will be scored and publicly reported as “Declined to Respond” for the High-Risk Deliveries measure.
Data will be available for review on the Leapfrog Hospital Survey Hospital Details Page beginning on July
12. The VON data Leapfrog obtains can be found in the Vermont Oxford Network’s Nightingale Internet
Reporting System. Leapfrog has provided instructions for using the VON Nightingale online tool on the
Survey and CPOE Materials webpage
. These instructions can be used to verify the data that Leapfrog is
obtaining from VON, which includes:
Variable
From the Vermont Oxford Network (SMR Report from Nightingale online tool)
Volume
From the latest 12-month standardized mortality or morbidity ratio (SMR) report for
Death or Morbidity, the hospital’s “N” for the volume of cases for the reporting period.
SMR 95%
(lower bound)
From the same report, the hospital’s “SMR 95% (lower)” for Death or Morbidity. This
represents the lower value of your hospital’s 95% confidence interval.
SMR
(shrunken)
From the same report, the hospital’s “SMR (shrunken)” for Death or Morbidity. This is
the weighted average of the hospital value and the population (Vermont Oxford
Network) mean value.
SMR 95%
(upper bound)
From the same report, the hospital’s “SMR 95% (upper)” for Death or Morbidity. This
represents the upper value of your hospital’s 95% confidence interval.
Deadlines and Reporting Periods
Leapfrog will update the VON data three times per Survey Cycle for all hospitals that have completed the
steps noted above according to the deadlines and reporting periods listed in the following table:
Complete and
submit Data
Sharing
Authorization to
VON by*
Data downloaded
from VON will be
scored and
publicly reported
for hospitals that
have submitted
Section 4 by
VON
Reporting
Period
Available on Hospital Details Page
and Public Reporting Website on
June 15, 2023 June 30, 2023 2021
July 12, 2023
Hospital Details Page
July 25, 2023
Public Reporting Website
August 15, 2023
August 31, 2023
2022**
September 8, 2023***
November 15, 2023
November 30, 2023
2022
December 7, 2023***
* Hospitals that successfully submitted a Data Sharing Authorization letter in previous years will not be
required to submit another letter in 2023.
**Anticipated release of 2022 VON data.
*** Available on Hospital Details Page on the same date as public release of Survey Results
See FAQs
for additional information about responding to the questions in this section.

2023 Leapfrog Hospital Survey – Hard Copy Section 4: Maternity Care
168 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Maternity Care Frequently Asked Questions (FAQs)
1. Under normal circumstances our hospital does not perform newborn deliveries. However,
during the reporting period we had a few emergency situations and/or transfers. Should
we indicate delivering newborns during the reporting period?
If your hospital does not electively perform newborn deliveries (i.e., no labor/delivery unit, only
admit in an emergency situation), you would respond “no” to question #2 in Section 4A, as
hospitals are instructed to report inborns only.
2. Should newborns that are delivered outside of the hospital that are brought to labor and
delivery be included in the total number of live births in Section 4A?
When calculating the total number of live births in Section 4A question #3, refer to the measure
specifications for Maternity Care Volume. Only inborns should be included in the total volume.
3. Should hospitals use delivery date or discharge date when including births/cases for the
measures reported in Section 4 Maternity Care?
The discharge date should be used to determine whether a case falls within the reporting period
specified.
4. In Section 4E, what tools should hospitals use for managing patients that receive a serum
or transcutaneous bilirubin screen to assess the risk of hyperbilirubinemia prior to
discharge?
One example a hospital can use is the Bhutani Nomogram. For an example, please see:
American Academy of Pediatrics Clinical Practice Guidelines: Management of Hyperbilirubinemia
in the Newborn Infant 35 or More Weeks of Gestation.
http://pediatrics.aappublications.org/content/114/1/297.full
Tip: To view any Figure in the reference, click on it to open, then again to enlarge.
Other tools may also be used if the serum or transcutaneous screen is conducted prior to
discharge and risk is managed appropriately.
5. In Section 4E, what codes or diagnoses should be used to identify severe congenital
anomalies for exclusions for newborn bilirubin screening prior to discharge?
Leapfrog’s Maternity Care Expert Panel has opted to not overcomplicate the measure by
providing ICD-codes for identifying congenital anomalies in the newborn. Instead, we would
suggest that you focus on identifying the following exclusions: documentation that the newborn
will receive comfort care measures only, which would accompany the prenatal documentation of
severe congenital anomalies in the newborn.
If you have concerns about the level of effort required to identify these exclusions, please note
that Leapfrog allows hospitals to report on a sample of 30 cases for those facilities that opt to
report on a sample instead of full case reporting. You can find those instructions in the
Maternity
Care Process Measure Specifications.

2023 Leapfrog Hospital Survey – Hard Copy Section 5: ICU Physician Staffing (IPS)
170 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
SECTION 5: ICU PHYSICIAN STAFFING (IPS)
This section includes questions and reference information for Section 5: ICU Physician Staffing (IPS).
Please carefully review the questions, endnotes, and reference information (e.g., measure specifications,
notes, and frequently asked questions) before you begin. Failure to review the reference information
could result in inaccurate responses.

2023 Leapfrog Hospital Survey – Hard Copy Section 5: ICU Physician Staffing (IPS)
171 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 5: 2023 ICU Physician Staffing (IPS) Standard
ICU Physician Staffing (IPS) Fact Sheet and Bibliography:
https://ratings.leapfroggroup.org/measure/hospital/2023/critical-care
Hospitals that do not admit critical care patients or operate an adult or pediatric general medical
and/or surgical ICU or neuro ICU should select “No” in question #2 and move on to the
Affirmation of Accuracy. Your hospital’s results will be displayed as “Does Not Apply” on
Leapfrog’s public reporting website.
Hospitals that regularly admit critical care patients to a non-critical care unit or mixed acuity unit,
should select “Yes” in question #2 and respond to all applicable questions. The standard applies
to all adult and pediatric general medical and/or surgical critical care patients and neuro critical
care patients (medical and surgical), including those patients in non-critical care and mixed acuity
units.
Section 5 includes questions about the management of critical care patients and the staffing structure of
your hospital’s pediatric and adult general medical and/or surgical ICUs and neuro ICUs.
Each hospital achieving the standard for ICU Physician Staffing assures that:
All critical care patients
33
in its adult or pediatric general medical and/or surgical ICUs and neuro ICUs
34
are managed or co-managed
35
by physicians certified in critical care medicine
36
who:
• Are ordinarily present in the ICU
37
during daytime hours for at least 8 hours per day, 7 days per week,
and during this time provide clinical care exclusively
37
in the ICU
OR
• Are present via telemedicine
38
, in combination with on-site intensivist coverage, for a total of 24 hours
per day, 7 days per week; meet all of Leapfrog’s ICU requirements for intensivist presence in the ICU
via telemedicine; and supported by an on-site intensivist who establishes and revises the daily care
plan for each ICU patient, and
• At other times*
• Return more than 95% of ICU calls within 5 minutes, based on a quantified analysis
39
of
notification device response time; and
• Can rely on a physician, physician assistant, nurse practitioner
42
, or an FCCS-certified nurse
“effector”
40
who is in the hospital and able to reach ICU patients within 5 minutes in more than
95% of cases, based on a quantified hospital analysis of response time of the effector reaching
the patient.
*Not applicable for hospitals with 24/7 intensivist coverage.
Download the 2023 Leapfrog Hospital Survey Scoring Algorithm on the
Scoring and Results
webpage.

2023 Leapfrog Hospital Survey – Hard Copy Section 5: ICU Physician Staffing (IPS)
172 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
5: ICU Physician Staffing (IPS)
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Notes:
Note 1: Each of the endnotes referenced in the questions below must be reviewed before responding to
each question.
Note 2: Some intensivist “presence” may be accomplished via tele-intensivists per Leapfrog’s
specifications (More Information
38
). However, at this time hospitals cannot achieve the standard through
the sole use of tele-intensivists.
Note 3: On an interim basis, other categories of physicians may be considered by Leapfrog to be
“certified in Critical Care Medicine” (More Information
36
).
Note 4: The standard applies to all adult and pediatric general medical and/or surgical critical care
patients and neuro critical care patients (medical and surgical), including those patients in non-critical
care and mixed acuity units.
1) What is the latest 3-month reporting period for which your
hospital is submitting responses to this section? 3 months
ending:
______
Format: Month/Year
2) Does your hospital operate any adult or pediatric general
medical and/or surgical ICUs or neuro ICUs
34
?
If your hospital has more than one applicable ICU, respond to all
questions in this section based on the ICU that has the lowest
level of staffing by physicians certified in critical care medicine
(
More Information
34
).
If your hospital does not operate an applicable ICU but regularly
admits critical care patients to non-critical care or mixed acuity
units, select “yes” and respond to the remaining questions in
Section 5.
If “no” to question #2, skip the remaining questions in Section 5
and go to the Affirmation of Accuracy. The hospital will be scored
as “Does Not Apply.”
Yes
No
Reporting Period: 3 months
Answer questions #1-15 based on the staffing structure currently in place at the time that you submit
this section of the Survey. The staffing structure should have been in place for at least the past 3
months and reflect the ordinary staffing structure for each applicable ICU.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 5: ICU Physician Staffing (IPS)
173 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
3) Is the ICU staffed with physicians who are certified in critical care
medicine
36
and present on-site or via telemedicine?
If “no” to question #3, skip the remaining questions in Section 5
and go to the Affirmation of Accuracy. This hospital will be
scored as “Limited Achievement.”
Yes, the ICU is staffed with
physicians certified in critical
care medicine
Yes, the ICU is staffed with
physicians certified in critical
care medicine based on
Leapfrog’s expanded definition
No, the ICU is not staffed with
any physicians certified in
critical care medicine
4) Do the physicians who are certified in critical care medicine
36
(whether present on-site or via telemedicine) manage or co-
manage
all critical care patients
33
in the ICU?
If “no” to question #4, skip questions #5-11 and continue to
question #12.
Yes, all patients are managed
or co-managed by a physician
certified in critical care
medicine when the physician is
present (on-site or via
telemedicine)
No, not all patients are
managed or co-managed by a
physician certified in critical
care medicine when the
physician is present (on-site or
via telemedicine)
There are currently two different options to achieve Leapfrog’s ICU Physician Standard: on-site intensivist
coverage for 8 hours a day/7 days per week or 24/7 tele-intensivist coverage with some daily on-site
intensivist coverage. Questions #5 and #6 are meant to differentiate between these two options; however,
they are both worth the same credit. Hospitals that have 24/7 on-site intensivist coverage, and who meet
all the criteria listed, should respond “yes” to question #5.
5) Are all critical care patients
33
in the ICU managed or co-managed
35
by one
or more physicians certified in critical care medicine
36
who meet all of the
following criteria:
• ordinarily present
37
on-site in the ICU during daytime hours
• for at least 8 hours per day, 7 days per week
• providing clinical care exclusively
37
in the ICU during these hours?
If “yes” to question #5, skip question #6 and continue to question #7.
Yes
No
6) Are all critical care patients
33
in the ICU managed or co-managed
35
by one
or more physicians certified in critical care medicine
36
who meet all of the
following criteria:
• present via telemedicine, in combination with on-site intensivist
coverage, for a total of 24 hours per day, 7 days per week
• meet all of Leapfrog’s ICU requirements for intensivist presence in
the ICU via telemedicine (More Information
38
)
• supported by an on-site intensivist who establishes and revises the
daily care plan for each ICU patient?
Yes
No
.
If “no” to question #5 and question #6, skip questions #7-8 and continue to question #9.
7) When the physicians (from question #3) are not present in the ICU on-site or
via telemedicine, do they return more than 95% of calls/pages/texts from
these units within five minutes, based on a quantified analysis
39
of
notification device response time? (More information on the use of
telemedicine to cover calls
41
)
Yes
No
Not applicable;
intensivists are
present on-site 24/7

2023 Leapfrog Hospital Survey – Hard Copy Section 5: ICU Physician Staffing (IPS)
174 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
8) When the physicians (from question #3) are not present on-site in the ICU or
not able to physically reach an ICU patient within 5 minutes, can they rely on
a physician, physician assistant, nurse practitioner
42
, or FCCS-certified
nurse or intern “effector”
40
who is in the hospital and able to reach these ICU
patients within five minutes in more than 95% of the cases, based on a
quantified analysis
39
of response time of the effector reaching the patient?
Yes
No
Not applicable;
intensivists are
present on-site 24/7
If “no” to either question #7 or #8 in this section, continue to questions #9-15. If “yes” or “not applicable;
intensivists are present on-site 24/7” to questions #7 and #8, skip the remaining questions in Section 5
and go to the Affirmation of Accuracy.
9) Are all critical care patients
33
in the ICU managed or co-managed
35
by one
or more physicians certified in critical care medicine
36
who meet all of the
following criteria:
• ordinarily present
37
on-site in the ICU during daytime hours
• for at least 8 hours per day, 4 days per week or 4 hours per day, 7
days per week
• providing clinical care exclusively
37
in the ICU during these hours?
Yes
No
10) Are all critical care patients
33
in the ICU managed or co-managed
35
by one
or more physicians certified in critical care medicine
36
who meet all of the
following criteria:
• present via telemedicine for 24 hours per day, 7 days per week
• meet all of Leapfrog’s modified ICU requirements for intensivist
presence in the ICU via telemedicine (More Information
43
)
• supported in the establishment and revision of daily care planning for
each ICU patient by an on-site intensivist, hospitalist, anesthesiologist,
or physician trained in emergency medicine?
Yes
No
11) Are all critical care patients
33
in the ICU managed or co-managed
35
by one
or more physicians certified in critical care medicine
36
who are:
• on-site at least 4 days per week to establish or revise daily care plans
for each critical care patient in the ICU?
Yes
No
If “yes” to question #9, #10, or #11, skip question #12 and continue to question #13.
12) If not all critical care patients
33
are managed or co-managed
35
by physicians
certified in critical care medicine
36
, either on-site or via telemedicine
38
, are
some critical care patients managed or co-managed by these physicians
who are:
• ordinarily present
37
on-site in the ICU during daytime hours
• for at least 8 hours per day, 4 days per week or 4 hours per day, 7
days per week
• providing clinical care exclusively
37
in the ICU during these hours?
Yes
No
13) Does an on-site clinical pharmacist do all the following:
• at least 5 days per week, makes daily on-site rounds on all critical care
patients
33
in the ICU
• on the other 2 days per week, returns more than 95% of
calls/pages/texts from the unit within 5 minutes, based on a
quantified
analysis
39
of notification device response time
OR
• makes daily on-site rounds on all critical care patients
33
in the ICU 7
days per week?
Yes
No
Clinical pharmacist
rounds 7 days per
week

2023 Leapfrog Hospital Survey – Hard Copy Section 5: ICU Physician Staffing (IPS)
175 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
14) Does a physician certified in critical care medicine
36
lead daily
interprofessional rounds on-site on all critical care patients
33
in the ICU 7
days per week?
Yes
No
15) Are physicians certified in critical care medicine
36
responsible for all ICU
admission and discharge decisions when they are:
• present on-site for at least 8 hours per day, 4 days per week or 4
hours per day, 7 days per week?
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy Section 5: ICU Physician Staffing (IPS)
176 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Affirmation of Accuracy:
As the hospital CEO or as an employee of the hospital to whom the hospital CEO has delegated
responsibility, I have reviewed this information pertaining to the ICU Physician Staffing Section at our
hospital, and I hereby certify that this information is true, accurate, and reflects the current, normal
operating circumstances at our hospital. I am authorized to make this certification on behalf of our
hospital.
The hospital and I understand that The Leapfrog Group, its members, the public and entities and persons
who contract or have other business dealings with The Leapfrog Group are relying on the truth and
accuracy of this information. The hospital and I also understand that The Leapfrog Group will make this
information and/or analyses of this information public through the Survey Results public reporting website,
The Leapfrog Group’s Hospital Safety Grade, and/or other Leapfrog Group products and services. This
information and/or analyses and all intellectual property rights therein shall be and remain the sole and
exclusive property of The Leapfrog Group in which The Leapfrog Group retains exclusive ownership. This
information does not infringe upon any third-party intellectual property rights or any other third-party rights
whatsoever and is free and clear of all encumbrances and liens of any kind. The hospital and I
acknowledge that The Leapfrog Group may use this information in a commercial manner for profit. The
hospital shall be liable for and shall hold harmless and indemnify The Leapfrog Group from any and all
damages, demands, costs, or causes of action resulting from any inaccuracies in the information or any
misrepresentations in this Affirmation of Accuracy. The Leapfrog Group and its members and entities and
persons who contract or have other business dealings with The Leapfrog Group reserve the right to omit
or disclaim information that is not current, accurate or truthful.
Affirmed by _____________________, the hospital’s ___________________________,
(first name and last name) (title)
on _______________________.
(date)

2023 Leapfrog Hospital Survey – Hard Copy Section 5: ICU Physician Staffing (IPS)
177 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 5: 2023 ICU Physician Staffing (IPS) Reference Information
What’s New in the 2023 Survey
Based on feedback from hospitals, Leapfrog is updating the wording of several questions in this section to
increase clarity regarding the criteria to respond “yes.”
First, question #3, which asks if physicians certified in critical care are managing or co-managing all
critical care patients in applicable intensive care units (ICUs), is being separated into two questions.
Second, we are updating questions #11 and #14 to specify the minimum number of hours required for
intensivist coverage.
Next, for hospitals that operate more than one type of ICU included in Leapfrog’s standard (adult or
pediatric general medical and/or surgical ICUs or neuro ICUs) with varying staffing models, Leapfrog is
adding a note to remind hospitals that they should respond to questions in this section based on the ICU
that has the lowest level of staffing by physicians certified in critical care medicine as described in
endnote #34. Additionally, we are updating the phrasing throughout the section to reference the ICU that
has the lowest level of staffing by physicians certified in critical care medicine more clearly.
While there are no changes to the scoring algorithm for Section 5: ICU Physician Staffing (IPS), Leapfrog
is updating question references in the scoring algorithm to reflect the updated question numbering
described above.
Change Summary Since Release
June 13, 2023 – Updated endnote #36 Certified in Critical Care Medicinef to reflect updated pathway to
certification offered by ABNS for neurointensivists. Please review updated endnote #36 on pages 311-
312 of the Survey.

2023 Leapfrog Hospital Survey – Hard Copy Section 5: ICU Physician Staffing (IPS)
178 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
ICU Physician Staffing (IPS) Frequently Asked Questions (FAQs)
General Questions
1. What is the reporting period for this measure?
Hospitals should report on Section 5 based on their staffing structure at the time they submit this
section of the Survey. The staffing structure must have been in place for at least the past 3
months and should reflect the ordinary staffing structure for the ICU.
2. How should hospitals report if they have more than one type of qualifying ICU?
If your hospital has more than one applicable ICU, respond to all questions in this section based
on the ICU that has the lowest level of staffing by physicians certified in critical care medicine.
Review endnote 34
for more information.
3. Does Leapfrog’s IPS standard apply to mixed acuity units? A multi-organizational service
unit (MOSU) unit?
The standard applies to all adult and pediatric general medical and/or surgical critical care
patients and neuro critical care patients (medical and surgical), including those patients in mixed
acuity units and other non-critical care units.
5. Are cardiac critical care patients in a general medical and/or surgical ICU required to be
co-managed by an intensivist?
Cardiac critical care patients in a general medical or surgical ICU that are in beds dedicated to
cardiac critical care patients and being cared for by dedicated cardiac physicians (e.g.,
cardiologist or cardiac surgeon) and nursing staff are not included in Leapfrog's ICU Physician
Staffing standard and do not need to be managed/co-managed by the intensivist. However,
cardiac critical care patients in a general medical or surgical ICU bed being cared for by general
medical or surgical critical care physicians and nursing staff are included in the standard and do
need to be managed/co-managed by the intensivist. Additional clarification is available
here.
Certification
5. Is there any empirical basis for specifying a minimum annual number of days of ICU
experience for each board-eligible physician providing ICU care?
No. Accordingly, if it is added to the Leapfrog standard in the future, it will be based on newly
published research and expert advice.
6. Do all intensivists serving as tele-intensivists need to meet Leapfrog’s definition of
“certified in critical care medicine”?
Yes. All intensivists who serve as tele-intensivists do need to meet Leapfrog’s definition
of
“certified in critical care medicine.”
7. Is a physician who is awaiting the results of the certification test considered “certified in
critical care medicine”?
A physician is considered certified in critical care medicine as long as the board that originally
certified the physician in critical care medicine deems that physician certified. Intensivists that are
close to having their certification lapse should check with the board that certified them to see how
exactly that board defines a lapsed certification.
8. How should intensivists trained in critical medicine in a foreign country be treated for
purposes of meeting the ICU Physician Staffing (IPS) Standard?
Foreign trained physicians who were certified in critical care medicine in the country in which they
trained would be considered certified for the purposes of the ICU Physician Staffing (IPS)
standard.

2023 Leapfrog Hospital Survey – Hard Copy Section 5: ICU Physician Staffing (IPS)
179 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Telemedicine
9. Can you clarify what you mean by combined presence of tele-intensivists and on-site
intensivists, as needed, for 24 hours per day/7 days per week in question #6?
Hospitals can achieve Leapfrog’s IPS Standard by having daily on-site intensivist coverage
combined with a telemedicine service that meets all ten of the Leapfrog requirements
. For
example, an on-site intensivist takes two hours in the morning to establish and revise the daily
care plan for each ICU patient, then a tele-intensivist picks up management or co-management of
the ICU patients and is continually monitoring all critical care patients for the remaining 22 hours,
for a combined total of 24 hours per day/7 days per week.
Hospitals should minimize the number of hand-offs
between the on-site intensivist and the tele-
intensivist during a 24-hour period and maximize the continuous time that one type of intensivist
is caring for critical care patients. Leapfrog does not recommend more than 4 hand-offs per
24 hours.
10. Our hospital uses a telemedicine service to provide coverage in our ICU for 16 hours/7
days per week coverage when the on-site intensivist is not present at the hospital. Can our
hospital still fully meet Leapfrog’s standard?
Hospitals that use telemedicine to cover “call” for the on-site intensivist are able to fully meet
Leapfrog’s standard if: (1) the telemedicine service meets all ten of the Leapfrog requirements
;
and (2) the hospital has an “effector” (physician/PA/NP/FCCS-certified nurse or intern) on-site
during that time period to carry out the tele-intensivist’s orders and can reach the ICU patient
within 5 minutes, 95% of the time.
Response Time
11. Does Leapfrog specify standards for second tier calls (e.g., the initial call to a physician is
not answered within 5 minutes)?
No. We do not intend to reach this level of detail in our specifications, absent a compelling case
that the gain would offset its added complexity.
12. Are there definitions for what constitutes high and low urgency calls/pages/texts?
No, and hospitals don’t have to focus only on high urgency calls/pages/texts, but some
notification device systems can make this differentiation, and, in these instances, low urgency
calls/pages/texts can be excluded from the analysis of response times.
13. If my hospital has few instances where there is no intensivist coverage, how should we
conduct the response time audit? Can we perform mock pages to satisfy the intent?
Unannounced mock pages would meet the intent. For the audit to be reliable, 20 unannounced
mock pages over 90 days should be evaluated.
14. When an intensivist is not on-site in the ICU, can hospitals use a non-FCCS-certified CRNA
as the “effector?”
No. To serve as the “effector,” CRNAs require FCCS-certification.
15. When an intensivist is not on-site in the ICU, can hospitals use FCCS-certified interns as
the “effector?”
Yes. An FCCS-certified intern can serve as the “effector.”
Rounding
16. How does Leapfrog define “makes on-site daily rounds” for clinical pharmacists in
question #13?
A clinical pharmacist would need to make on-site daily rounds (5 or 7 days per week) on all
critical care patients (not some or most critical care patients) in all applicable ICUs (adult or
pediatric general medical and/or surgical ICUs and neuro ICUs).

2023 Leapfrog Hospital Survey – Hard Copy Section 5: ICU Physician Staffing (IPS)
180 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Please note that if your clinical pharmacist is rounding 5 days per week, then, during the other 2
days per week, a clinical pharmacist still needs to respond to calls/texts/pages within 5 minutes,
as determined by a response time audit.
17. Are pharmacy residents considered clinical pharmacists in question #13?
To be considered a clinical pharmacist, Leapfrog only requires that the pharmacist have a Doctor
of Pharmacy (PharmD) degree. Therefore, a PharmD graduate completing a residency would
meet Leapfrog’s criterion to be considered a clinical pharmacist.
18. What roles should be included in interprofessional rounds in question #14?
For rounds to be considered interprofessional, the team should include 3 or more persons.
Typical personnel that would be part of the rounding team include physician, nurse, pharmacist,
physical and/or occupational therapist, and nutritionist.

2023 Leapfrog Hospital Survey – Hard Copy
182 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
SECTION 6: PATIENT SAFETY PRACTICES
This section includes questions and reference information for Section 6: Patient Safety Practices. Please
carefully review the questions, endnotes, and reference information (e.g., measure specifications, notes,
and frequently asked questions) before you begin. Failure to review the reference information could result
in inaccurate responses.

2023 Leapfrog Hospital Survey – Hard Copy
183 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 6: 2023 Patient Safety Practices
NQF Safe Practices Fact Sheets:
https://ratings.leapfroggroup.org/measure/hospital/2023/effective-
leadership-prevent-errors and https://ratings.leapfroggroup.org/measure/hospital/2023/staff-work-
together-prevent-errors
Hand Hygiene Fact Sheet and Bibliography:
https://ratings.leapfroggroup.org/measure/hospital/2023/handwashing
Nursing Workforce Fact Sheet and Bibliography:
https://ratings.leapfroggroup.org/measure/hospital/2023/nursing-workforce
Section 6 includes questions about your hospital’s adherence to two National Quality Forum-endorsed
Safe Practices, questions about your hospital’s nursing staffing and skill mix, and questions about hand
hygiene practices. In 2023, the subsection on nurse staffing and skill mix (6C) will be scored and publicly
reported.
Each hospital achieving the standard for NQF Safe Practice #1 – Culture of Safety Leadership
Structures and Systems and NQF Safe Practice #2 – Culture Measurement, Feedback, and
Intervention:
Has earned 100% of points (adopted all elements) for the NQF Safe Practice.
Each hospital achieving the standard for Total Nursing Care Hours per Patient Day:
Has a ratio of total nursing care hours per patient day that is greater than or equal to the 50th percentile
(where higher is better) for that hospital’s cohort (small teaching, large teaching, non-teaching, pediatric,
or critical access hospital).
Each hospital achieving the standard for RN Hours per Patient Day:
Has a ratio of RN hours per patient day that is greater than or equal to the 50th percentile (where higher
is better) for that hospital’s cohort (small teaching, large teaching, non-teaching, pediatric, or critical
access hospital).
Each hospital achieving the standard for Nursing Skill Mix:
Has a percentage of total productive nursing hours worked by RN nursing staff that is higher than or
equal to the 50
th
percentile (where higher is better) for the hospital’s cohort (small teaching, large
teaching, non-teaching, pediatric, or critical access hospitals).
Each hospital achieving the standard for Percentage of RNs who are BSN-Prepared:
Has a percentage of RNs who are BSN-prepared that is greater than or equal to 80%.
Each hospital achieving the standard for Hand Hygiene:
Has met all elements for the Monitoring domain including collecting compliance data on at least 200 hand
hygiene opportunities* each month in each patient care unit. Has also met all elements for the Feedback
domain, as well as 2 of the 3 remaining domains for hand hygiene:
• Training and Education Domain
• Infrastructure Domain
• Culture Domain
OR
Has met all elements for the Monitoring domain including collecting compliance data on at least 100 hand
hygiene opportunities* each month in each patient care unit, as well as all 4 remaining domains for hand
hygiene:
• Feedback Domain
• Training and Education Domain
• Infrastructure Domain
• Culture Domain

2023 Leapfrog Hospital Survey – Hard Copy
184 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
*or at least the number of hand hygiene opportunities outlined based on the unit type in Tables 1-3 or
Tables 4-6.
Download the 2023 Leapfrog Hospital Survey Scoring Algorithm on the Scoring and Results
webpage.
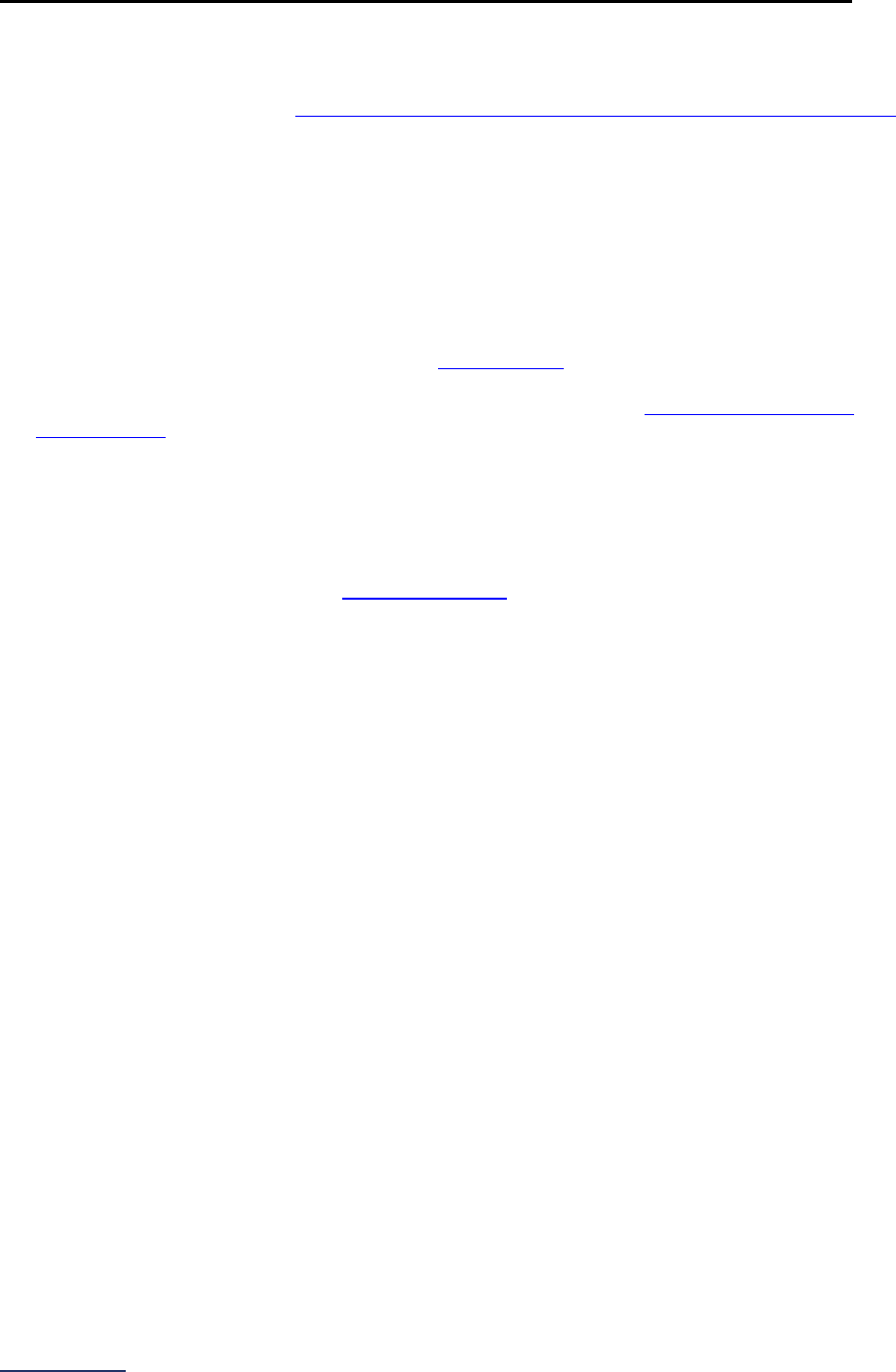
2023 Leapfrog Hospital Survey – Hard Copy
185 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Instructions for Reporting on Section 6: Patient Safety Practices
1. Prepare:
a. Download and review a copy of the National Quality Forum’s Safe Practices for Better
Healthcare – 2010 Update report for reporting on subsections 6A, 6B, and NQF Safe Practice
#9 in 6C (see link on http://leapfroggroup.org/survey-materials/survey-and-cpoe-materials
).
b. Print and review a hard copy of (1) the Survey questions, (2) the practice-specific FAQs, and
(3) the scoring algorithm.
2. Identify Individuals to Assist: Decide who should participate on your team to assist in collection of
the documentation for assessment.
3. Plan: The team should be briefed and assigned duties to help capture the key information necessary
for submission of this section.
4. Collect and Maintain: Key documentation must be collected to support answering the questions in
this section of the Survey. Please refer to the Survey Binder
for examples of acceptable
documentation. Documentation should be maintained to ensure that your hospital can respond to
Leapfrog’s request for documentation should you be selected for our Monthly Documentation
Requirements. Reviews are performed every month during the Survey Cycle (April 1 to November 30)
and throughout the Corrections Period). In addition, the documentation can be helpful if your hospital
is planning to update and resubmit this section of the Survey prior to November 30.
5. Assess: When all the supporting documents are assembled, it is recommended that hospitals review
their final responses to Section 6 with the CEO and/or responsible leadership. Hospitals should
update their answers online as they adopt additional practices throughout the Survey Cycle (April 1 to
November 30). As a reminder, the Corrections Period
(December 1-January 31) is reserved for
corrections to previously submitted Surveys only. Any updates made to reflect a change in
performance must be made prior to the November 30 Late Submission and Performance Update
Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.
6. Submit: Section 6 must be completed and affirmed before it can be submitted with the Survey.
Important Note: In the Online Hospital Survey Tool, make sure to click the “Review of this Practice
Complete” checkbox at the bottom of each safe practice (subsections 6A-6B only) even if no items
are checked, to mark the Safe Practice as complete. This checkbox must be checked for NQF-endorsed
Safe Practices #1 and #2 in order to affirm Section 6 in the Online Hospital Survey Tool.

2023 Leapfrog Hospital Survey – Hard Copy
186 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
6A: NQF Safe Practice #1 – Culture of Safety Leadership Structures
and Systems
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Notes:
Note 1: Page numbers throughout this subsection refer to the
NQF Safe Practices for Better Healthcare –
2010 Update report, not this document.
Note 2: Hyperlinks throughout this subsection refer to the Patient Safety Practices FAQs
beginning on
page 213, not to endnotes. These hyperlinks are not included in the Online Survey Tool.
Check all boxes that apply.
1.1
Within the last 12 months, in regard to raising the awareness of key stakeholders to our
organization’s efforts to improve patient safety, the following actions related to the
identification and mitigation of risks and hazards have been taken:
AWARENESS
a
board (governance) minutes reflect regular communication regarding all three of the
following:
• risks and hazards (as defined by NQF Safe Practice #4 – Identification and Mitigation
of Risks and Hazards);
• culture measurement (as defined by NQF Safe Practice #2 – Culture Measurement,
Feedback, and Intervention); and,
• progress towards resolution of safety and quality problems. (p.75)
b
patients and/or families of patients are active participants in the hospital-wide safety and
quality committee that meets on a regularly scheduled basis (e.g., biannually or quarterly).
(p.75)
c
steps have been taken to report ongoing efforts to improve safety and quality in the
organization and the results of these efforts to the community. (p.75)
d
all staff and independent practitioners were made aware of ongoing efforts to reduce risks
and hazards and to improve patient safety and quality in the organization. (p.75)
1.2
Within the last 12 months, in regard to holding the board, senior administrative leadership,
midlevel management, nursing leadership, physician leadership, and frontline caregivers
directly accountable for results related to the identification and mitigation of risks and
hazards, the organization has done the following:
ACCOUNTABILITY
a
an integrated patient safety program has been in place for the entire reporting period,
providing oversight and alignment of safe practice activities. (p.76)
b
a Patient Safety Officer (PSO) has been appointed and communicates regularly with the
board (governance) and senior administrative leadership; the PSO is the primary point of
contact of the integrated patient safety program. (p.76)
c
performance has been documented in performance reviews and/or compensation incentives
for all levels of hospital management and hospital-employed caregivers noted above. (p.76)

2023 Leapfrog Hospital Survey – Hard Copy
187 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
d
the interdisciplinary patient safety team communicated regularly with senior administrative
leadership regarding both of the following and documented these communications in
meeting minutes (pp. 76-77):
• progress in meeting safety goals; and
• provide team training
to caregivers.
e
the hospital reported adverse events to external mandatory or voluntary programs. (p.77)
1.3
Within the last 12 months, in regard to implementation of the patient safety program, the
board (governance) and senior administrative leadership have provided resources to cover
the implementation as evidenced by:
ABILITY
a
dedicated patient safety program budgets to support the program, staffing, and technology
investment. (p.77)
1.4
Within the last 12 months, structures and systems have been in place to ensure that senior
administrative leadership is taking direct action, as evidenced by:
ACTION
a
CEO and senior administrative leadership are personally engaged in reinforcing patient
safety improvements, e.g., “walk-arounds,” and reporting to the board (governance).
Calendars reflect allocated time. (p.78)
b
CEO has actively engaged leaders from service lines, midlevel management, nursing
leadership, and physician leadership in patient safety improvement actions. (p.79)
c
hospital has established a structure for input into the patient safety program by licensed
independent practitioners and the organized medical staff and physician leadership. Input
documented in meeting minutes or materials. (p.79)
1.5
Review of this Safe Practice is complete.
This checkbox is in the Online Hospital Survey Tool to ensure that your hospital has
reviewed the data entry for the above questions. This question must be marked, even if no
items are checked.

2023 Leapfrog Hospital Survey – Hard Copy
188 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
6B: NQF Safe Practice #2 – Culture Measurement, Feedback, and
Intervention
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Notes:
Note 1: Page numbers throughout this subsection refer to the
NQF Safe Practices for Better Healthcare –
2010 Update report, not this document.
Note 2: Hyperlinks throughout this subsection refer to the Patient Safety Practices FAQs
beginning on
page 213, not to endnotes. These hyperlinks are not included in the Online Survey Tool.
Note 3: Throughout this subsection, the culture of safety survey referenced in each practice element
refers to one administered in the last 24 months as indicated in 2.1a. This means that the same culture of
safety survey should be used when responding to each practice element.
Check all boxes that apply.
2.1
Within the last 24 months, in regard to culture measurement, our organization has done the
following:
AWARENESS
a
conducted a culture of safety survey of our employees using a nationally recognized tool that
has demonstrated validity, consistency, and reliability. The units surveyed account for at least
50% of the aggregated care delivered to patients within the hospital and include the high
patient safety risk units or departments
. (p.88)
If item ‘a’ is not checked, no other items in NQF Safe Practice #2 may be checked.
b
portrayed the results of the culture of safety survey in a report, which reflects both hospital-
wide and individual unit level results, as applicable. (p.88)
c
benchmarked results of the culture of safety survey against external organizations, such as
“like” hospitals or other hospitals within the same health system.
d
compared results of the culture of safety surveys across roles and staff levels.
e
service line, midlevel managers, or senior administrative leaders used the results of the
culture of safety survey to debrief at the relevant unit level, using semi-structured approaches
for the debriefings and presenting results in aggregate form to ensure the anonymity of
survey respondents.
2.2
Within the last 24 months, in regard to accountability for improvements in culture
measurement, our organization has done the following:
ACCOUNTABILITY
a
shared the results of the culture of safety survey with the board (governance) and senior
administrative leadership in a formal report and discussion. (p.88)
b
included in performance evaluation criteria for senior administrative leadership both the
response rates to the culture of safety survey and the use of the culture of safety survey
results in the improvement efforts.

2023 Leapfrog Hospital Survey – Hard Copy
189 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
2.3
Within the last 12 months, in regard to culture measurement, the organization has done the
following (or has had the following in place):
ABILITY
a
conducted staff education program(s) on methods to improve the culture of safety, tailored to
the organization’s culture of safety survey results.
b
included the costs of culture measurement/follow-up activities in the patient safety program
budget.
2.4
Within the last 12 months, in regard to culture measurement, feedback, and interventions, our
organization has done the following (or has had the following in place):
ACTION
a
developed or implemented explicit, hospital-wide organizational policies and procedures for
regular culture measurement. (p.88)
b
disseminated the results of the culture of safety survey widely across the institution, and
senior administrative leadership held follow-up meetings with the sampled units to discuss
the unit’s results and concerns. (p.88)
c
identified performance improvement interventions based on the culture of safety survey
results, which were shared with senior administrative leadership and subsequently measured
and monitored. (p.88)
2.5
Review of this Safe Practice is complete.
This checkbox is in the Online Hospital Survey Tool to ensure that your hospital has reviewed
the data entry for the above questions. This question must be marked, even if no items are
checked.

2023 Leapfrog Hospital Survey – Hard Copy
190 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
6C: Nursing Workforce
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Specifications: See Nursing Workforce Measure Specifications
in the Reference Information
beginning on page 201.
Nurse Staffing and Skill Level
1) 12-month reporting period used:
01/01/2022 – 12/31/2022
07/01/2022 – 06/30/2023
2) Does your hospital submit data on Total Nursing Care Hours
per Patient Day, RN Hours per Patient Day, Nursing Skill Mix,
and/or Percentage of RNs who are BSN-Prepared to any of
the following nursing databases?
Select all that apply.
National Database of Nursing
Quality Indicators (NDNQI)
PatientCareLink
Other
None of the above
3) Did your hospital calculate the Total Nursing Care Hours per
Patient Day, RN Hours per Patient Day, and Nursing Skill Mix
measures for the reporting period, and do you choose to
report those data to this Survey?
If “no” to question #3, skip questions #4-10 and continue to
question #11. The hospital will be scored as “Limited
Achievement.”
Yes
No
4) Which method did your hospital use to calculate the total
number of patient days for each medical, surgical and/or med-
surg unit?
Midnight census
Patient days from actual hours
Patient days from multiple census
reports
Midnight census and patient days
from actual hours for short stay
patients
Other
Total Nursing Care Hours per Patient Day, RN Hours per Patient Day, and Nursing Skill
Mix
5) Does your hospital operate any adult and/or pediatric Medical
Units (excluding step-down and progressive units)?
If “no” to question #5, skip question #6 and continue to
question #7.
Yes
No
Reporting Period: 12 months
• Survey submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys submitted on or after September 1:
o 07/01/2022 – 06/30/2023
Note: As a reminder, the Corrections Period
(December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy
191 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
6) Enter your hospital’s responses below by quarter across all adult and/or pediatric Medical Units
(excluding step-down and progressive units) for the reporting period selected in question #1:
(a) Total number of
patient days:
(b) Total number of
productive hours worked by
employee or contract
nursing staff with direct
patient care responsibilities
(RN, LPN/LVN, and UAP):
(c) Total number of
productive hours worked by
RN nursing staff with direct
patient care responsibilities:
Quarter 1
Quarter 2
Quarter 3
Quarter 4
7) Does your hospital operate any adult and/or pediatric
Surgical
Units (excluding step-down and progressive units)?
If “no” to question #7, skip question #8 and continue to
question #9.
Yes
No
8) Enter your hospital’s responses below by quarter across all adult and/or pediatric
Surgical Units
(excluding step-down and progressive units) for the reporting period selected in question #1:
(a) Total number of
patient days:
(b) Total number of
productive hours worked
by employee or contract
nursing staff with direct
patient care responsibilities
(RN, LPN/LVN, and UAP):
(c) Total number of productive
hours worked by RN nursing
staff with direct patient care
responsibilities:
Quarter 1
Quarter 2
Quarter 3
Quarter 4
9) Does your hospital operate any adult and/or pediatric
Med-
Surg Units (excluding step-down and progressive units)?
If “no” to question #9, skip question #10 and continue to
question #11.
Yes
No
10) Enter your hospital’s responses below by quarter across all adult and/or pediatric
Med-Surg Units
(excluding step-down and progressive units) for the reporting period selected in question #1:
(a) Total number of
patient days:
(b) Total number of
productive hours worked
by employee or contract
nursing staff with direct
patient care responsibilities
(RN, LPN/LVN, and UAP):
(c) Total number of productive
hours worked by RN nursing
staff with direct patient care
responsibilities:
Quarter 1
Quarter 2
Quarter 3
Quarter 4

2023 Leapfrog Hospital Survey – Hard Copy
192 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Percentage of RNs who are BSN-Prepared
11) Did your hospital calculate the Percentage of RNs who are
BSN-Prepared measure for the reporting period, and do you
choose to report those data to this Survey?
If “no” to question #11, skip questions #12-13 and continue to
question #14. The hospital will be scored as “Limited
Achievement.”
Yes
No
12) Total number of employed RN nursing staff at the hospital with
direct patient care responsibilities:
______
13) Total number of employed RN nursing staff at the hospital with
direct patient care responsibilities who have a BSN degree or
higher (e.g., MSN, DNP, PhD):
______
NQF Safe Practice #9 – Nursing Workforce
Important Note: Hyperlinks in question #15 refer to the Patient Safety Practices FAQs
beginning on page
213, not to endnotes. These hyperlinks are not included in the Online Survey Tool.
14) Is your hospital currently recognized as an
American Nurses Credentialing Center (ANCC)
Magnet
®
organization or a 2020 Pathway to
Excellence
®
organization?
If “yes, our hospital is a current American
Nurses Credentialing Center (ANCC) Magnet
®
organization” or “yes, our hospital is a 2020
Pathway to Excellence
®
organization,” skip
question #15, and continue to the next
subsection.
Pathway to Excellence
®
hospitals that received
the designation prior to 2020 must select “No.”
Yes, our hospital is a current American Nurses
Credentialing Center (ANCC) Magnet
®
organization
Yes, our hospital is a 2020 Pathway to
Excellence
®
organization
No
15) Within the last 12 months, to ensure adequate and competent nursing staff service and nursing
leadership at all levels, our organization has:
a. held nursing leadership directly
accountable for improvements in
performance through performance
reviews or compensation.
Yes
No
b. included nursing leadership as part of
the hospital senior administrative
leadership team.
Yes
No
Reporting Period: 12 months
Answer questions #11-13 based on the most recent day within the last 12-months for which you have
complete data.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy
193 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
c. held the board (governance) and senior
administrative leadership accountable
for the provision of financial resources
to ensure adequate nurse staffing
levels.
Yes
No
d. budgeted financial resources for
balancing staffing levels and skill levels
to improve performance.
Yes
No
e. developed a staffing plan, with input
from nurses, to ensure that adequate
nursing staff-to-patient ratios are
achieved.
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy
194 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
6D: Hand Hygiene
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Notes:
Note 1: Question #22 will not be scored or publicly reported.
Note 2: Hyperlinks not followed by a superscript throughout this subsection refer to the
Patient Safety
Practices FAQs beginning on page 213. These hyperlinks are not included in the Online Survey Tool.
Note 3: The framework and questions in this subsection are modeled after the World Health
Organization’s Hand Hygiene Self-Assessment Framework
.
Note 4: Hospital responses should reflect patient care units
only, including all inpatient units, outpatient
units (pre-operative and post-operative), observation units, and emergency department units. Only
include outpatient locations that share your hospital’s license and/or CMS Certification Number (CCN).
Specifications: See Hand Hygiene Measure Specifications
in the Reference Information beginning on
page 205.
Training and Education
1) Do individuals who touch patients or who touch items that will
be used by patients
44
in your patient care units receive hand
hygiene training from a professional with appropriate training
and skills
45
at both:
• the time of onboarding, and
• annually thereafter?
If “no” to question #1, skip questions #2-3 and continue to
question #4.
Yes
No
2) In order to pass the initial hand hygiene training, do
individuals who touch patients or who touch items that will be
used by patients
44
in your patient care units need to physically
demonstrate proper hand hygiene with soap and water and
alcohol-based hand sanitizer?
Yes
No
3) Are all six of the following topics included in your hospital’s
initial and annual hand hygiene training?
• Evidence linking hand hygiene and infection
prevention
• When
individuals who touch patients or who touch
items that will be used by patients
44
should perform
hand hygiene (e.g., WHO's 5 Moments for Hand
Hygiene, CDC’s Guideline for Hand Hygiene)
• How individuals who touch patients or who touch
items that will be used by patients
44
should clean their
Yes
No
Reporting Period: Answer questions #1-22 based on the practices currently in place at the time you
submit this section of the Survey.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy
195 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
hands with alcohol-based hand sanitizer and soap
and water as to ensure they cover all surfaces of
hands and fingers, including thumbs and fingernails
• When gloves should be used in addition to hand
washing (e.g., caring for C. diff. patients) and how
hand hygiene should be performed when gloves are
used
• The minimum time that should be spent performing
hand hygiene with soap and water and alcohol-based
hand sanitizer
• How hand hygiene compliance is monitored
Infrastructure
4) Does your hospital have a process in place to ensure that all
the following are done, as necessary, and quarterly audits are
conducted on a sample of dispensers in your patient care
units to ensure that the process is followed?
• Refill paper towels, soap dispensers, and alcohol-
based hand sanitizer dispensers when they are empty
or near empty
• Replace batteries in automated paper towel
dispensers, soap dispensers, and alcohol-based hand
sanitizer dispensers (if automated dispensers are
used in the patient care units)
Yes
No
5) Do all rooms and bed spaces in your patient care units have:
• an alcohol-based hand sanitizer dispenser located at
the entrance to the room or bed space, and
• alcohol-based hand sanitizer dispenser(s) located
inside the room or bed space that are equally
accessible to the location of all patients in the room or
bed space?
Yes
No
6) Does your hospital conduct audits of the volume of alcohol-
based hand sanitizer that is delivered with each activation of a
wall-mounted dispenser (manual and automated) on a sample
of dispensers in your patient care units at all the following
times:
• upon installation,
• whenever the brand of product or system changes,
and
• whenever adjustments are made to the dispensers?
OR
Has your hospital conducted an audit of the volume of alcohol-
based hand sanitizer that is delivered with each activation of a
wall-mounted dispenser (manual and automated) on a sample
of your hospital’s existing dispensers if there have been no
changes to any dispensers?
If “no” or “does not apply, wall-mounted dispensers are not
used,” skip question #7 and continue to question #8.
Yes
No
Does not apply, wall-mounted
dispensers are not used
7) Do all of the audited dispensers deliver, with one activation,
1.0 mL of alcohol-based hand sanitizer OR a volume of
alcohol-based hand sanitizer that covers the hands completely
and requires 15 or more seconds for hands to dry (on
average)?
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy
196 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Monitoring
8) Does your hospital collect hand hygiene compliance data on at
least 200 hand hygiene opportunities, or at least the number
of hand hygiene opportunities outlined based on the unit type
in Tables 1-3, each month in each patient care unit?
If “yes” to question #8, skip questions #9-10 and continue to
question #11.
Yes, using an electronic
compliance monitoring system
throughout all patient care units
Yes, using an electronic
compliance monitoring system
throughout some patient care
units and only direct observation
in all other patient care units
Yes, using only direct observation
throughout all patient care units
No
9) Does your hospital collect hand hygiene compliance data on at
least 100 hand hygiene opportunities, or at least the number
of hand hygiene opportunities outlined based on the unit type
in Tables 4-6, each month in each patient care unit?
If “yes” to question #9, skip question #10 and continue to
question #11.
Yes, using an electronic
compliance monitoring system
throughout all patient care units
Yes, using an electronic
compliance monitoring system
throughout some patient care
units and only direct observation
in all other patient care units
Yes, using only direct observation
throughout all patient care units
No
10) Does your hospital collect hand hygiene compliance data on at
least 100 hand hygiene opportunities each quarter in each
patient care unit?
If “no” to question #10, skip questions #11-19 and continue to
question #20.
Yes, using an electronic
compliance monitoring system
throughout all patient care units
Yes, using an electronic
compliance monitoring system
throughout some patient care
units and only direct observation
in all other patient care units
Yes, using only direct observation
throughout all patient care units
No
11) Does your hospital use hand hygiene coaches or compliance
observers to provide individuals who touch patients or who
touch items that will be used by patients
44
in your patient care
units with feedback on both when they are and are not
compliant with performing hand hygiene?
Yes
No
Direct Monitoring – Electronic Compliance Monitoring System
If “yes, using an electronic compliance monitoring system throughout all patient care units” or “yes, using
an electronic compliance monitoring system throughout some patient care units and only direct
observation in all other patient care units” to question #8, question #9, or question #10, answer questions
#12-13 based on the units that use an electronic compliance monitoring system.
12) In those patient care units where an electronic compliance
monitoring system is used, does the monitoring system used
meet both of the following criteria?
• The system can identify both opportunities for hand
hygiene and that hand hygiene was performed
• The hospital itself has validated the accuracy of the
data collected by the electronic compliance monitoring
system
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy
197 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
13) In those patient care units where an electronic compliance
monitoring system is used, are direct observations also
conducted for coaching and intervention purposes that meet
all the following criteria?
• Observers immediately intervene prior to any harm
occurring to provide non-compliant individuals with
immediate feedback
• Observations identify both opportunities for hand
hygiene and compliance with those opportunities
• Observations determine who practiced hand hygiene,
verify when they practiced it, and whether their
technique was correct
• Observations within a unit are conducted weekly or
monthly across all shifts and on all days of the week
proportional to the number of
individuals who touch
patients or who touch items that will be used by
patients
44
on duty for that shift
• Observations capture a representative sample of the
different roles of
individuals who touch patients or who
touch items that will be used by patients
44
(e.g.,
nurses, physicians, techs, environmental services
workers)
Yes
No
Direct Monitoring – Direct Observation
If “yes, using an electronic compliance monitoring system throughout some patient care units and only
direct observation in all other patient care units” or “yes, using only direct observation” to question #8,
question #9, or question #10 answer questions #14-15 based on the units that do NOT use an electronic
compliance monitoring system.
14) In those patient care units where an electronic compliance
monitoring system is NOT used, do the direct observations
meet all the following criteria?
• Observations identify both opportunities for hand
hygiene and compliance with those opportunities
• Observations determine who practiced hand hygiene,
verify when they practiced it, and whether their
technique was correct
• Observations within a unit are conducted weekly or
monthly across all shifts and on all days of the week
proportional to the number of
individuals who touch
patients or who touch items that will be used by
patients
44
on duty for that shift
• Observations are conducted to capture a
representative sample of the different roles of
individuals who touch patients or who touch items that
will be used by patients
44
(e.g., nurses, physicians,
techs, environmental services workers)
Yes
No
15) Does your hospital have a system in place for both the initial
and recurrent training and validation of hand hygiene
compliance observers?
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy
198 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Feedback
16) Are unit-level hand hygiene compliance data fed back to
individuals who touch patients or who touch items that will be
used by patients
44
at least monthly for improvement work?
Yes
No
17) Are unit-level hand hygiene compliance data used for creating
unit-level action plans?
Yes
No
18) Is regular (at least every 6 months) feedback of hand hygiene
compliance data, with demonstration of trends over time, given
to:
• senior administrative leadership, physician leadership,
and nursing leadership,
• the board (governance), and
• the medical executive committee?
If “no” to question #18, skip question #19 and continue to
question #20.
Yes
No
19) If “yes” to question #18, is senior administrative leadership,
physician leadership, and nursing leadership held directly
accountable for hand hygiene performance through
performance reviews or compensation?
Yes
No
Culture
20) Are patients and visitors invited to remind
individuals who
touch patients or who touch items that will be used by
patients
44
to perform hand hygiene?
Yes
No
21) Have all of the following individuals (or their equivalents)
demonstrated a commitment to support hand hygiene
improvement in the last year (e.g., a written or verbal
commitment delivered to those individuals who touch patients
or who touch items that will be used by patients
44
)?
• Chief Executive Officer
• Chief Medical Officer
• Chief Nursing Officer
Yes
No
22) Do all rooms and bed spaces in your patient care units have a
sink for hand washing within 20 feet of the patient’s bed that is
easily accessible to
individuals who touch patients or who
touch items that will be used by patients
44
?
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy
199 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Affirmation of Accuracy
As the hospital CEO or as an employee of the hospital to whom the hospital CEO has delegated
responsibility, I have reviewed this information pertaining to the Patient Safety Practices Section at our
hospital, and I hereby certify that this information is true, accurate, and reflects the current, normal
operating circumstances at our hospital. I am authorized to make this certification on behalf of our
hospital.
The hospital and I understand that The Leapfrog Group, its members, the public and entities and persons
who contract or have other business dealings with The Leapfrog Group are relying on the truth and
accuracy of this information. The hospital and I also understand that The Leapfrog Group will make this
information and/or analyses of this information public through the Survey Results public reporting website,
The Leapfrog Group’s Hospital Safety Grade, and/or other Leapfrog Group products and services. This
information and/or analyses and all intellectual property rights therein shall be and remain the sole and
exclusive property of The Leapfrog Group in which The Leapfrog Group retains exclusive ownership. This
information does not infringe upon any third-party intellectual property rights or any other third-party rights
whatsoever and is free and clear of all encumbrances and liens of any kind. The hospital and I
acknowledge that The Leapfrog Group may use this information in a commercial manner for profit. The
hospital shall be liable for and shall hold harmless and indemnify The Leapfrog Group from any and all
damages, demands, costs, or causes of action resulting from any inaccuracies in the information or any
misrepresentations in this Affirmation of Accuracy. The Leapfrog Group and its members and entities and
persons who contract or have other business dealings with The Leapfrog Group reserve the right to omit
or disclaim information that is not current, accurate or truthful.
Affirmed by _____________________, the hospital’s ___________________________,
(first name and last name) (title)
on _______________________.
(date)

2023 Leapfrog Hospital Survey – Hard Copy
200 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 6: 2023 Patient Safety Practices Reference Information
What’s New in the 2023 Survey
Section 6C: Nursing Workforce
Leapfrog is scoring and publicly reporting results for the Nurse Staffing and Skill Level measures (Total
Nursing Care Hours per Patient Day, RN Hours per Patient Day, Nursing Skill Mix, and Percentage of
RNs who are BSN-Prepared) and is moving these measures to Section 6C: Nursing Workforce. In
preparation for scoring and public reporting, we are also adding a question regarding which method was
used to calculate the total number of patient days for each medical, surgical, and/or med-surg unit.
Leapfrog is also updating the measure specifications for Total Nursing Care Hours per Patient Day, RN
Hours per Patient Day, and Nursing Skill Mix to more closely align with the National Database of Nursing
Quality Indicators’ (NDNQI) definitions of medical, surgical, and medical/surgical units. Previously
hospitals were instructed to exclude units from reporting if at least 90% of the patients in the unit require
telemetry, or the primary reason for admission to the unit is the patient’s need for telemetry. That
exclusion has been removed for 2023.
Leapfrog has also published
benchmarking data for all new nurse staffing and skill mix measures so that
hospitals can assess how they compare nationally on these four measures.
Further, given that Leapfrog has asked hospitals to report their progress in implementing the elements of
NQF Safe Practice #9 – Nursing Workforce for over 15 years, we are now only asking hospitals to report
on five of the seventeen practice elements not directly captured through the Nurse Staffing and Skill Level
measures. The five practice elements include:
• 9.2a: held nursing leadership directly accountable for improvements in performance through
performance reviews or compensation.
• 9.2b: included nursing leadership as part of the hospital senior administrative leadership team.
• 9.2d: held the board (governance) and senior administrative leadership accountable for the
provision of financial resources to ensure adequate nurse staffing levels.
• 9.3d: budgeted financial resources for balancing staffing levels and skill levels to improve
performance.
• 9.4a: developed a staffing plan, with input from nurses, to ensure that adequate nursing staff-to-
patient ratios are achieved.
Hospitals recognized as an American Nurses Credentialing Center (ANCC) Magnet® hospital or a 2020
Pathway to Excellence® hospital will receive full credit for the five practice elements. However, NQF Safe
Practice #9 is not being scored and publicly reported as a stand-alone measure. Instead, the NQF Safe
Practice #9 – Nursing Workforce measure will only be used in scoring if the hospital scores in the bottom
performance category (“Limited Achievement”) on the Nurse Staffing and Skill Mix measures (Total
Nursing Care Hours per Patient Day, RN Hours per Patient Day, and Nursing Skill Mix) to bump the
hospital’s performance category up from “Limited Achievement” to “Some Achievement.”
Change Summary Since Release
July 21, 2023 – Section 6C Nursing Workforce – Total Nursing Care Hours per Patient Day, RN Hours per
Patient Day, and Nursing Skill Mix: Updated exclusions for medical, surgical or med-surg units with fewer
than 15 patient days per month for all three months during a quarter and units that were closed for an
entire quarter of the reporting period. Please review updated specifications on page 202 of the Survey.

2023 Leapfrog Hospital Survey – Hard Copy
201 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Patient Safety Practices Measure Specifications
Nursing Workforce
Important Note: Hospitals that share a CMS Certification Number are required to report by facility.
Please carefully review Leapfrog’s Multi-Campus Reporting Policy
.
Total Nursing Care Hours per Patient Day, RN Hours per Patient Day, and Nursing Skill
Mix
Source: American Nurses Association (ANA) (National Quality Forum #0204 and #0205)
Reporting Period: 12 months
• Survey submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys submitted on or after September 1:
o
07/01/2022 – 06/30/2023
Hospitals that report this data to NDNQI (National Database of Nursing Quality Indicators):
Hospitals that report to NDNQI (National Database of Nursing Quality Indicators) will be able to respond
to questions #4 - 10 in Section 6C using data that is available upon request for system product users.
Hospitals should email NDNQISupport@PressGaney.com
or call 855-304-9788 to connect with one of
NDNQI’s team members and specify that they are requesting support for extracting data from the data
summary report or system data export for the purposes of reporting on Total Nursing Care Hours per
Patient Day, RN Hours per Patient Day, and Nursing Skill Mix in the Leapfrog Hospital Survey.
Question #4: Based on your hospital’s current method for counting patient days, select one of the five
(5) methods below to calculate the total number of patient days for all medical, surgical, and med-surg
units:
Midnight Census
If your hospital has a daily midnight census count, sum the daily midnight census counts (the number of
patients on the unit at midnight each day) during the reporting period.
Patient Days from Actual Hours
If your hospital has an accounting system that tracks the actual time spent in the hospital by each
patient, sum the actual hours for all patients during the reporting period and divide by 24.
Patient Days from Multiple Census Reports
If your hospital collects censuses multiple times per day (e.g., every 4 hours or each shift), sum the
daily average censuses to determine patient days for the reporting period.
Midnight Census and Patient Days from Actual Hours for Short Stay Patients
If your hospital uses a combination of Midnight Census and Patient Days from Actual Hours, select this
option.
Other
If your hospital does not use any of the methods above, select this option.
Patient days should be calculated to include all patients in inpatient medical, surgical, and med-surg
units. This includes patients that do not have inpatient status (i.e., observation patients).
Questions #5, #7, and #9:
Medical, Surgical, and Med-Surg Units: For the purposes of Total Nursing Care Hours per Patient
Day, RN Hours per Patient Days, and Nursing Skill Mix, Leapfrog has aligned with the National
Database of Nursing Quality Indicators’ (NDNQI) unit definitions below.
Medical Units (adult and pediatric): A single acuity general care unit in which at least 90% of the
patients are admitted to medical services, such as internal medicine, oncology, or cardiology. Medical
specialty units such as Cardiac Medical, GI Medical, Infectious Disease Medical, Neurology Medical,

2023 Leapfrog Hospital Survey – Hard Copy
202 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Hematology/Oncology Medical, Renal Medical, and Respiratory Medical, are also included.
Surgical Units (adult and pediatric): A single acuity general care unit in which at least 90% of the
patients are admitted to surgical services, such as general surgery, neurosurgery, or orthopedics.
Surgical specialty units such as Bariatric Surgery, Cardio-thoracic Surgery, Gynecologic Surgery,
Neurosurgery, Orthopedic Surgery, Plastic Surgery, Transplant Surgery, and Trauma Surgery, are also
included.
Med-Surg Combined Units (adult and pediatric): A single acuity general care unit that cares for
patients admitted for medical, surgical, or family practice services and does not meet the 90% criteria
for Medical or Surgical unit type. Specialty med-surg units such as Cardiac Med-Surg,
Neuro/Neurosurgery Med-Surg, and Hematology/Oncology Med-Surg, are also included.
Exclusions:
• Critical care units (adult, pediatric, and neonatal)
• Step-down and progressive units
• Mixed acuity units
• Units that were closed for an entire quarter of the reporting period
• Units with fewer than 15 patient days per month for all three months during a quarter of the
reporting period
Questions #6, #8, and #10 (a): Total number of patient days in all medical units (when responding to
question #6a), surgical units (question #8a) and med-surg units (question #10a) for each quarter based
on your hospital’s current method for counting patient days.
Questions #6, #8, and #10 (b): Total number of productive hours worked by employee or contract
nursing staff with direct patient care responsibilities (RN, LPN/LVN, and UAP) in all medical units (when
responding to question #6b), surgical units (question #8b) and med-surg units (question #10b) in each
quarter.
Sum the total number of productive hours worked by nursing staff with direct patient care
responsibilities (RN, LPN/LVN, or UAP) in each medical, surgical, and med-surg unit.
Productive Hours are actual direct patient care hours worked by nursing staff including overtime, not
budgeted or scheduled hours. Vacation, sick time, orientation periods for new hires, education leave, or
committee time are considered non-productive hours. However, orientation programs for new hires vary
from hospital to hospital. Once orientees reach the point where they are considered part of the staffing
matrix, their work hours are charged to the unit, and they would be replaced if they call in sick, then
their hours are counted as productive, even if they are in an orientation period.
Nursing Staff include both staff employed by the hospital or temporary staff who are not employed by
the hospital (i.e., contracted/agency staff). Nursing Staff also include float staff - who are assigned to a
unit other than their unit of employment on an as-needed basis – if they are assigned direct patient care
responsibilities.
Included nursing staff:
• Staff who are counted in the unit’s staffing matrix,
• Are replaced if they call in sick, and
• Work hours are charged to the unit’s cost center.
Excluded nursing staff:
• Persons whose primary responsibility is administrative in nature (at least 50% of their time is
administrative)
• Specialty teams, patient educators, or case managers who are not assigned to a specific unit
• Unit secretaries or clerks, monitor technicians, and others with no direct patient care
responsibilities

2023 Leapfrog Hospital Survey – Hard Copy
203 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Direct Patient Care Responsibilities are defined as patient centered nursing activities by hospital unit-
based staff in the presence of the patient and activities that occur away from the patient that are patient
related:
• Medication administration
• Nursing treatments
• Nursing rounds
• Admission, transfer, discharge activities
• Patient teaching
• Patient communication
• Coordination of patient care
• Documentation time
• Treatment planning
• Patient screening (e.g., fall risk) and assessment
Questions #6, #8, and #10 (c): Total number of productive hours worked by RN nursing staff with
direct patient care responsibilities in all medical units (when responding to question #6c), surgical units
(question #8c) and med-surg units (question #10c) in each quarter.
Sum the total number of productive hours worked by RN nursing staff with direct patient care
responsibilities in each medical, surgical, and medical/surgical unit.
RN Nursing Staff include both staff employed by the hospital or temporary staff who are not employed
by the hospital (i.e., contracted/agency staff). RN Nursing Staff also include float staff - who are
assigned to a unit other than their unit of employment on an as-needed basis – if they are assigned
direct patient care responsibilities.
Percentage of RNs who are BSN-Prepared
Source: The Leapfrog Group
Reporting Period: Answer questions #11-13 based on the most recent day within the last 12-months for
which you have complete data.
Question #12 (denominator): Total number of employed RN nursing staff at the hospital with direct
patient care responsibilities.
RN Nursing Staff include staff employed by the hospital but exclude temporary staff who are not
employed by the hospital (i.e., contracted/agency staff). Nursing Staff also include float staff - who are
assigned to a unit other than their unit of employment on an as-needed basis – if they are assigned
direct patient care responsibilities.
Include employed RN nursing staff in most all hospital units, including observation, outpatient units, and
emergency departments who:
• Are counted in the unit’s staffing matrix, and
• Are replaced if they call in sick, and
• Whose work hours are charged to the unit’s cost center.
Exclude nursing staff who:
• Are not employed by the hospital (i.e., contracted/agency staff)
• Are exclusively assigned to skilled nursing units or mixed acuity neonatal units
• Are part of specialty teams or are patient educators or case managers who are not assigned to a
specific unit
• Are unit secretaries or clerks or others with no direct patient care responsibilities
• Are primarily responsible for administrative tasks (at least 50% of their time is administrative)
Direct Patient Care Responsibilities are defined as patient centered nursing activities by hospital unit-
based staff in the presence of the patient and activities that occur away from the patient that are patient
related:

2023 Leapfrog Hospital Survey – Hard Copy
204 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
• Medication administration
• Nursing treatments
• Nursing rounds
• Admission, transfer, discharge activities
• Patient teaching
• Patient communication
• Coordination of patient care
• Documentation time
• Treatment planning
• Patient screening (e.g., fall risk) and assessment
Question #13 (numerator): Total number of employed RN nursing staff at the hospital with direct
patient care responsibilities who have a BSN degree or higher (e.g., MSN, DNP, and PhD).
Note: Hospitals can use any method to identify RN nursing staff who have a BSN degree or higher,
including:
• Information from HR or credentialing office, or
• Staff survey via SurveyMonkey or other free data collection tool.

2023 Leapfrog Hospital Survey – Hard Copy
205 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Hand Hygiene
Important Note: Hospitals that share a CMS Certification Number are required to report by facility.
Please carefully review Leapfrog’s Multi-Campus Reporting Policy
.
Source: The framework and questions in this subsection are modeled after the World Health
Organization’s Hand Hygiene Self-Assessment Framework
.
Reporting Period: Answer questions #1-22 based on the practices currently in place at the time you
submit this section of the Survey.
Note: For monitoring, this means that the monthly sample size (or quarterly sample size if answering
question #10) would need to be met at least once in each patient care unit the month (or quarter)
preceding the time of the submission of the Survey and there must be a process in place to meet the
monthly (or quarterly) sample size thereafter in each patient care unit every month/quarter.
If the monitoring sample size is not met at any point after submission, the Survey must be updated and
re-submitted. More information about updating a Survey can be found at
https://www.leapfroggroup.org/survey-materials/updating-your-hospital-survey
.
As a reminder, Leapfrog randomly requests documentation on a monthly basis and key documentation
must be collected and maintained to support responses to all questions. Hospitals are urged to
regularly review their documentation, including reports supporting their monitoring of hand hygiene, and
maintain copies throughout the Survey Cycle. Please refer to the
Survey Binder for examples.
Patient Care Units: Include only the following patient care units when reporting on the questions in this
subsection:
• inpatient units:
o medical and/or surgical units (including telemetry/step-down/progressive units)
19
o pediatric units
o labor and delivery units
o mother/baby units (e.g., nursery, etc.)
o intensive care units (adult, pediatric, and/or neonatal)
o pre-operative and post-operative units (e.g., PACUs, etc.)
• outpatient units, including free-standing hospital outpatient departments and surgery centers
that are reported on in Section 9 Outpatient Procedures, that share your hospital’s license
and/or CMS Certification Number:
o pre-operative and pre-procedural units/areas
o post-operative and post-procedural units/areas
• observation units
• emergency department units
Infrastructure
Question #4: Does your hospital have a process in place to ensure that all the following are done, as
necessary, and quarterly audits are conducted on a sample of dispensers in your patient care units to
ensure that the process is followed?
• Refill paper towels, soap dispensers, and alcohol-based hand sanitizer dispensers when they
are empty or near empty
• Replace batteries in automated paper towel dispensers, soap dispensers, and alcohol-based
hand sanitizer dispensers (if automated dispensers are used in the patient care units)
In order to respond “yes” to question #4, a process must be in place to ensure that paper towel, soap,
and alcohol-based hand sanitizer dispensers are refilled when they are empty or near empty and
batteries are replaced in automated paper towel, soap, and alcohol-based hand sanitizer dispensers (if
automated dispensers are used in patient care units).
In addition, a quarterly audit must be conducted on a sample of paper towel, soap, and alcohol-based
hand sanitizer dispensers to include checking that dispensers are refilled and that batteries in
automated dispensers are replaced. The quarterly audit should be a supplement to a system that

2023 Leapfrog Hospital Survey – Hard Copy
206 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
checks these supplies on a routine basis (e.g., environmental services check with their regular
cleaning).
Sampling Instructions: The sample must be based on a random or systematic sampling procedure,
where the sampling plan assures wide sampling (i.e., the same places would not always be monitored)
and includes at least 5% of the dispensers in 20% of the patient care units.
Question #6: Does your hospital conduct audits of the volume of alcohol-based hand sanitizer that is
delivered with each activation of a wall-mounted dispenser (manual and automated) on a sample of
dispensers in your patient care units at all the following times:
• upon installation;
• whenever the brand of product or system changes; and
• whenever adjustments are made to the dispensers;
OR
Has your hospital conducted an audit of the volume of alcohol-based hand sanitizer that is delivered
with each activation of a wall-mounted dispenser (manual and automated) on a sample of your
hospital’s existing dispensers if there have been no changes to any dispensers?
In order to respond “yes” to question #6, a volume audit on a sample of wall-mounted alcohol-based
hand sanitizer dispensers (manual and automated) in your patient care units must be conducted at
least once (upon installation, whenever the brand of product or system changes, and whenever
adjustments are made to the dispensers) using one of the below methods. Prior volume audits are
acceptable if they were conducted using the instructions in Leapfrog’s 2019-2022 Surveys,
documentation has been maintained, and no changes have been made to the dispensers since the
audit.
Sampling Instructions: The sample must be based on a random or systematic sampling procedure,
where the sampling plan assures wide sampling (i.e., the same places would not always be monitored)
and includes at least 5% of the dispensers.
Method #1 - Auditing Liquid Volume: To audit the liquid volume of alcohol-based hand sanitizer that
is delivered with each activation of a wall-mounted dispenser (manual and automated), use the
following process:
1. Select a sample of dispensers based on a random or systematic sampling procedure.
2. Take a small, graduated plastic medicine cup and have the dispenser deliver 10 doses of alcohol-
based hand sanitizer.
3. Divide the total volume dispensed by 10 to get an average of the amount dispensed.
NOTE: Hospitals using foam alcohol-based hand sanitizer will need to follow the steps above, but will
instead need to weigh the sample, divide the results by 10 to obtain the average output of the sample,
and then convert the weight in grams to milliliters by dividing the weight by the density (which can be
provided by your vendor for your specific product).
Method #2 - Auditing Average Hand Rubbing Time: To audit the average hand rubbing time of
alcohol-based hand sanitizer that is delivered with each activation of a wall-mounted dispenser (manual
and automated), use the following process:
1. Select a sample of dispensers based on a random or systematic sampling procedure.
2. Identify multiple individuals (at least 10) with varying hand sizes (by quick observation).
3. For each sampled dispenser, have each of the individuals identified in step #1 dispense a volume
of alcohol-based hand sanitizer.
4. For each individual, have a separate individual time the amount of hand rubbing time required for
hands to dry completely.
5. Repeat this process for each individual and calculate an average time based on the ten
observations conducted.
6. Repeat this process for each sampled dispenser.
Question #7: Do all the audited dispensers deliver, with one activation, 1.0 mL of alcohol-based hand
sanitizer OR a volume of alcohol-based hand sanitizer that covers the hands completely and requires
15 or more seconds for hands to dry (on average)?

2023 Leapfrog Hospital Survey – Hard Copy
207 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
In order to respond “yes” to question #7, the average liquid volume for each sampled dispenser needs
to be at least 1.0 mL (if using method #1) or the average hand rubbing time for each sampled
dispenser needs to be at least 15 seconds (if using method #2).
Monitoring
Hand hygiene opportunities: Hand hygiene opportunities are the number of times that an individual
who touches patients or who touches items used by patients should have cleaned their hands given the
hand hygiene framework your hospital has adopted (e.g., WHO’s “5 moments”, Ontario’s 4 moments,
CDC’s guidelines, etc.). In terms of determining opportunities to monitor, this would depend on the
guidelines the hospital chooses to follow.
For example, many facilities choose to audit before and after patient contact or room entry and exit
because this is operationally the simplest method. Auditing opportunities before and after dirty tasks is
operationally difficult. There is some evidence that measuring adherence on room entry and exit may
be an acceptable stand-in for other opportunities within the patient encounter.
Question #8: Does your hospital collect hand hygiene compliance data on at least 200 hand hygiene
opportunities, or at least the number of hand hygiene opportunities outlined based on the unit type in
Tables 1-3, each month in each patient care unit?
In order to respond “yes” to question #8, your hospital must monitor at least 200 hand hygiene
opportunities, or at least the number of hand hygiene opportunities outlined based on the unit type in
Tables 1-3, each month in each patient care unit using either:
• An electronic compliance monitoring system throughout all patient care units
• An electronic compliance monitoring system throughout some patient care units and only direct
observation in all other patient care units
• Only direct observation throughout all patient care units
Refer to the following tables to determine how many hand hygiene opportunities must be monitored in
each patient care unit monthly for question #8. Historical data (e.g., past 3 months, 6 months, 12
months, etc.) on the monthly occupancy rates or procedure/patient volumes should be used.
Table 1: Units where the monthly occupancy rate can be calculated
If your unit’s average daily census is…
Your patient care unit needs to collect
hand hygiene compliance data for at least
this number of hand hygiene opportunities
per month for question #8…
13 patients or higher
200
10-12 patients
150
7-9 patients
100
5-6 patients
75
3-4 patients
45
1-2 patients
15
Table 2: Units where the monthly occupancy rate cannot be calculated (e.g., PACU, labor and
delivery, outpatient units)
If your unit’s average number of
procedures in a month is…
Your patient care unit needs to collect
hand hygiene compliance data for at least
this number of hand hygiene opportunities
per month for question #8…
400 procedures or greater
200
320-399 procedures
150
240-319 procedures
100
160-239 procedures
75
120-159 procedures
50
60-119 procedures
30
30-59 procedures
15
<30 procedures
5

2023 Leapfrog Hospital Survey – Hard Copy
208 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Table 3: Emergency department units
If your emergency department’s average
number of visits in a month is…
Your patient care unit needs to collect
hand hygiene compliance data for at least
this number of hand hygiene opportunities
per month for question #8…
2000 visits or greater
200
1500-1999 visits
150
1000-1499 visits
100
750-999 visits
75
500-749 visits
50
250-499 visits
25
150-249 visits
15
<150 visits
5
Question #9: Does your hospital collect hand hygiene compliance data on at least 100 hand hygiene
opportunities, or at least the number of hand hygiene opportunities outlined based on the unit type in
Tables 4-6, each month in each patient care unit?
In order to respond “yes” to question #9, your hospital must monitor at least 100 hand hygiene
opportunities, or at least the number of hand hygiene opportunities outlined based on the unit type in
Tables 4-6, each month in each patient care unit using either:
• An electronic compliance monitoring system throughout all patient care units
• An electronic compliance monitoring system throughout some patient care units and only direct
observation in all other patient care units
• Only direct observation throughout all patient care units
Refer to the following tables to determine how many hand hygiene opportunities must be monitored in
each patient care unit monthly for question #9. Historical data (e.g., past 3 months, 6 months, 12
months, etc.) on the monthly occupancy rates or procedure/patient volumes should be used.
Table 4: Units where the monthly occupancy rate can be calculated
If your unit’s average daily census is…
Your patient care unit needs to collect
hand hygiene compliance data for at least
this number of hand hygiene opportunities
per month for question #9…
13 patients or higher
100
10-12 patients
75
7-9 patients
50
5-6 patients
37
3-4 patients
22
1-2 patients
7
Table 5: Units where the monthly occupancy rate cannot be calculated (e.g., PACU, labor and
delivery, outpatient units)
If your unit’s average number of
procedures in a month is…
Your patient care unit needs to collect
hand hygiene compliance data for at least
this number of hand hygiene opportunities
per month for question #9…
400 procedures or greater
100
320-399 procedures
75
240-319 procedures
50
160-239 procedures
37
120-159 procedures
25
60-119 procedures
15
30-59 procedures
7
<30 procedures
2

2023 Leapfrog Hospital Survey – Hard Copy
209 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Table 6: Emergency department units
If your emergency department’s average
number of visits in a month is…
Your patient care unit needs to collect
hand hygiene compliance data for at least
this number of hand hygiene opportunities
per month for question #9…
2000 visits or greater
100
1500-1999 visits
75
1000-1499 visits
50
750-999 visits
37
500-749 visits
25
250-499 visits
15
150-249 visits
7
Question #10: Does your hospital collect hand hygiene compliance data on at least 100 hand hygiene
opportunities each quarter in each patient care unit?
In order to respond “yes” to question #10, your hospital must monitor at least 100 hand hygiene
opportunities each quarter in each patient care unit using either:
• An electronic compliance monitoring system throughout all patient care units
• An electronic compliance monitoring system throughout some patient care units and only direct
observation in all other patient care units
• Only direct observation throughout all patient care units
There are no alternate sample sizes for the quarterly requirement. Hospitals trying to meet the quarterly
requirement in question #10 will need to monitor 100 hand hygiene opportunities a quarter.
Question #12: In those patient care units where an electronic compliance monitoring system is used,
does the monitoring system used meet both of the following criteria?
• The system can identify both opportunities for hand hygiene and that hand hygiene was
performed
• The hospital itself has validated the accuracy of the data collected by the electronic compliance
monitoring system
In order to respond “yes” to question #12, the electronic monitoring system in use must identify both
opportunities and that hand hygiene was performed, which could include both group monitoring
systems and badge-based systems.
For example, an electronic monitoring system that records when an individual (not identified) enters
and exits a room and also records if a dispenser was used within the same time frame, would qualify as
the entry and exit is used as a proxy for a hand hygiene opportunity (before and after touching a
patient) and the dispenser use is used as a proxy for a hand hygiene event. This data can be adjusted
to take visitors into account and used to estimate hand hygiene compliance. Another example would be
a badge-based system where individuals or their roles can be identified.
In addition, validation of the accuracy of the data collected by the electronic compliance monitoring
system must be performed by hospital personnel or independent third-party personnel, in addition to
any validation conducted by the manufacturer. It needs to include both a "planned path” phase where
the researcher(s) make timed observations of room entries and exits and use of dispensers and
compare their results to data recorded by the electronic compliance monitoring system. Followed by a
"behavioral path" phase where observers record the same variables when individuals who touch
patients or who touch items that will be used by patients are performing their usual duties, as this tends
to be more chaotic and variable. A general validation protocol that can be used for both group
monitoring systems and badge-based systems has been described in a fair amount of detail in the 2016
article by Limper H et al. Similar methods for conducting validation studies of badge-based system
have been described by Pineles LL, et al. in 2014, and by Doll ME et al. in 2019.

2023 Leapfrog Hospital Survey – Hard Copy
210 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Question #13: In those patient care units where an electronic compliance monitoring system is used,
are direct observations also conducted for coaching and intervention purposes that meet all of the
following criteria?
• Observers immediately intervene prior to any harm occurring to provide non-compliant
individuals with immediate feedback
• Observations identify both opportunities for hand hygiene and compliance with those
opportunities
• Observations determine who practiced hand hygiene, verify when they practiced it, and
whether their technique was correct
• Observations within a unit are conducted weekly or monthly across all shifts and on all days of
the week proportional to the number of
individuals who touch patients or who touch items that
will be used by patients
44
on duty for that shift
• Observations capture a representative sample of the different roles of individuals who touch
patients or who touch items that will be used by patients
44
(e.g., nurses, physicians, techs,
environmental services workers)
In order to respond “yes” to question #13, direct observations must be conducted for coaching and
intervention purposes and meet the following sample sizes at a minimum:
In units with low hand hygiene compliance* and/or units with high infection rates**:
• On a monthly basis, hospitals will need to perform 20 direct observations.
• If after collecting those observations, more than 50% of the observations were NOT compliant
and/or did NOT demonstrate proper technique, the hospital will need to perform an additional
20 observations the following month (40 total observations per unit with low hand hygiene
compliance or high infection rates).
• T
he hospital must continue to collect 40 observations per month until the number of
observations that are NOT compliant and/or did not demonstrate proper technique is reduced
to less than 50%.
Compliance and infection rates must be assessed at least quarterly and additional observations need to
be collected on a monthly basis for the entire quarter if warranted based on the above rules. If a
standardized infection ratio cannot be calculated for the quarter, units with 2 or more observed
infections should do the additional collection of direct observations.
*Low hand hygiene compliance is defined as two or more standard deviations below the hospital's
overall mean hand hygiene compliance rate.
**High infection rates are defined as two or more standard deviations above the hospital's standardized
infection ratio for any of the following healthcare-associated infection measures: CLABSI, CAUTI,
MRSA, and C.diff.
In all other units:
• On a quarterly basis, hospitals using ECM need to perform 10% of the observations noted in
Tables 1-3 in ALL patient care units included in Leapfrog’s Hand Hygiene Standard (e.g., if the
unit would require 200 direct observations without ECM, then with the use of ECM, they need to
collect 20 direct observations).
• Units in which direct observation data is being collected monthly (i.e., units with low hand
hygiene compliance and/or high infection rates) do not require the additional quarterly data
collection.
All direct observations conducted must meet the following criteria:
• Observers immediately intervene prior to any harm occurring to provide non-compliant
individuals with immediate feedback
• Observations identify both opportunities for hand hygiene and compliance with those
opportunities
• Observations determine who practiced hand hygiene, verify when they practiced it, and
whether their technique was correct

2023 Leapfrog Hospital Survey – Hard Copy
211 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
• Observations within a unit are conducted weekly or monthly across all shifts and on all days of
the week proportional to the number of individuals who touch patients or who touch items that
will be used by patients
44
on duty for that shift
Observations capture a representative sample of the different roles of individuals who touch
patients or who touch items that will be used by patients
44
(e.g., nurses, physicians, techs,
environmental services workers)
Observers must record at least the following:
• The date as well as the start and end time of the observation session (or the date and shift
being observed
• The unit where the observation session is being conducted
• The role of the individual being observed (e.g., nurse, physician, etc.)
• The indication (or moment) for performing hand hygiene (e.g., before/after touching a patient,
before/after a procedure, before/after touching patient surroundings, etc.)
• Whether hand hygiene was performed or not performed based on the indication noted and if
the technique was correct
Question #14: In those patient care units where an electronic compliance monitoring system is NOT
used, do the direct observations meet all of the following criteria?
• Observations identify both opportunities for hand hygiene and compliance with those
opportunities
• Observations determine who practiced hand hygiene, verify when they practiced it, and
whether their technique was correct
• Observations within a unit are conducted weekly or monthly across all shifts and on all days of
the week proportional to the number of
individuals who touch patients or who touch items that
will be used by patients
44
on duty for that shift
• Observations capture a representative sample of the different roles of individuals who touch
patients or who touch items that will be used by patients
44
(e.g., nurses, physicians, techs,
environmental services workers)
In order to respond “yes” to question #14, direct observations must be conducted in patient care units
that do not use an electronic compliance monitoring system and must meet the following criteria:
• Observations identify both opportunities for hand hygiene and compliance with those
opportunities
• Observations determine who practiced hand hygiene, verify when they practiced it, and
whether their technique was correct
• Observations within a unit are conducted weekly or monthly across all shifts and on all days of
the week proportional to the number of
individuals who touch patients or who touch items that
will be used by patients
44
on duty for that shift
• Observations capture a representative sample of the different roles of individuals who touch
patients or who touch items that will be used by patients
44
(e.g., nurses, physicians, techs,
environmental services workers)
Observers must record at least the following:
• The date as well as the start and end time of the observation session (or the date and shift
being observed)
• The unit where the observation session is being conducted
• The role of the individual being observed (e.g., nurse, physician, etc.)
• The indication (or moment) for performing hand hygiene (e.g., before/after touching a patient,
before/after a procedure, before/after touching patient surroundings, etc.)
• Whether hand hygiene was performed or not performed based on the indication noted and if
the technique was correct
Question #15: Does your hospital have a system in place for both the initial and recurrent training and
validation of hand hygiene compliance observers?
In order to respond “yes” to question #15, your hospital must regularly monitor the quality and accuracy
of observations that are collected by each observer through initial and recurrent validation (at least
once a year) of hand hygiene compliance observers. This would include having an individual trained in

2023 Leapfrog Hospital Survey – Hard Copy
212 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
infection control simultaneously collecting data with the hand hygiene compliance observers and
comparing results. Alternatively, videos which include an interactive assessment and completion of an
observation form, such as the
WHO Hand Hygiene Training Film and Slides Accompanying the Training
Films, Videos from Hand Hygiene Australia, or internally developed videos with assessment, would also
be sufficient for validating hand hygiene compliance observers. Hospitals must expand the testing
scenarios that are included in the videos (i.e., the videos should be expanded to include: various types
of individuals who touch patients or who touch items that will be used by patients, a larger number of
scenarios where individuals are adherent and non-adherent, the inclusion of all moments observed,
etc.).
See FAQs
for additional information about responding to the questions in this section.

2023 Leapfrog Hospital Survey – Hard Copy
213 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Patient Safety Practices Frequently Asked Questions (FAQs)
General Questions
1. For the purposes of reporting on Section 6 of the Leapfrog Hospital Survey:
• Frontline caregivers include, but are not necessarily limited to employed physicians, mid-
levels (NPs, PAs), nurses, environmental services staff, allied health professionals, and
occupational and physical therapists.
• Service line management refers to those who manage all functions within a specific service
line (e.g., oncology, cardiac, women's, orthopedics). These individuals may be managing a
departmental sub-function within a broader department (e.g., cardiac care within the
Department of Medicine).
• Midlevel management includes the intermediate management of an organization that is
subordinate to the executive management and responsible for at least two lower levels of staff
below them. An example would include a Director of Nursing, who might oversee Nursing
Managers, who oversee the nurses who care directly for patients.
• Physician leadership refers to physicians who serve in a leadership role in the
organization. Titles may include positions such as Chief Medical Officer, Vice President for
Medical Affairs, Medical Director, and/or Department Chair.
• Nursing leadership refers to nurses who serve in a leadership role (e.g., Chief Nursing
Officer, Vice President/Assistant Vice President of Nursing, Vice President/Assistant Vice
President for Clinical Operations, etc.)
• Senior administrative leadership refers to administrators who are responsible for hospital-
wide departments or services (e.g., Chief Executive Officer, Chief Administrative Officer, Chief
Nursing Officer, Chief Medical Officer, etc.).
• Patient Safety Officer refers to the patient safety leader (who may or may not have the title
“Patient Safety Officer”) who has responsibility for multiple and integrated areas of patient
safety. The organization may appoint an officer who may have other assigned duties or may
specifically employ a Patient Safety Officer designated with this responsibility. Multiple
executives who are responsible for individual areas (i.e., risk, quality, infection prevention,
etc.), but do not assess the integrated safety issues, would not qualify.
• Board (governance) refers to the individual hospital’s board of directors, or the board that
governs the hospital and has the ability to pass policies that impact the hospital, or a
committee of the board (such as a board-appointed, hospital-wide Patient Safety and Quality
Committee, which includes board members).
• Medical executive committee refers to a primary governance committee for medical staff.
The medical executive committee makes leadership decisions related to medical staff policies,
procedures, and rules, with an emphasis on quality control and quality improvement. They also
adopt and implement these policies and procedures and are responsible for medical staff
appointment and reappointment.
2. Why is it necessary to continue to review a safe practice once it has been implemented?
All too often in the hectic pace of providing patient care in a hospital, with frequent staff turnover
and lots of part-time employees, it is difficult to get a change in practice well-established. Annual
review with monitoring and tracking of the safe practices will ensure that they are embedded in the
operations of the hospital and not lost in the transition of new staff coming in or part-time
employees coming and going.

2023 Leapfrog Hospital Survey – Hard Copy
214 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
3. The phrase “performance reviews or compensation” is used throughout Section 6 within
many Accountable elements. Do performance reviews and compensation plans need to
have specific language about the Safe Practice or can a set of patient safety goals related to
the specific Safe Practice be attached?
A performance review or compensation plan should include specific language about a Safe
Practice. A list of Safe Practices and related goals may be incorporated into the performance
review and/or compensation plan or formalized programs whereby a measure of success of those
activities or programs is tied to individual performance reviews or compensation incentive plans of
executives.
Every employee should have a patient safety component included in their annual review. Another
option is to include in the employee’s competency review (OPPE, FPPE).
4. There are several references to hospital budgets throughout Section 6 within many Ability
elements. How can hospitals meet the intent of these elements?
The intent of these elements is to verify that actions specific to the Safe Practices have been
included in hospital budgets. To meet the intent of these elements, hospitals should ensure that
these actions can be identified within a department budget or hospital budget. If the budget
includes categories that address the Safe Practice but do not specifically name the Safe Practice,
then the intent of the element is met.
Further, if a hospital has not allocated budget dollars for activities tied to a Safe Practice but can
document expenses specific to the Safe Practice during the reporting period, the intent of the
element is met. Plans to allocate specific budget dollars for a Safe Practice should be incorporated
into the next upcoming budget year as an ongoing process.
Hospitals may also document training or education expenditures specific to the Safe Practice or
expenditures on educational materials that are specific to the Safe Practice.
Hospitals that have invested in in-house staff educators and who include in their job descriptions
the coordination and delivery of training and education to appropriate hospital staff on specific Safe
Practices meet the intent of this element. For example, if the position description for an in-house
educator, such as the Quality Director, includes the coordination and delivery of in-service training
and educational sessions related to improving the culture of safety based on the organization’s
culture of safety survey results, then the intent of this practice is met. Specific time allocations are
not required as long as there is documentation of staff participation through dated meeting minutes
or attendance records. Training can be in-person or virtual/ computer-based.
5. How should staff education be measured?
Educational meetings should clearly address the subject matter pertinent to adverse events and
performance improvement targeted by the specific Safe Practice. Hospitals should track meeting
dates, frequency of training sessions provided, attendance records or completion records, and the
percentage of the total staff who received the information. Training can be in-person or virtual/
computer-based.
6. There are several references to developing or implementing Performance Improvement
Programs throughout Section 6 within many Action elements. How can hospitals meet the
intent of these elements?
At a minimum, performance improvement programs should include all the following five criteria:
• Education regarding the pertinent adverse event frequency, severity, and/or impact of best
practices. These expenses should be budgeted and tracked to meet the budget elements
of the Safe Practices.
• Skill building in use of performance improvement tools. These expenses should be
budgeted and tracked to meet the budget elements of the Safe Practices.
• Measurement of process measures or outcomes measures. The “Outcome, Process,
Structure, and Patient-Centered Measures” section at the end of each Safe Practice in the

2023 Leapfrog Hospital Survey – Hard Copy
215 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
NQF Safe Practices for Better Healthcare 2010 Update suggests performance measures
that can be used to support measuring and monitoring quality improvement efforts.
• Process improvement and interventions.
• Reporting of performance outcomes.
A “campaign” such as an awareness campaign would not meet the intent of these elements.
NQF Safe Practice #1 – Leadership Structures and Systems
7. 1.1a, 1.2b, and 1.2d: Several elements within NQF Safe Practice #1 mention that “regular
communication” is required. How does Leapfrog define “regular communication?”
Regular communication means more than once a year. Some hospitals may discuss these items
quarterly or even monthly. Hospitals can document that these communications took place through
dated meeting minutes. We would urge hospitals to improve the detail of their board and other
meeting minutes to ensure they are able to clearly document that the issues were discussed.
The discussion of these items can be a general note in the minutes, without specific details.
However, hospitals should maintain copies of dated presentations and reports related to these
agenda items in order to document adherence to these elements. Meetings can be in-person or
virtual.
8. 1.1a: If NQF Safe Practice #4 – Identification and Mitigation of Risks and Hazards has been
removed from the Leapfrog Hospital Survey, are hospital boards still expected to
communicate regularly regarding risks and hazards?
Though we are not asking hospitals to report on this Safe Practice, it remains important to
communicate the actions you are taking to identify and mitigate risks and hazards to patients to
your board.
9. 1.1b: What is meant by “patients and/or families of patients are active participants in the
hospital-wide safety and quality committee?”
To meet the intent of this element, hospitals must have patients and/or families of patients
participate on the hospital-wide safety and quality committee. The safety and quality committee
should have influence over hospital-wide quality and safety issues, not just a particular department
or service line. Meetings should be formal, and minutes should be taken. Topics covered should be
related to broad oversight of hospital-wide patient safety and quality issues and what is being done
to effect changes. An example would be tracking and preventing adverse events.
In most hospitals, due to the scope of issues discussed at Patient and Family Advisory Council
(PFAC) meetings, having a PFAC would not meet the criteria for a safety and quality committee. If
your hospital has a PFAC member on the hospital-wide patient safety and quality committee, then
your hospital is meeting the intent of this safe practice.
Patients and/or families of patients can participate in these meetings in person, via conference call,
or via video conference. Hospitals do not meet the intent of this element if the patients and/or
families of patients are invited but do not regularly attend. It is the responsibility of the hospital to
ensure that patients and/or families of patients can provide their perspectives to other committee
members during meetings. Hospitals should identify people who are not Board members or
employees to serve on the committee so the participant can represent the views of patients and
without conflict. Board members have a fiduciary responsibility to the organization, and therefore
may have a potential conflict representing the views of patients and/or families of patients.
Hospitals can document adherence to this element by maintaining committee rosters and meeting
minutes with attendance and participation noted. Patients and/or families of patients should have
the opportunity to present or co-present a topic, lead or co-lead a discussion, or co-chair the
committee, and this should be noted in the meeting minutes. Patients and/or families should have
attended at least one meeting prior to Survey submission.

2023 Leapfrog Hospital Survey – Hard Copy
216 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Hospitals in the process of adding patients/families of patients to the hospital-wide safety and
quality committee can refer to AHRQ’s toolkit for engaging patients and families in hospital
improvement work for more information at
https://www.ahrq.gov/patient-safety/patients-
families/engagingfamilies/index.html.
10. 1.1c: What are some examples of how a hospital can report to the community ongoing
efforts and results of these efforts to improve safety and quality?
Hospitals can utilize several communication vehicles to reach the broader community, including
webpages that are prominent from the organization’s homepage, electronic newsletters, mailings or
annual reports, or an ad in the local paper. The communication must include both efforts the
hospital is taking to improve safety and quality and the results of those efforts. As the sole focus of
the NQF Safe Practices for Better Health Care report is reducing or preventing adverse events
(refer to NQF list of adverse events at
https://www.qualityforum.org/topics/sres/serious_reportable_events.aspx), patient safety and
quality efforts reported to the community must have this focus as well. In other words, efforts the
hospital is taking to improve safety and quality should be related to reducing or preventing these
adverse events and the results of those efforts would be the measurable outcomes.
Meetings where a few community members are present would not meet the intent of this practice
as the intent is to reach a larger audience.
Each hospital within the same health care system would need to report the results to the
community for each hospital in the system because the results of efforts to improve safety and
quality would be different at each hospital.
11. 1.2a, 1.3a, 1.4c: What are the minimum requirements to qualify as a “patient safety
program?”
As part of accreditation through The Joint Commission, hospitals are required to meet standard
LD.03.09.01, which identifies the elements that must be included in an integrated patient safety
program (see pages PS-28 to PS-30 in Patient Safety Systems chapter of the CAMH
). Hospitals
that are not accredited by The Joint Commission can use these elements as a guide as well.
12. 1.2d: What is the role of an interdisciplinary patient safety committee?
An interdisciplinary patient safety committee is an internal hospital committee that oversees the
activities defined in the NQF Safe Practice #1 Practice Element Specifications and develops action
plans to create solutions and changes in performance.
13. 1.2d: What is an example of team training that is appropriate for caregivers?
Hospitals can utilize TeamSTEPPS
, a comprehensive, evidence-based training program for
healthcare professionals. At a minimum, the elements of basic teamwork training should be met as
described on page 96 of the Safe Practices for Better Healthcare– 2010 Update, which is available
for download here:
http://www.leapfroggroup.org/survey-materials/survey-and-cpoe-materials.
Team training to caregivers would need to be provided and then reported to senior administrative
leadership by the interdisciplinary patient safety team to meet the intent of Safe Practice 1.2d.
14. 1.2e: How can hospitals that have not had any adverse events during the reporting period
earn credit for this element?
First, we urge your hospital to reassess its conclusion that no adverse events occurred; that would
be highly unusual. Following the reassessment, if no adverse events were identified and the
hospital can document that it has policies in place to report such events when they do occur (to a
mandatory or voluntary program), the hospital would meet the intent of this element. Please see
Section 7A: Never Events for a list of adverse events and components of a Never Events Policy.

2023 Leapfrog Hospital Survey – Hard Copy
217 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
15. 1.4a: What are some examples of how the CEO and senior administrative leadership can be
personally engaged in reinforcing patient safety improvements?
Executive walk-arounds are an example of how the CEO and senior administrative leadership can
be personally engaged in reinforcing patient safety improvements. The executive walk-arounds
provide staff with visibility and access to senior management. They also provide the CEO and
senior administrative leadership with the opportunity to address issues and concerns in various
departments in real-time, rather than in monthly meetings. Monthly meetings with staff in a
centralized location do not meet the intent of this Safe Practice.
Progress on the implementation of walk-arounds can be measured by tracking the number of walk-
arounds performed per unit or clinical area for designated time periods as shown in the calendars
of the CEO and senior administrative leadership. Some progressive hospitals have tied incentives
to regular executive walk-rounds and to reliable exchange of information on clinical unit
performance. Some hospitals have established a feedback loop between the CEO and senior
administrative leadership and staff to measure the implementation of performance improvement
ideas that were generated during executive walk-arounds.
16. 1.4b: What are some examples of how the CEO can actively engage leaders from service
lines, midlevel management, clinical leadership, and physician leadership in patient safety
improvement actions?
Hospitals can refer to the American College of Healthcare Executives
professional policy
statement, which includes examples of how leaders should be engaged in patient safety and
quality.
17. 1.4c: What are some examples of how hospitals can engage the medical staff as direct
contributors to the patient safety program?
Examples may include:
• Senior leadership requests time on Medical Staff Department standing agendas to provide
patient safety updates and elicit direct feedback on specific areas as well as “what keeps the
medical staff up at night.”
• Medical staff are invited and encouraged to be active participants in clinical unit meetings
where patient safety is addressed.
• The board appoints a community-based active medical staff member to represent the
organization on a regional patient safety initiative.
18. 1.4c: In a hospital where all medical staff is employed, how do we answer this question?
The intent of this element is to ensure that physicians and medical staff have the opportunity to
provide input on the hospital’s patient safety plan because often they do not have a significant
position in the hierarchal structure of an organization but carry a great deal of influence over how
the organization is run. Thus, they are informal leaders who can be change agents and
“accelerators or barriers for improvement.” If the organization's board and senior administrative
leadership seek and document input from physicians and medical staff regarding patient safety
programs, the intent of this element has been met.
NQF Safe Practice #2 – Culture Measurement, Feedback, and Intervention
19. Why are two different reporting periods used in NQF Safe Practice #2?
Within the Awareness and Accountability elements, a 24-month reporting period is used because
these elements are related to conducting the culture of safety survey, which is typically conducted
every other year. Within the Ability and Action elements, a 12-month reporting period is used
because these practices are related to follow-up activities that would be completed after the results
from the culture of safety survey are available.
20. 2.1a: What are the requirements to qualify as a “culture of safety survey?”
Several surveys are readily available that specifically address culture, safety climate, and
teamwork. These surveys incorporate all the additional specifications as outlined in NQF Safe
Practice #2 (see 2010 NQF Safe Practice Report).

2023 Leapfrog Hospital Survey – Hard Copy
218 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Hospitals that do not use a nationally recognized culture of safety tool must ensure that their
culture of safety survey meets Leapfrog’s guidelines for what constitutes a valid, consistent, and
reliable survey tool. These guidelines were developed in consultation with Leapfrog’s Culture of
Safety Expert Panel. The guidelines can be found on the Survey and CPOE Materials webpage
under supporting materials for Section 6.
A general employee satisfaction survey that has a small component of the survey addressing
organizational culture does not qualify.
21. 2.1a Can an employee engagement survey and nationally recognized culture of safety tool
be conducted at the same time?
Yes, if the culture of safety tool is unaltered and administered in its entirety.
22. 2.1a: What does “50% of the aggregated care delivered to patients within the hospital”
mean?
As described on page 88 of the NQF Safe Practices for Better Healthcare Report, “a census of
units or service areas that in aggregate deliver care to more than 50 percent of the patients
receiving care should be surveyed, service lines or units where there is a high patient safety risk
should be measured, and there should be a valid sample to allow for unit-level analysis and
facilitate improvement.”
23. 2.1b: For reporting individual unit level results, what is the minimum number of responses a
hospital should have?
Most major vendors use a threshold of 5 or more responses and a 40% response rate. For larger
units, a lower response rate may be acceptable. If a unit does not meet these thresholds, your
hospital could aggregate the results of “like” units together (e.g., medical/surgical units, ICUs,
ORs). Hospitals should not combine results across “unlike” units.
24. 2.1c: What is meant by “like” hospitals? Can hospitals benchmark against those within the
same network or system? How would a pediatric hospital benchmark against other pediatric
hospitals?
Hospitals should benchmark their results against hospitals with similar demographics, such as
hospital type, number of beds, number of admissions, urban/rural designation, etc. Hospitals in
systems or healthcare networks should benchmark throughout the health system, but not within the
same region.
Pediatric hospitals utilizing the AHRQ Hospital Survey on Patient Safety Culture can benchmark
their results using the AHRQ’s User Comparative Database Report
(refer to instructions starting on
page 29).
25. 2.1d: What is meant by roles and staff levels?
Roles are job types (e.g., surgeons, nurses, hospitalists, physician assistants, or clinical and non-
clinical, etc.). Staff levels are defined within the organization’s hierarchy (e.g., senior
administrators, directors, managers, etc.).
26. 2.2b: Does performance evaluation criteria for senior administrative leadership need to
include the actual targeted response rate to the culture of safety survey?
Yes. The organization’s targeted response rate to the culture of safety survey should be included in
performance evaluation criteria for senior administrative leadership. Criteria for using the survey
results in improvement efforts should also be included to meet the intent of this element.
27. 2.3a: Which employees should be included in the staff education program? Employees in all
units or just those in low-performing units?
Staff education needs to include education for all levels of staff, from senior administrative and
clinical leadership to frontline caregivers. In addition, because all units have opportunities for
improvement, the staff education should include all units but can focus on deficiencies of a specific
unit or department.

2023 Leapfrog Hospital Survey – Hard Copy
219 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
28. 2.1e and 2.4b: What is the difference between 2.1e and 2.4b? Both seem to focus on sharing
and discussing the Culture of Safety Survey results.
While both elements focus on sharing and discussing Culture of Safety Survey results, these
elements focus on different audiences and activities, and the result is that different kinds of
feedback are collected. Safe Practice 2.1e requires local leaders (e.g., unit/department manager)
to engage their unit/departments in a discussion about the survey results, while Safe Practice 2.4b
requires senior administrative leadership to engage each sampled unit in a discussion about the
survey results and their concerns.
Nurse Staffing and Skill Level
29. Should we include nursing hours from days when a unit was open and staffed but had zero
patients?
No. Nursing hours from days when the unit was open and staffed but had zero patients should not
be included as these would not be considered direct patient care hours. Additionally, if these
nursing hours cannot be removed, then the unit should not be included.
As a reminder, the Percentage of RNs that are BSN-Prepared measure is a hospital-wide measure
and is not unit specific (as described in measure specifications).
30. Why has Leapfrog aligned their unit type definitions with NDNQI?
Though NDNQI participation is not required to report on the Total Nursing Care Hours per Patient
Day, RN Hours per Patient Day, or Nursing Skill Mix measures, a number of hospitals participate in
NDNQI and have data available to report to the Leapfrog Hospital Survey.
31. Should medical, surgical, or med-surg units equipped with telemetry, and/or units that
include patients needing telemetry be excluded when reporting on the Total Nursing Care
Hours per Patient Day, RN Hours per Patient Day, and Nursing Skill Mix measures?
Not necessarily. For the purposes of these measures, Leapfrog has aligned with NDNQI, which
defines units based on the acuity of the patients being cared for in the unit, and not the equipment
being used. Telemetry is considered a monitoring tool rather than an evaluation of a patient’s level
of acuity. For example, a unit that has all telemetry patients may still have a general population
level of patients that would meet NDNQI’s definition of a medical, surgical, or med-surg unit.
Hospitals should evaluate the actual acuity of the patients in each unit to determine the appropriate
unit designation.
32. What is the definition of UAP?
UAP is any unlicensed person, regardless of title, who performs tasks delegated by a nurse. This
includes certified nursing aides/assistants (CNAs), patient care assistants (PCAs), patient care
technicians (PCTs), state-tested nursing assistants (STNA), nursing assistants-registered (NA/Rs)
or certified medication aides/assistants (MA-Cs).
33. Are Advanced Practice Registered Nurses (APRNs), which includes certified nurse-
midwifes, clinical nurse specialists, certified nurse practitioners, and certified registered
nurse anesthetists, included when calculating total nursing care hours (question #6b, #8b
and #10b), RN hours (question #6c, #8c and #10c), and total number of employed RNs in the
hospital (question #12)?
No. APRNs do not provide direct patient care responsibilities as defined in the measure
specifications. APRNs provide a higher level of care, such as diagnosing illnesses and prescribing
treatments and medications.
34. Should RNs with direct patient care responsibilities exclusively assigned to outpatient units
or hospital clinics be included when reporting on the Percentage of RNs who are BSN-
Prepared measure?

2023 Leapfrog Hospital Survey – Hard Copy
220 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Yes. The total number of RN nursing staff employed at the hospital should include nurses with
direct care responsibilities in most hospital units, including observation, co-located
30
outpatient
units, and emergency departments. Only employed RNs exclusively assigned to Skilled Nursing
units or Mixed Acuity Neonatal units should be excluded when reporting on the measure.
NQF Safe Practice #9 – Nursing Workforce
35. 15e: If the state has set minimum nurse to patient staffing ratios, do hospitals in that state
automatically meet the intent of this element?
No. Minimum ratios do not necessarily address the “adequacy” issue, as the make-up of your
hospital’s patient population may require more intensive staffing than are prescribed by the state’s
minimum.
36. 15e: What are the minimum requirements to qualify as a “staffing plan?”
“A staffing plan” refers to nursing policies and procedures or a specific process used by the
organization to pre-determine appropriate staffing patterns based on usual patient mix and nursing
qualifications. A hospital must demonstrate full achievement of its targets.
Organizations must integrate several data sets into a staffing system that predefines and quantifies
appropriate staffing targets. These data sets include:
• Historical Data (e.g., patient volumes, acuity levels, and staff volumes of direct
caregivers)
• Comparative Data (e.g., comparisons between similar units internally and comparative
external data from hospitals of like size and geographic location)
• Clinical Outcomes
• Skill Mix of Staff (e.g., licensing levels and educational training, years of experience, and
volume of new graduates on a unit)
• Physical environment (e.g., distance staff have to travel to access support equipment,
visibility of patients, locations of nursing stations to patient rooms, etc.)
• Type of patient care needs
• Support services available
Daily monitoring should take place to determine variances between predetermined staffing patterns
and actual staffing patterns. If necessary, corrective action should be taken. Regular monitoring
should take place to determine the accuracy of targets established and determine adjustments as
needed.
Hand Hygiene
Training and Education
37. Are online training modules acceptable for the purposes of question #1 and question #3?
Online training modules are acceptable for the purposes of answering question #1 and question #3
if they meet all requirements outlined in the question.
For question #1, the online training must be done at the frequency specified and would need to be
delivered and/or developed by a professional with appropriate training and skills
. For question #3,
the online training must meet all six topics outlined in the question.
Physical demonstration (question #2) cannot be done using an online training module.
38. What are examples of what can count as “physically demonstrating” proper hand hygiene
during the initial hand hygiene training?
Before new individuals to your hospital have contact with patients and the patient care space, they
will need to demonstrate proper hand hygiene with soap and water and alcohol-based hand

2023 Leapfrog Hospital Survey – Hard Copy
221 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
sanitizer. This demonstration could be done as part of other onboarding activities, during
occupational health activities as part of the TB test, during department orientations, in small
groups, etc. A group “teach-back” would be acceptable, but with no more than 10 students per one
trainer/monitor. An online or in-person “simulation” would not be sufficient for this purpose.
Computer-based assessments of an individual’s hand hygiene technique are acceptable if the
assessment tracks an individual’s hand motions, is done without providing instructions to the
individual during the assessment, and feedback is given to them at the end.
Hospitals that are starting to implement this component should add physical demonstration to their
initial training for any new hires. Leapfrog is not asking hospitals to retroactively train individuals.
Infrastructure
39. What does Leapfrog mean by “equally accessible to the location of all patients in the room
or bed space” for the purposes of question #5?
Equally means the same distance from any patient's bed, which can be measured in steps.
Leapfrog is not looking for an exact distance, but rather the goal is to ensure that hand hygiene can
be easily performed regardless of the location of the patient being cared for in the room or bed
space.
Monitoring
40. Why did Leapfrog select 200 hand hygiene opportunities for monthly monitoring in question
#8?
200 hand hygiene opportunities were chosen as the sample size based on a study by Yin et. al
which showed that 180-195 opportunities would need to be monitored to accurately observe a 10%
change in hand hygiene compliance (Yin et al.). The additional sample sizes outlined in the
measure specifications are for smaller patient care units where monitoring 200 opportunities may
not be feasible.
References:
Steed C, Kelly JW, Blackhurst D, Boeker S, Diller T, Alper P, Larson E. Hospital hand hygiene
opportunities: where and when (HOW2)? The HOW2 Benchmark Study. American Journal of
Infection control. 2011 Feb 1;39(1):19-26.
Jun Yin MS, Heather Schacht Reisinger PhD, Mark Vander Weg PhD, Marin L. Schweizer PhD,
Andrew Jesson, Daniel J. Morgan MD MS, Graeme Forrest MD, Margaret Graham, Lisa Pineles
MA and Eli N. Perencevich MD MS. Establishing evidence-based criteria for directly observed
hand hygiene compliance monitoring programs: a prospective, multicenter cohort study. Infection
Control and Hospital Epidemiology Vol. 35, No. 9 2014 Sep, pp. 1163-8.
41. My hospital uses an electronic compliance monitoring system, but it does not meet all the
criteria outlined in questions #12-13. Can I report on the hand hygiene compliance data we
collect via direct observation instead?
Yes. If your hospital also uses direct observation to collect hand hygiene compliance data (not just
for coaching/intervention) throughout all patient care units (including those with the electronic
compliance monitoring system), you can select “yes, using only direct observation” in either
question #8, question #9, or question #10 and report on your adherence to the direct observation
criteria only. Otherwise, you will need to respond “no” to question #12.
42. Is Leapfrog encouraging hospitals to implement electronic compliance monitoring? These
systems can be costly, and the technology still needs to advance.
The questions in the hand hygiene standard ask about a variety of strategies that can be used to
monitor and improve hand hygiene. Leapfrog is encouraging hospitals to take a multimodal
approach. Regarding monitoring, while hospitals can achieve the Leapfrog standard with direct

2023 Leapfrog Hospital Survey – Hard Copy
222 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
observation alone, Leapfrog is communicating a strong preference for use of electronic monitoring
(implemented according to evidence-based principles). In addition to literature suggesting
electronic monitoring works better to pinpoint compliance issues, the difference in the sheer
number of hand hygiene opportunities covered by the two monitoring strategies represent powerful
evidence in favor of electronic monitoring. Electronic monitoring allows facilities to monitor virtually
every patient encounter, while direct observation monitors a selection. Based on the evidence, our
standard calls for monitoring 200 hand hygiene opportunities per unit per month, which is a small
subset of overall hand hygiene opportunities. Even beyond capturing more encounters aligned with
the evidence, electronic monitoring alleviates the ethical quandary of an observer watching patient
harm without intervening.
As with Computerized Physician Order Entry (CPOE) systems and Bar Code Medication
Administration (BCMA) systems, we anticipate that electronic compliance monitoring technology
will improve over time and become an important component of a comprehensive hand hygiene
program. Electronic monitoring is a routine component of public safety in other industries where
compliance is critical, so health care can and should achieve those standards for its patients.
All items included in Section 6D are based on the evidence review and recommendations from
Leapfrog's national Hand Hygiene Expert Panel
and others. We have included in the Hand Hygiene
bibliography several peer-reviewed studies that have examined the benefits of using electronic
monitoring systems over direct observation. The bibliography is available at
https://ratings.leapfroggroup.org/measure/hospital/handwashing.
43. Are online training modules acceptable for the purposes of training hand hygiene
compliance observers in question #15?
Online training can be used for the initial and recurrent training of hand hygiene compliance
observers. Please refer to the Hand Hygiene Measure Specifications for more information on the
requirements for the validation of hand hygiene compliance observers.
Feedback
44. For the purposes of responding to question #19, what are some examples of how hospital
leadership can be held accountable through performance reviews or compensation?
A performance review or compensation plan should include specific language about hand hygiene
performance. A list of hand hygiene practices and related goals may be incorporated into the
performance review and/or compensation plan or formalized programs whereby a measure of
success of those activities or programs is tied to individual performance reviews or compensation
incentive plans of executives. Examples include meeting targets for hand hygiene compliance
rates, having bonuses tied to structural changes like the implementation of electronic compliance
monitoring systems, etc. Language pertaining solely to infection control practices and performance
would NOT be sufficient.
Culture
45. What are some examples of how patients and visitors can be invited to remind individuals
who touch patients or who touch items that will be used by patients to perform hand
hygiene?
Patients and visitors can be invited to remind individuals who touch patients or who touch items
that will be used by patients to perform hand hygiene with posters placed in patient care units,
bedside placards, buttons worn by the staff, etc.
46. What are some examples of demonstrating a commitment to hand hygiene improvement as
referenced in question #21?
Some examples of how individuals can demonstrate a commitment to support hand hygiene
improvement are written or verbal commitments given during town hall meetings, videos, e-mails
from the CEO, public comments to staff, etc. This needs to be a verbal or written commitment that

2023 Leapfrog Hospital Survey – Hard Copy Section 7: Managing Serious Errors
225 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
SECTION 7: MANAGING SERIOUS ERRORS
This section includes questions and reference information for Section 7: Managing Serious Errors. Please
carefully review the questions, endnotes, and reference information (e.g., measure specifications, notes,
and frequently asked questions) before you begin. Failure to review the reference information could result
in inaccurate responses.

2023 Leapfrog Hospital Survey – Hard Copy Section 7: Managing Serious Errors
226 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 7: 2023 Managing Serious Errors
Never Events Fact Sheet:
https://ratings.leapfroggroup.org/measure/hospital/2023/responding-never-
events
Section 7 includes questions about your hospital’s response to Never Events. In addition, Leapfrog
collects information via its NHSN Group about five healthcare-associated infections (CLABSI, CAUTI,
MRSA, C. diff., and SSI: Colon). Hospitals reporting on Section 7B: Healthcare-Associated Infections are
required to join Leapfrog’s NHSN Group. Important information and deadlines are available on the
Join
NHSN Group webpage.
Each hospital achieving the standard for Never Events:
Has a policy that includes the nine principles of Leapfrog’s Never Events policy and will implement this
policy if a “never event” occurs within their hospital.
Each hospital achieving the standards for Healthcare-Associated Infections:
1. Has a CLABSI standardized infection ratio of less than or equal to 0.413 for ICU and select ward
inpatients.
2. Has a CAUTI standardized infection ratio of less than or equal to 0.427 for ICU and select ward
inpatients.
3. Has a MRSA standardized infection ratio of less than or equal to 0.496 for facility-wide inpatients.
4. Has a C. diff. standardized infection ratio of less than or equal to 0.621 for facility-wide inpatients.
5. Has an SSI: Colon standardized infection ratio of less than or equal to 0.349 for inpatients following
eligible colon procedures.
Download the 2023 Leapfrog Hospital Survey Scoring Algorithm on the
Scoring and Results
webpage.

2023 Leapfrog Hospital Survey – Hard Copy Section 7: Managing Serious Errors
227 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
7A: Never Events
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Note: To earn credit for these questions, hospitals must have a policy in place that addresses
the National Quality Forum’s list of Serious Reportable Events. All references to “never event” or “serious
reportable event” are specific to the National Quality Forum list available at
https://www.qualityforum.org/topics/sres/serious_reportable_events.aspx
.
Below are the nine elements which make up The Leapfrog Group’s Policy Statement regarding
never
events.
46
Indicate which of the following principles are included in your hospital’s current never events
policy.
1) We apologize to the patient
47
and/or family affected by the never event
46
.
Yes
No
2) We report the event to at least one of the following external agencies
48
within 15 business days of becoming aware that the never event
46
has
occurred:
√ Joint Commission, as part of its Sentinel Events policy
√ DNV GL Healthcare
√ State reporting program for medical errors
√ Patient Safety Organization (as defined in The Patient Safety and
Quality Improvement Act of 2005)
Yes
No
3) We perform a root cause analysis
49
, which at a minimum, includes the
elements required by the chosen external reporting agency.
Yes
No
4) We waive all costs directly related to the never event
46
.
In order to respond “Yes” to this question, all costs directly related to the
never event must be waived to both the patient and the payor.
Yes
No
5) We make a copy of this policy available to patients, patients’ family
members, and payers upon request.
Yes
No
6) We interview patients and/or families, who are willing and able, to gather
evidence for the root cause analysis
49
.
Yes
No
7) We inform the patient and/or the patient’s family of the action(s) that our
hospital will take to prevent future recurrences of similar events based on
the findings from the root cause analysis
49
.
Yes
No
8) We have a protocol in place to provide support for caregivers involved in
never events
46
and make that protocol known to all caregivers and
affiliated clinicians.
Yes
No
9) We perform an annual review to ensure compliance with each element of
Leapfrog’s Never Events Policy for each never event
46
that occurred.
Cannot respond “yes” to this question, unless “yes” to questions #1-8.
Yes
No
Reporting Period:
Answer questions #1-9 based on the principles currently included in your hospital’s never events
policy at the time you submit this section of the Survey.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 7: Managing Serious Errors
228 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
7B: Healthcare-Associated Infections
Hospitals that share a CMS Certification Number, must have a unique NHSN ID as required by NHSN.
Please carefully review Leapfrog’s NHSN Instructions webpage
.
Specifications: See Healthcare-Associated Infections Measure Specifications
in the Reference
Information beginning on page 231.
Leapfrog obtains standardized infection ratios (SIRs) for each of the following applicable infection
measures directly from the CDC’s National Healthcare Safety Network (NHSN):
• CLABSI in ICUs and select wards
• CAUTI in ICUs and select wards
• Facility-wide inpatient MRSA Blood Laboratory-identified Events
• Facility-wide inpatient C. diff. Laboratory-identified Events
• SSI: Colon
In order for Leapfrog to obtain the SIRs for each applicable infection from NHSN, hospitals must complete
the following steps:
1. Join* Leapfrog’s NHSN Group by the published deadlines using the checklist
in the Healthcare-
Associated Infections Measure Specifications,
2. Provide an accurate NHSN ID in the Hospital Profile, and
3. Submit the 2023 Leapfrog Hospital Survey.
*Hospitals are not required to “re-join” Leapfrog’s NHSN Group if they joined and conferred rights in
previous Leapfrog Hospital Survey Cycles. However, all hospitals in Leapfrog’s NHSN Group must review
their Rights Acceptance Report annually (by the published join-by deadlines
) to ensure that Leapfrog has
access to the data from all of the locations that were active during the reporting period, even if those
locations are no longer active, to ensure that Leapfrog obtains the appropriate SIR.
Hospitals that join Leapfrog’s NHSN group, but do not provide an accurate NHSN ID in the Hospital
Profile or do not submit the 2023 Leapfrog Hospital Survey, will be scored and publicly reported as
“Declined to Respond” for each of the five infection measures.
For all other deadlines, please refer to the “Deadlines and Reporting Periods” table provided in the
Healthcare-Associated Infections Measure Specifications, as well as online
.
Reporting Period: 12 months
• June and August Data Downloads: 01/01/2022 – 12/31/2022
• October and December Data Downloads: 07/01/2022 – 06/30/2023
Leapfrog will update data four times per Survey Cycle for all members of our NHSN group that have provided an accurate
NHSN ID in the Hospital Profile and submitted the 2023 Leapfrog Hospital Survey.
Visit the Join NHSN Group webpage for important information on deadlines for joining Leapfrog’s NHSN Group.

2023 Leapfrog Hospital Survey – Hard Copy Section 7: Managing Serious Errors
229 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Affirmation of Accuracy
As the hospital CEO or as an employee of the hospital to whom the hospital CEO has delegated
responsibility, I have reviewed this information pertaining to the Managing Serious Errors Section at our
hospital, and I hereby certify that this information is true, accurate, and reflects the current, normal
operating circumstances at our hospital. I am authorized to make this certification on behalf of our
hospital.
The hospital and I understand that The Leapfrog Group, its members, the public and entities and persons
who contract or have other business dealing with The Leapfrog Group are relying on the truth and
accuracy of this information. The hospital and I also understand that The Leapfrog Group will make this
information and/or analyses of this information public through the Survey Results public reporting website,
The Leapfrog Group’s Hospital Safety Grade, and/or other Leapfrog Group products and services. This
information and/or analyses and all intellectual property rights therein shall be and remain the sole and
exclusive property of The Leapfrog Group in which The Leapfrog Group retains exclusive ownership. This
information does not infringe upon any third-party intellectual property rights or any other third-party rights
whatsoever and is free and clear of all encumbrances and liens of any kind. The hospital and I
acknowledge that The Leapfrog Group may use this information in a commercial manner for profit. The
hospital shall be liable for and shall hold harmless and indemnify The Leapfrog Group from any and all
damages, demands, costs, or causes of action resulting from any inaccuracies in the information or any
misrepresentations in this Affirmation of Accuracy. The Leapfrog Group and its members and entities and
persons who contract or have other business dealing with The Leapfrog Group reserve the right to omit or
disclaim information that is not current, accurate or truthful.
Affirmed by _____________________, the hospital’s ___________________________,
(first name and last name) (title)
on _______________________.
(date)

2023 Leapfrog Hospital Survey – Hard Copy Section 7: Managing Serious Errors
230 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 7: 2023 Managing Serious Errors Reference Information
What’s New in the 2023 Survey
We are adding Section 7: Managing Serious Errors to the list of sections required to submit the 2023
Leapfrog Hospital Survey.
Section 7A: Never Events
Leapfrog is clarifying question #4, which asks if hospitals waive all costs related to all never events
, by
stating that costs must be waived to both the patient and the payor.
Change Summary Since Release
None. If substantive changes are made to this section of the Survey after release on April 1, 2023, they
will be documented in this Change Summary section.

2023 Leapfrog Hospital Survey – Hard Copy Section 7: Managing Serious Errors
231 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Healthcare-Associated Infections Measure Specifications
Important Note: Hospitals that share a CMS Certification Number must have a unique NHSN ID as
required by NHSN. Please carefully review Leapfrog’s NHSN Instructions webpage
.
Checklist for Joining Leapfrog’s NHSN Group and Ensuring the Data are Accurate
Join or verify that you are in Leapfrog’s NHSN Group by the join-by dates
o Instructions for joining or verifying that you are in Leapfrog’s NHSN Group are available here in
the “NHSN Guidance: Join the Group, Review/Accept Data Rights Template, and Downloading
Reports” document.
o Join-by dates are listed in the “Deadlines and Reporting Periods” table below.
Review and accept Leapfrog’s Data Rights Template within NHSN
o All hospitals are required to review and accept the Data Rights Template at least annually
before the June 22, 2023, NHSN join-by date and whenever updates are made to their location
mapping in NHSN.
Include any locations that were active during the reporting period even if they are
currently inactive to ensure that Leapfrog obtains the appropriate SIR.
Confirm that you have given Leapfrog access to data from your 2022 NHSN Patient
Safety Component - Annual Hospital Survey. Surveillance data from the 2022 NHSN
Annual Hospital Survey is used by NHSN to risk adjust SIRs; SIRs cannot be calculated
by NHSN or downloaded by Leapfrog if you restrict access to this data.
Failure to review and update (as needed) your Data Rights Template may result in
Leapfrog pulling incorrect data for your hospital.
o Instructions for reviewing and accepting the Data Rights Template are available here
in the
“NHSN Guidance: Join the Group, Review/Accept Data Rights Template, and Downloading
Reports” document.
Generate datasets and download reports within NHSN on the same day as Leapfrog
o All hospitals are required to (a) generate datasets within NHSN, (b) download CMS IPPS reports,
and (c) download a copy of your 2022 Patient Safety Component - Annual Hospital Survey from
NHSN on the same day
that Leapfrog will be downloading the data from NHSN for all current
group members.
o Instructions for generating datasets and downloading these reports from NHSN are available here
in the “NHSN Guidance: Join the Group, Data Rights Template, and Downloading Reports”
document.
o NHSN data download dates are listed in the “Deadlines and Reporting Periods” table below
(please note that there are four NHSN data download dates per Survey Cycle)
o By generating datasets and downloading reports within NHSN on the same day as Leapfrog,
hospitals can confirm that their data matches the data Leapfrog has obtained.
o If hospitals do not generate datasets and download reports on the same day as Leapfrog, the
Help Desk will not review any discrepancies.
Review SIRs
o For verification purposes, hospitals are required to download reports from NHSN on the same
day, as described above.
o Once Leapfrog has published the healthcare-associated infection Survey Results on the Hospital
Details Page, hospitals are urged to compare the Survey Results to their NHSN reports that were
pulled on the same day as Leapfrog.
o Dates on which the Survey Results will be available on the Hospital Details page are listed in the
“Deadlines and Reporting Periods” table below.
Report discrepancies
If, while comparing your NHSN reports to your Leapfrog Hospital Survey Results, you find a
discrepancy, you must contact Leapfrog’s Help Desk
immediately. If you do not contact Leapfrog by

2023 Leapfrog Hospital Survey – Hard Copy Section 7: Managing Serious Errors
232 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
the end of the month in which scored results are available on your Hospital Details Page (i.e., July,
September, November, and January, respectively), the issue will not be investigated by the Help
Desk.
Deadlines and Reporting Periods
Join Leapfrog’s
NSHN Group by
Leapfrog will
download data from
NHSN for all current
group members on
Data downloaded
from NHSN will be
scored and
publicly reported
for hospitals that
have submitted
Section 7 by
HAI
Reporting
Period
Available on Hospital
Details Page and Public
Reporting Website on
June 22, 2023 June 23, 2023 June 30, 2023
01/01/2022 –
12/31/2022
July 12, 2023
Hospital Details Page
July 25, 2023
Public Reporting Website
August 23, 2023 August 24, 2023 August 31, 2023
01/01/2022 –
12/31/2022
September 8, 2023
October 23, 2023 October 24, 2023 October 31, 2023
07/01/2022 –
06/30/2023
November 7, 2023
December 20, 2023 December 21, 2023* November 30, 2023
07/01/2022 –
06/30/2023
January 8, 2024
*The Leapfrog Hospital Survey closes on November 30, 2023. The last NHSN data download is on
December 21, 2023, to incorporate any facilities and corrections from facilities that joined by the last join
date of December 20, 2023.

2023 Leapfrog Hospital Survey – Hard Copy Sect. 8 – Medication Safety
233 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Never Events Frequently Asked Questions (FAQs)
1. When reporting Never Events, what “state reporting program for medical errors” applies in
my state?
Congress has passed legislation requiring all states to develop a reporting program for medical
errors. At this time, many states have already enacted or adopted some requirement that
hospitals report serious medical errors or similar adverse events to a state agency. Others are
still implementing legislation or regulations that define that requirement. States that have
developed programs may also define reportable events differently.
2. What if there is no “state reporting program for medical errors” in my state? Do we still
have to report Never Events to meet Leapfrog principles for this policy? To whom?
Hospitals in states that do not have a state reporting program or requirement in effect can meet
the reporting requirement of Leapfrog’s principles for implementation of a Never Events policy by
reporting all Never Events voluntarily to The Joint Commission, DNV GL Healthcare, or a Patient
Safety Organization.
If there is no state-required reporting program in effect, no available Patient Safety Organization
to which your hospital can report, and your hospital is not Joint Commission or DNV GL
Healthcare accredited, the Leapfrog requirement for reporting to an external agency is amended.
Hospitals must report the Never-Event to their governance board and must still perform a root-
cause analysis internally of each Never Event to meet Leapfrog’s principle for full implementation
of its Never Events policy.
3. The reportable adverse events defined by our state’s reporting program don’t include all
twenty-nine (29) Never Events endorsed by the National Quality Forum (NQF) and adopted
in the Leapfrog policy. Will reporting only the state-required reportable events to the state
agency suffice for meeting Leapfrog’s requirement for reporting Never Events to an
external agency? Does our hospital have to report other Never Events, as defined by
NQF/Leapfrog, to that state agency even though not required by our state’s reporting
program?
Hospitals should report all their state-required reportable events to the state agency. All other
Never Events, as defined by NQF’s list of Serious Reportable Events, that cannot be reported to
the state agency, should be reported to another external agency (e.g., accreditor, Patient Safety
Organization), if possible. If reporting those events to another external agency is not possible, the
final option is to report those events to the hospital’s governance board.
4. Won’t Leapfrog’s request to have hospitals apologize to the patient put the hospital at risk
for liability?
Not necessarily. Research indicates that malpractice suits are often the result of a failure on the
hospital’s part to communicate openly with the patient and apologize for its error. Patients feel the
most anger when they perceive that no one is willing to take responsibility for the adverse event
that has occurred. A sincere apology from the responsible hospital staff can help to heal the
breach of trust between doctor/hospital and patient. (When Things Go Wrong: Responding to
Adverse Events. Boston, 2006. Mass Coalition for the Prevention of Medical Errors)
5. How does Leapfrog define “waive cost?”
At its core, Leapfrog’s approach to never events is about improving patient care. While the policy
asks hospitals to refrain from billing either the patient or a third-party payer, such as a health plan
or employer company, for any costs directly related to a serious reportable adverse event,
Leapfrog understands that, due to the wide array of circumstances surrounding never events,
specific details of what constitutes “waiving cost” should be handled on a case-by-case basis by
the parties involved. For an example, please see “
Lessons learned from implementing a
principled approach to resolution following patient harm” by Smith et. al.

2023 Leapfrog Hospital Survey – Hard Copy Sect. 8 – Medication Safety
234 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
6. Does Leapfrog recommend any resources for hospitals looking to adhere to Leapfrog’s
Never Events principles?
Yes, the Agency for Healthcare Research and Quality (AHRQ) has developed and tested the
Communication and Optimal Resolution (CANDOR) Toolkit
, which outlines a process for
hospitals and practitioners to respond to unexpected events in a timely, thorough, and just way.
The National Patient Safety Foundation (NPSF) has issued a report titled RCA
2
: Improving Root
Cause Analyses and Actions to Prevent Harm, which examines best practices and provides
guidelines to help standardize and improve Root Cause Analysis. In addition, hospitals can
download tips and tools for interviewing patients and families for the Root Cause Analysis on the
Survey and CPOE Materials webpage.

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
236 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
SECTION 8: PEDIATRIC CARE
This section includes questions and reference information for Section 8: Pediatric Care. Please carefully
review the questions, endnotes, and reference information (e.g., measure specifications, notes, and
frequently asked questions) before you begin. Failure to review the reference information could result in
inaccurate responses.

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
237 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 8: 2023 Pediatric Care
Pediatric Care Fact Sheet: https://ratings.leapfroggroup.org/measure/hospital/2023/pediatric-care
Hospitals that do not care for (or perform CT scans on) patients 17 years of age or younger should
respond “no” to question #2 in 8A and 8B and move on to the Affirmation of Accuracy. Your
hospital’s results will be displayed as “Does Not Apply” on Leapfrog’s public reporting website.
Section 8 includes questions about patient experience (CAHPS Child Hospital Survey) and Computed
Tomography (CT) radiation dose for pediatric patients. The subsection on CT dose (8B) includes a set of
optional fact-finding questions that will not be scored or publicly reported in 2023.
Each hospital achieving the standard for Patient Experience (CAHPS Child Hospital Survey):
Performed in the top quartile for at least 4 of the 5 CAHPS Child Hospital Survey domains (a subset of
the 18 total domains), listed below:
• Communication with Parent – Communication about your child’s medicines
• Communication with Parent – Keeping you informed about your child’s care
• Communication with Child – How well nurses communicate with your child
• Communication with Child – How well doctors communicate with your child
• Attention to Safety and Comfort – Preventing mistakes and helping you report concerns
Each hospital achieving the standard for Pediatric Computed Tomography (CT) Dose:
Received 75% or more of the possible points based on comparing CT radiation doses across two
anatomic areas (head and abdomen/pelvis) and five age strata to national benchmarks.
Download the 2023 Leapfrog Hospital Survey Scoring Algorithm on the
Scoring and Results
webpage.

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
238 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
8A: Patient Experience (CAHPS Child Hospital Survey)
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Notes:
Note 1: This subsection is only applicable to hospitals that care for patients 17 years of age or younger.
Hospitals that do not care for patients 17 years of age or younger should respond “no” to question #2 in
8A and move on to 8B. Your hospital’s results will be displayed a “Does Not Apply” on Leapfrog’s public
reporting website.
Note 2: To help ensure that the Top Box Scores
50
represent an appropriate sample of patients, hospitals
must have at least 100 pediatric acute-care admissions to inpatient units other than a neonatal ICU
(NICU). Otherwise, they should respond “Yes, but fewer than 100 pediatric admissions were for non-
NICU patients” to question #2 and skip the remaining questions in Section 8A. The hospital will be scored
as “Does Not Apply.” For example, if your hospital had 600 pediatric acute-care admissions, and 550 of
those admissions were to a neonatal ICU, you should respond “Yes, but fewer than 100 pediatric
admissions were for non-NICU patients” to question #2 as your hospital only has 50 admissions to
inpatient units other than a neonatal ICU.
Specifications: See Patient Experience (CAHPS Child Hospital Survey) Measure Specifications
in
the Reference Information beginning on page 246.
1) What is the latest 12-month reporting period for which your hospital is
submitting responses to this section? 12-month reporting period ending:
______
Format: Month/Year
2) Did your hospital have at least 500 pediatric acute-care admissions
during the 12-month period referenced in Section 1, question #5?
Refer to your responses to questions #5 and #11 in
Section 1A: Basic
Hospital Information.
If “no” or “yes, but fewer than 100 pediatric admissions were for non-
NICU patients,” skip questions #3-12 and continue to the next
subsection. The hospital will be scored as “Does Not Apply.”
Yes
Yes, but fewer than 100
pediatric admissions were for
non-NICU patients
No
3) Has your hospital administered, or started to administer the CAHPS
Child Hospital Survey during the reporting period?
If “no” to question #3, skip questions #4-12 and continue to the next
subsection. The hospital will be scored as “Limited Achievement.”
Yes
No
4) Did your hospital administer the full CAHPS Child Hospital Survey or a
truncated version?
If administering a truncated version, hospitals must follow the
specifications for truncating the survey provided in the
Patient
Experience (CAHPS Child Hospital Survey) Measure Specifications.
Full version
Truncated version
Reporting Period: 12 months
Answer questions #1-12 for the latest 12-month period prior to the submission of this section of the Survey.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted Surveys only.
Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission and Performance
Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
239 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
5) How many surveys were returned during the reporting period?
If less than 100, skip questions #6-12 and continue to the next
subsection. The hospital will be scored as “Unable to Calculate Score.”
______
6) Which of the following modes were used to administer the survey?
Select all that apply.
Mail
Phone
Email
Tablet
7) Which of the following times were surveys administered during the
reporting period?
Select all that apply.
Day of discharge
After discharge
In questions #8-12, report your hospital’s Top Box Score
50
(rounded to the nearest whole number) from
each of the five publicly reported patient experience measures from your 12-month vendor report that
matches the reporting period that you selected in question #1.
8) Communication with Parent – Communication about your child’s
medicines
______
9) Communication with Parent – Keeping you informed about your child’s
care
______
10) Communication with Child – How well nurses communicate with your
child
______
11) Communication with Child – How well doctors communicate with your
child
______
12) Attention to Safety and Comfort – Preventing mistakes and helping you
report concerns
______
2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
240 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
8B: Pediatric Computed Tomography (CT) Radiation Dose
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Notes:
Note 1: All pediatric patient (ages 17 years and younger) scans performed at your hospital should be
included when reporting on this measure, including cases that were never admitted to an inpatient ward.
Note 2: This section is only applicable to hospitals that perform CT scans on patients 17 years of age or
younger. Hospitals that do not perform CT scans on patients 17 years of age or younger should respond
“no” to question #2 in 8B and move on to the Affirmation of Accuracy. Your hospital’s results will be
displayed as “Does Not Apply” on Leapfrog’s public reporting website.
Specifications: See Pediatric Computed Tomography (CT) Radiation Dose Measure Specifications
in the Reference Information beginning on page 247.
Sufficient Sample: See
Pediatric Computed Tomography (CT) Radiation Dose Measure
Specifications for instructions on identifying a sufficient sample for questions #2-7.
1) 12-month reporting period used:
01/01/2022 – 12/31/2022
07/01/2022 – 06/30/2023
2) Does your hospital perform CT scans on pediatric patients?
If “no” to question #2, skip the remaining questions in Section 8B, and go
to the Affirmation of Accuracy. The hospital will be scored as “Does Not
Apply.”
Yes
No
3) Did your hospital calculate its distribution of CT radiation doses for
pediatric patients over the reporting period, and do you choose to report
those data to this Survey?
If “no” to question #3, skip the remaining questions in Section 8B, and go
to the Affirmation of Accuracy. The hospital will be scored as “Limited
Achievement.”
Yes
No
4) Which of the following is your hospital using to report the CT radiation
dose length product (DLP)?
If “manual data collection” is not selected, skip question #5 and continue
to question #6.
Manual Data Collection
Dose Monitoring Software
ACR National Radiology Data
Registry Report
Reporting Period: 12 months
Answer questions #1-9 based on all cases (or a sufficient sample of them)
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o 07/01/2022 – 06/30/2023
Note: As a reminder, the Corrections Period
(December 1-January 31) is reserved for corrections to previously submitted Surveys only.
Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission and Performance
Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
241 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
5) If using manual data collection, do the responses in questions #6 and #7
represent a sample?
If your hospital reviewed all cases for age strata with fewer than 30
encounters and sampled for those age strata with greater than 30
encounters, select “yes.”
Yes
No
6) Enter your hospital’s 25
th
, 50
th
, and 75
th
percentiles for CT radiation dose length product (DLP) in routine
head scans for pediatric patients for each age stratum standardized to 16cm phantoms.
If the number of encounters for an age stratum is less than 10 (in column a), skip columns b, c, and d and
then move to the next age stratum. If zero, enter “0” in column a. The hospital will be scored as “Unable to
Calculate Score” if all age strata have less than 10 encounters.
Age Stratum
HEAD
(a)
Number of
encounters
(b)
25
th
Percentile
(c)
50
th
Percentile
(d)
75
th
Percentile
< 1 year
1 - 4
5 - 9
10 - 14
15 - 17
7) Enter your hospital’s 25
th
, 50
th
, and 75
th
percentiles for CT radiation dose length product (DLP) in routine
abdomen/pelvis scans for pediatric patients for each age stratum standardized to 32cm phantoms.
If the number of encounters for an age stratum is less than 10 (in column a), skip columns b, c, and d and
then move to the next age stratum. If zero, enter “0” in column a. The hospital will be scored as “Unable to
Calculate Score” if all age strata have less than 10 encounters.
Age Stratum
ABDOMEN/PELVIS
(a)
Number of
encounters
(b)
25
th
Percentile
(c)
50
th
Percentile
(d)
75
th
Percentile
< 1 year
1 - 4
5 - 9
10 - 14
15 - 17
8) Which of the following CT machines are used for pediatric CT scans
reported on in questions #1-7:
Select all that apply.
Analogic
Canon
GE
Hitachi
Neusoft
Neurologica
Philips
Siemens
Toshiba
United Imaging
9) In what year was your CT machine manufactured?
Please review the manufacturer’s gantry label to determine this
response. If using more than one machine for pediatric CT scans, enter
the year of the oldest machine.
______

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
242 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Additional Questions (Optional – Fact Finding Only)
10) For the routine head and routine abdomen/pelvis scans included in Section 8B, estimate the
percent of total scans that is more than one phase (e.g., pre contrast and post contrast or multiple
post contrast phases).
a) Routine head scans that are more than
one phase:
b) Routine abdomen/pelvis scans that are
more than one phase:
11) For the routine head scans included in Section 8B, how many age or size
categories has your hospital developed protocols for?
If “1,” continue to question #11a and skip questions #11b and #11c.
If “2” or greater, skip question #11a and continue to questions #11b and #11c.
1
2
3
4
5
More than 5
11a) If one protocol, what kilovoltage (kV) is used for routine head scans?
70
80
90
100
110
120
130
140
150
11b) If two or more protocols, what is the lowest kilovoltage (kV) used for routine
head scans?
70
80
90
100
110
120
130
140
150
11c) If two or more protocols, what is the highest kilovoltage (kV) used for routine
head scans?
70
80
90
100
110
120
130
140
150
12) For the routine abdomen/pelvis scans included in Section 8B, how many age
or size categories has your hospital developed protocols for?
If “1,” continue to question #12a and skip questions #12b and #12c.
If “2” or greater, skip question #12a and continue to questions #12b and #12c.
1
2
3
4
5
More than 5
12a) If one protocol, what kilovoltage (kV) is used for routine abdomen/pelvis
scans?
70
80
90
100
110
120
130
140

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
243 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
150
12b) If two or more protocols, what is the lowest kilovoltage (kV) used for routine
abdomen/pelvis scans?
70
80
90
100
110
120
130
140
150
12c) If two or more protocols, what is the highest kilovoltage (kV) used for routine
abdomen/pelvis scans?
70
80
90
100
110
120
130
140
150
13) Are CT protocols for routine head and routine abdomen/pelvis scans included
in Section 8B reviewed and updated?
If “no, skip the remaining questions in Section 8B and go to the Affirmation of
Accuracy.
Yes
No
13a) If “yes,” how often are CT protocols reviewed and updated?
Monthly
Quarterly
Biannually
Annually
Every 2 years
Every 3-5 years
13b) If “yes,” who is involved in the process of reviewing and updating CT
protocols?
Select all that apply.
Technologist
Medical
physicist
Radiologist
Other

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
244 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Affirmation of Accuracy
As the hospital CEO or as an employee of the hospital to whom the hospital CEO has delegated
responsibility, I have reviewed this information pertaining to the Pediatric Care Section at our hospital,
and I hereby certify that this information is true, accurate, and reflects the current, normal operating
circumstances at our hospital. I am authorized to make this certification on behalf of our hospital.
The hospital and I understand that The Leapfrog Group, its members, the public and entities and persons
who contract or have other business dealings with The Leapfrog Group are relying on the truth and
accuracy of this information. The hospital and I also understand that The Leapfrog Group will make this
information and/or analyses of this information public through the Survey Results public reporting website,
The Leapfrog Group’s Hospital Safety Grade, and/or other Leapfrog Group products and services. This
information and/or analyses and all intellectual property rights therein shall be and remain the sole and
exclusive property of The Leapfrog Group in which The Leapfrog Group retains exclusive ownership. This
information does not infringe upon any third-party intellectual property rights or any other third-party rights
whatsoever and is free and clear of all encumbrances and liens of any kind. The hospital and I
acknowledge that The Leapfrog Group may use this information in a commercial manner for profit. The
hospital shall be liable for and shall hold harmless and indemnify The Leapfrog Group from any and all
damages, demands, costs, or causes of action resulting from any inaccuracies in the information or any
misrepresentations in this Affirmation of Accuracy. The Leapfrog Group and its members and entities and
persons who contract or have other business dealings with The Leapfrog Group reserve the right to omit
or disclaim information that is not current, accurate or truthful.
Affirmed by _____________________, the hospital’s ___________________________,
(first name and last name) (title)
on _______________________.
(date)

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
245 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 8: 2023 Pediatric Care Reference Information
What’s New in the 2023 Survey
Section 8A: Patient Experience (CAHPS Child Hospital Survey)
For hospitals administering the CAHPS Child Hospital Survey, Leapfrog is asking an additional question
to assess whether the full CAHPS Child Hospital Survey or a truncated version is being administered.
Additionally, we are updating the measure specifications to clarify that hospitals administering the
truncated version of the CAHPS Child Hospital Survey must retain the demographic questions in their
original order and unaltered. This requirement supports Leapfrog’s efforts to have hospitals stratify quality
measures for the purpose of identifying healthcare disparities.
There are no changes to the scoring algorithm for Section 8A: Patient Experience (CAHPS Child Hospital
Survey).
Section 8B: Pediatric Computed Tomography (CT) Radiation Dose
Based on feedback from hospitals and industry experts, Leapfrog is adding a new unscored question to
collect the age of the CT machine being used for pediatric CT scans. We are also adding a question
asking for the name of the CT machine’s manufacturer. This new information will be used to analyze the
relationship between machine age and dose and used to inform thresholds used in Leapfrog’s data
verification protocols
.
There are no changes to the scoring algorithm for Section 8B: Pediatric Computed Tomography (CT)
Radiation Dose.
Change Summary Since Release
None. If substantive changes are made to this section of the Survey after release on April 1, 2023, they
will be documented in this Change Summary section.

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
246 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Pediatric Care Measure Specifications
Patient Experience (CAHPS Child Hospital Survey)
Important Note: Hospitals that share a CMS Certification Number are required to report by facility.
Please carefully review Leapfrog’s Multi-Campus Reporting Policy
.
Source: Agency for Healthcare Quality and Research (AHRQ) (NQF #2548)
Reporting Period: The latest 12-month period prior to the submission of this section of the Survey.
Hospitals can elect to use either survey return date or discharge date to pull reports for this measure.
This section of the Leapfrog Hospital Survey asks hospitals who care for pediatric patients about their
results from the CAHPS Child Hospital Survey. The first several questions are designed to learn more
about the current administration of the survey. The last five questions collect the “Top Box”
50
score for
each of the following five domains:
• Communication with Parent – Communication about your child’s medicines
• Communication with Parent – Keeping you informed about your child’s care
• Communication with Child – How well nurses communicate with your child
• Communication with Child – How well doctors communicate with your child
• Attention to Safety and Comfort – Preventing mistakes and helping you report concerns
Hospitals using Press Ganey, NRC, or PRC to administer the CAHPS Child Hospital Survey can use
the AHRQ CAHPS Crosswalk below to ensure they are reporting on the correct domains. The
Crosswalk is designed to help translate the standard vendor report to the AHRQ Domains used in
questions #8-12 of the Leapfrog Hospital Survey.
Hospitals that opt to truncate the CAHPS Child Hospital Survey after question 40, which is the last
question required to report on the five domains listed above, must ensure that all questions prior to and
including question 40 remain in order and unaltered. In addition, hospitals opting to truncate the survey
must retain questions 50-54 and 56-57 (in order and unaltered) which ask for the child and survey
participant’s demographic information. Note: Hospitals that collect information captured in questions
51-52 administratively are not required to retained them.
For more information regarding which questions from the CAHPS Child Hospital Survey correspond to
the five domains listed above, visit
https://www.ahrq.gov/cahps/surveys-guidance/hp/about/child-
survey-measures.html.
CAHPS Child Hospital Survey Domain Crosswalk
AHRQ Domain Name
Press Ganey
Domain Name
NRC Domain Name
PRC Domain
Name
Communication with Parent –
Communication about your
child’s medicines
Communication
Child's Med
Communication
About Meds
Communication
About Medicines
Communication with Parent –
Keeping you informed about
your child’s care
Informed
Child's Care
Keeping You Informed
About Your Child’s
Care
Keeping Parent
Informed
Communication with Child –
How well nurses communicate
with your child
Nurses
Communicate
Child
Nurse Communication
With Child
Nurses – Child
Communication with Child –
How well doctors communicate
with your child
Doctors
Communicate
Child
Doctor
Communication With
Child
Doctors – Child
Attention to Safety and Comfort
– Preventing mistakes and
helping you report concerns
Prevent
Mistakes Rpt
Conc
Preventing Mistakes
and Helping You
Report Concerns
Preventing
Mistakes
See FAQs for additional information about responding to the questions in this section.

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
247 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Pediatric Computed Tomography (CT) Radiation Dose
Important Notes:
Note 1: Hospitals that share a CMS Certification Number are required to report by facility. Please carefully
review Leapfrog’s Multi-Campus Reporting Policy
.
Note 2: For purposes of this measure, an “encounter” consists of a full examination and any CT scans
performed within one hour of each other involving the designated anatomic area (i.e., head or
abdomen/pelvis). For example, two CT scans conducted 30-minutes apart on the same patient’s head are
considered one “encounter.” CT scans of the same anatomic area performed with and without contrast
are considered one “encounter.” Scans of two different anatomic areas performed within one hour would
not be considered the same “encounter.” Scans of the same anatomic area performed greater than 60
minutes apart would also not be considered the same “encounter.”
Note 3: This measure includes two sets of instructions in the table below: one for hospitals using dose
monitoring software and one for hospitals that are not using dose monitoring software. Please be sure to
use the correct set of instructions.
Note 4: Hospitals performing manual data collection must use the CT Dose Workbook provided on the
Survey and CPOE Materials webpage
to standardize their CT radiation dose length product (DLP) for
head scans to 16cm phantoms and abdomen/pelvis scans to 32cm phantoms.
Source:
University of California, San Francisco (NQF #2820)
Reporting Period: 12 months
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o 07/01/2022 – 06/30/2023
Hospitals participating in the ACR National Radiology Data Registry:
Data for this measure can be obtained directly from a special Leapfrog report. See details below.
Hospitals that report to the American College of Radiology (ACR) and receive reports through the
National Radiology Data Registry (NRDR) will be able to respond to questions #6-7 in Section 8B using
a specialized report that will be made available with the quarterly Dose Index Registry Executive
Summary reports. This separate “Leapfrog” report will be available for download through the NRDR
portal and will contain data according to the age ranges and measure specifications of the Leapfrog
Hospital Survey. Enter values from the “Leapfrog” tab using the “Table: Pediatric Exams for
Leapfrog Survey” and the appropriate percentiles in the Head and Abdomen Pelvis columns
rounded to the nearest whole number directly into the Online Hospital Survey Tool. While this
report should be from the most recent 12 months, it does not need to include the full 12 months.
Hospitals using dose monitoring software:
Data for this measure can be obtained using dose monitoring software. See instructions below.
Question #6, column a: Total Number of Encounters
Using your dose monitoring software, obtain the total number of encounters in routine head scans for
each age stratum (<1, 1-4, 5-9, 10-14, 15-17). Enter these values into the Survey.
Inclusions:
CT HEAD BRN WO IVCON
CT HEAD WO IVCON
CT PEDS HEAD WO IVCON
CT HEAD
CT PEDS HEAD BRN WO IVCON
CT BRN WO IVCON
CT HEAD BRN
CT HEAD BRN W IVCON

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
248 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
CT HEAD W IVCON
CT HEAD WO & W IVCON
CT HEAD BRN WO & W IVCON
CT BRN WO & W IVCON
CT BRN W IVCON
Exclusions:
• Encounters involving facial bones or sinuses
• Encounters where the indication is for a biopsy, including prop studies and stereotactic studies
and procedures
• Encounters that cross multiple anatomic areas should be excluded. For example, encounters
involving both the head and neck are excluded from the “head” anatomic region.
• Encounters where the documented age is missing or listed as the date of admission (i.e., an
accurate birthday could not be obtained and therefore the admission date was used), as is the
case for some trauma patients
Sampling Cases:
Hospitals using dose monitoring software must report on all encounters in the 12-month reporting
period.
Hospitals using Radimetrics Dose Monitoring Software:
• If your hospital is using unique study instance UIDs for every scan performed, review your data
to ensure that any scans performed on the same patient and same anatomic area within a one-
hour timeframe are being combined into one encounter. The DLPs for all scans within the one-
hour timeframe should be combined and that combined value used to calculate the percentiles
reported to Leapfrog.
• If your hospital is NOT using unique study instance UIDs and is combining DLPs for all scans
performed on the same patient and same anatomic area within one hour into one study
instance UID, no review is required as this adheres to the definition of encounter. However,
these hospitals should review any repeat or delayed scans to ensure repeat or delayed scans
performed greater than 60 minutes apart are not being combined into the same study instance
UID.
Question #6, columns b, c, and d: 25
th
, 50
th
, and 75
th
Percentiles
Based on the encounters identified for each age stratum (column a), use your dose monitoring
software to calculate the 25
th
percentile (column b), the 50
th
percentile (column c), and the 75
th
percentile (column d) for CT radiation dose length product (DLP) in routine head scans standardized
to 16cm phantoms. Enter these values into the Survey rounded to the nearest whole number.
If the number of encounters for an age stratum (i.e., <1 or 1-4, etc.) is less than 10 (column a), skip
columns b, c, and d. If the number of encounters for an age stratum is zero, enter “0” in column a, and
skip columns b, c, and d. You cannot leave any rows in column a blank.
Phantom Dose Specifications:
For head scans, use a 16 cm phantom dose value. The green box in the screenshot below the table is
an example of a Dose Report which shows the phantom dose value used. The phantom dose value is
used to estimate the radiation dose to the patient.
Scout Doses (Topograms):
Scout doses should not be included when calculating the 25
th
, 50
th
, or 75
th
percentiles.
Question #7 column a: Total Number of Encounters
Using your dose monitoring software, obtain the total number of encounters in routine abdomen/pelvis
for each age stratum (<1, 1-4, 5-9, 10-14, 15-17). Enter these values into the Survey.
Inclusions:
CT ABD PELVIS W IVCON
CT ABD/PEL W IVCON
CT PEDS ABD PELVIS

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
249 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
CT ABD PELVIS WO IVCON
CT ABD/PEL WO IVCON
CT ABD/PEL
CT PEDS ABD/PEL WO IVCON
CT ABD PELVIS WO & W IVCON
CT ABD/PEL WO & W IVCON
CT ABD PELVIS
CT ABD PELVIS WO IVCON
Exclusions:
• Encounters where the indication is for a biopsy, including prop studies and stereotactic studies
and procedures
• Encounters that cross multiple anatomic areas should be excluded. For example, encounters
involving both the chest and abdomen/pelvis are excluded from the “abdomen/pelvis” anatomic
region.
• Encounters where the documented age is missing or listed as the date of admission (i.e., an
accurate birthday could not be obtained and therefore the admission date was used), as is the
case for some trauma patients
Sampling Cases:
Hospitals using dose monitoring software must report on all encounters in the 12-month reporting
period.
Question #7, columns b, c, and d: 25
th
, 50
th
, and 75
th
Percentiles
Based on the encounters identified for each age stratum (column a), use your dose monitoring
software to calculate the 25
th
percentile (column b), the 50
th
percentile (column c), and the 75
th
percentile (column d) for CT radiation dose length product (DLP) in routine abdomen/pelvis scans
standardized to 32cm phantoms. Enter these values into the Survey rounded to the nearest whole
number.
If the number of encounters for an age stratum (i.e., <1 or 1-4, etc.) is less than 10 (column a), skip
columns b, c, and d. If the number of encounters for an age stratum is zero, enter “0” in column a, and
skip columns b, c, and d. You cannot leave any rows in column a blank.
Phantom Dose Specifications:
For abdomen/pelvis scans, use a 32cm phantom dose value. The green box in the screenshot below
the table is an example of a Dose Report which shows the phantom dose value used. The phantom
dose value is used to estimate the radiation dose to the patient.
Scout Doses (Topograms):
Scout doses should not be included when calculating the 25
th
, 50
th
, or 75
th
percentiles.
Hospitals not using dose monitoring software:
Data for this measure can be obtained from Dose Reports that come directly from the CT Machine and
are sent along with the images to the Picture Archiving and Communications (PACS) used to review
the images. See instructions below.

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
250 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
CT Dose Excel Workbook
To assist hospitals who do not use dose monitoring software in calculating the responses to questions
#6 and #7, Leapfrog has developed a CT Dose Workbook to assist hospitals in standardizing their CT
dose data based on these specifications. The workbook includes 12 tabs: Instructions, Summary, and
10 Data Entry tabs for each anatomic area/age stratum combination. The tabs for head scans are red
and the tabs for abdomen/pelvis scans are blue. Once you enter your hospital’s CT radiation dose
length product (DLP) data into the appropriate tab, the workbook will automatically calculate your
responses to questions #6 and #7 in the Summary tab, and those values should be entered in the
Survey.
The CT Dose Workbook is available on the Survey and CPOE Materials webpage
and should be used
when reporting on this measure.
Question #6, column a: Total Number of Encounters
To determine the total number of encounters in routine head scans for each age stratum (<1, 1-4, 5-9,
10-14, 15-17), you will need to obtain dose reports. See sampling instructions below.
Inclusions:
CT HEAD BRN WO IVCON
CT HEAD WO IVCON
CT PEDS HEAD WO IVCON
CT HEAD
CT PEDS HEAD BRN WO IVCON
CT BRN WO IVCON
CT HEAD BRN
CT HEAD BRN W IVCON
CT HEAD W IVCON
CT HEAD WO & W IVCON
CT HEAD BRN WO & W IVCON
CT BRN WO & W IVCON
CT BRN W IVCON
Exclusions:
• Encounters involving facial bones or sinuses
• Encounters where the indication is for a biopsy, including prop studies and stereotactic studies
and procedures
• Encounters that cross multiple anatomic areas should be excluded. For example, encounters
involving both the head and neck are excluded from the “head” anatomic region.
• Encounters where the documented age is missing or listed as the date of admission (i.e., an
accurate birthday could not be obtained and therefore the admission date was used), as is the
case for some trauma patients
Sampling Cases:
Hospitals that are using information stored in the CT Machine have the option of reporting on all
encounters or a sample of encounters. Hospitals opting to identify a sample of encounters for this
measure should follow these instructions:
• Review your hospital’s scans starting on January 15, 2022 (or July 15, 2022 if (re)submitting a
Survey on or after September 1, 2023).
• Work sequentially until a sample of at least 30 encounters per anatomic area and age
strata combination (i.e., head <1; head 1-4, etc.) is reached, or all cases in the reporting
period are reviewed, whichever comes first.

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
251 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Question #6, columns b, c, and d: 25
th
, 50
th
, and 75
th
Percentiles
Using your dose reports, enter the phantom dose and Total DLP (mGY-cm) for each encounter into
each of the 5 “Head” tabs of the CT Dose Workbook
. Be sure to review the instructions tab carefully
before you begin entering data. The worksheet will automatically standardize your dose data to a 16cm
phantom, if necessary. The worksheet will then automatically calculate the total number of encounters,
as well as the 25
th
, 50
th
, and 75
th
percentiles for each anatomic area and age stratum.
See the example CT Dose Report from a CT scanner below this table:
• The green box in the screenshot below the table is an example of a Dose Report which shows
the phantom dose value used. The phantom dose value is used to estimate the radiation dose
to the patient.
• The red box highlights the Total DLP. Note that your CT scanner may have a differently
formatted Dose Report.
Scout Doses (Topograms):
Scout doses should not be included when entering the Total DLP for each encounter into the CT Dose
Workbook.
Question #7 column a: Total Number of Encounters
To determine the total number of encounters in routine abdomen/pelvis scans for each age stratum
(<1, 1-4, 5-9, 10-14, 15-17), you will need to obtain dose reports. See sampling instructions below.
Inclusions:
CT ABD PELVIS W IVCON
CT ABD/PEL W IVCON
CT PEDS ABD PELVIS
CT ABD PELVIS WO IVCON
CT ABD/PEL WO IVCON
CT ABD/PEL
CT PEDS ABD/PEL WO IVCON
CT ABD PELVIS WO & W IVCON
CT ABD/PEL WO & W IVCON
CT ABD PELVIS
CT ABD PELVIS WO IVCON
Exclusions:
• Encounters where the indication is for a biopsy, including prop studies and stereotactic studies
and procedures
• Encounters that cross multiple anatomic areas should be excluded. For example, encounters
involving both the chest and abdomen/pelvis are excluded from the “abdomen/pelvis” anatomic
region.
• Encounters where the documented age is missing or listed as the date of admission (i.e., an
accurate birthday could not be obtained and therefore the admission date was used), as is the
case for some trauma patients

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
252 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Question #7 columns b, c, and d: 25
th
, 50
th
, and 75
th
Percentiles
Using your dose reports, enter the phantom dose and Total DLP (mGY-cm) for each encounter into
each of the 5 “Abdomen Pelvis” tabs of the CT Dose Workbook
. Be sure to review the instructions tab
carefully before you begin entering data. The worksheet will automatically standardize your dose data
to a 32cm phantom, if necessary. The worksheet will then automatically calculate the total number of
encounters, as well as the 25
th
, 50
th
, and 75
th
percentiles for each anatomic area and age stratum.
See the example CT Dose Report from a CT scanner below this table:
• The green box in the screenshot below the table is an example of a Dose Report which shows
the phantom dose value used. The phantom dose value is used to estimate the radiation dose
to the patient.
• The red box highlights the Total DLP. Note that your CT scanner may have a differently
formatted Dose Report.
Scout Doses (Topograms):
Scout doses should not be included when entering the Total DLP for each encounter into the CT Dose
Workbook.
Example of Dose Report:
See FAQs for additional information about responding to the questions in this section.

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
253 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Pediatric Care Frequently Asked Questions (FAQs)
Patient Experience (CAHPS Child Hospital Survey)
1. Why does Leapfrog refer to two different reporting periods in Section 8A question #2: one
reporting period which refers to the number of pediatric admissions during the 12 months
from Section 1 and one reporting period which refers to the results of our CAHPS Child
Hospital Survey?
These two reporting periods are different because hospitals that have not historically had 500
pediatric admissions annually may not have begun to administer the CAHPS Survey to their
patients and therefore it would not be appropriate to ask these facilities to report on their current
results in Section 8A.
2. Do I need to include neonatal ICU (NICU) discharges when administering the CAHPS Child
Hospital Survey and reporting those results to Leapfrog?
The Child CAHPS Hospital Survey was designed to be administered to pediatric discharges
including NICU discharges. Additional details on fielding the CAHPS Child Hospital Survey can
be found here
.
In 2023, hospitals that have been administering the CAHPS survey without including NICU
discharges in their sample can report those results to Leapfrog, provided they meet the minimum
sample size and timing requirements in the Leapfrog Hospital Survey. However, we are urging
those hospitals to begin including NICU discharges-- per the manual guidelines-- immediately, as
CAHPS is designed to include those patients. Hospitals that are just starting to administer the
survey in 2023 should include NICU discharges in their sample per the sampling framework
detailed in the manual.
3. Can we use other lower cost modes for administering the CAHPS Child Hospital Survey?
Survey administration is costly, and our hospital has low response rates.
Some hospitals have asked about the use of alternative, lower cost modes of survey
administration, such as administering paper surveys at discharge that can then be batched and
mailed to a vendor to calculate results. This approach is potentially an opportunity both to lower
the cost of administration and to increase response rates.
Leapfrog’s Pediatric Expert Panel
has noted that while administering the CAHPS Child Hospital
Survey using paper forms at discharge is not on the list of AHRQ-approved modes, hospitals that
are trying to find ways to administer the survey and increase response rate should be able to
submit results to the 2023 Leapfrog Hospital Survey. That said, the Pediatric Expert Panel has
expressed a desire for these different modes to be tested, and so we cannot guarantee that you
will be able to submit these results for future Leapfrog Hospital Surveys.
4. Is Interactive Voice Response (IVR) or texting a link to an online survey an acceptable
mode for administering the CAHPS Child Hospital Survey?
Hospitals that administer the CAHPS Child Hospital Survey via IVR should select “phone” in
question #6. Hospitals that administer the CAHPS Child Hospital Survey by texting a link to an
online survey to the patient should select “email.”
Pediatric Computed Tomography (CT) Radiation Dose
5. Is this measure only applicable to pediatric inpatients, or should all pediatric scans be
included?
All pediatric patient (ages 17 years and younger) scans should be included when reporting on this
measure, including cases that were never admitted to an inpatient ward.

2023 Leapfrog Hospital Survey – Hard Copy Section 8: Pediatric Care
254 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
6. Should multiple phase scans be included in the reporting?
Yes, the intent of this measure is to capture the entire dose a patient receives, even if this
radiation is received over multiple phase scans.
7. Should any CT encounters involving anatomic areas not listed in the Survey questions
(i.e., head or abdomen/pelvis) be included in the reporting?
No. When reporting CT encounters in the Survey, only encounters involving the head or
abdomen/pelvis should be included. Encounters involving any other anatomic area should not be
reported. For example, encounters involving both the head and neck are excluded from the
“head” anatomic region.
8. Are the CT doses adjusted for any factors other than age, such as height and weight?
No, CT doses are only stratified by age and anatomic region. The pediatric CT radiation dose
measure is an NQF-endorsed measure, developed so that all hospitals in the country who do
pediatric CT scans can report. Its known limitation is that it does not consider patient size (e.g.,
height and weight) but instead uses age as a proxy for size. Part of the reason is that unless
hospitals use dose monitoring software, which not all hospitals currently do due to cost, they
would not have this information readily available to them. However, to align with the American
College of Radiology (ACR), head scans are standardized to 16cm phantoms and
abdomen/pelvis scans are standardized to 32cm phantoms.
9. If our CT machine was completely refurbished, should we indicate that year in question #9
for year of manufacture?
Yes, if your hospital’s CT machine has been completely refurbished, you should enter the year it
was refurbished for manufacture year.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
256 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
SECTION 9: OUTPATIENT PROCEDURES
This section includes questions and reference information for Section 9: Outpatient Procedures. Please
carefully review the questions, endnotes, and reference information (e.g., measure specifications, notes,
and frequently asked questions) before you begin. Failure to review the reference information could result
in inaccurate responses.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
257 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 9: 2023 Outpatient Procedures
Outpatient Procedures Fact Sheet and Bibliography:
https://ratings.leapfroggroup.org/measure/hospital/2023/care-elective-outpatient-surgery-patients
Section 9 includes questions about the volume and safety of same-day procedures performed in hospital
outpatient departments, as well as the experience of patients who had a same-day surgery performed.
Hospitals will not be able to access Section 9 until the American Medical Association’s Terms of
Use are completed via the CPT Code Workbook button on the Survey Dashboard, and the
appropriate CPT Code Workbook is downloaded.
Each hospital achieving the standards for Certified Clinicians Present While Patients Are
Recovering:
1. Has an ACLS certified clinician, plus a second clinician, present at all times and immediately
available in the building while adult patients are present in the hospital outpatient department and
has a physician or CRNA present at all times and immediately available in the building until all
adult patients are physically discharged from the hospital outpatient department.
2. Has a PALS certified clinician, plus a second clinician, present at all times and immediately
available in the building while pediatric patients are present in the hospital outpatient department
and has a physician or CRNA present at all times and immediately available in the building until
all pediatric patients are physically discharged from the hospital outpatient department.
Each hospital achieving the standard for Patient Follow-up:
Provided an accurate CCN in the Hospital Profile, reported volume for adult lower gastrointestinal
endoscopy procedures in Section 9C, and is in the top quartile of performance for OP-32, Rate of
Unplanned Hospital Visits After an Outpatient Colonoscopy, based on responses to the 2022 Leapfrog
ASC Survey and Section 9 of the 2022 Leapfrog Hospital Survey submitted by June 30, 2022.
Each hospital achieving the standard for the Safe Surgery Checklist:
1. Uses a safe surgery checklist on all patients undergoing an applicable procedure (reported on in
Section 9C) that includes all safe surgery checklist elements, and
2. Verbalizes all elements of the checklist in the presence of the appropriate personnel, and
3. Completes an audit of a sufficient sample of patients to document adherence to the checklist, and
4. Has documented adherence to the checklist for at least 90% of the patients included in the audit.
Each hospital achieving the standard for Medication Safety for Outpatient Procedures:
Has met the 90% target for documenting all three components: home medications, visit medications, and
allergies/adverse reaction(s) in the clinical record.
Each hospital achieving the standard for Patient Experience (OAS CAHPS):
Performed in the top quartile based on responses to the 2020 Leapfrog ASC and Hospital Surveys
submitted by August 31, 2020, for the four OAS CAHPS domains, listed below:
a) Facilities and Staff
b) Communication About Your Procedure
c) Patients’ Rating of the Facility
d) Patients Recommending the Facility
Download the 2023 Leapfrog Hospital Survey Scoring Algorithm on the
Scoring and Results
webpage.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
258 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
9A: Basic Outpatient Department Information
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Notes:
Note 1: In order to access this section in the Online Survey Tool, hospitals must complete the American
Medical Association’s Terms of Use via the CPT Code Workbook button next to Section 9 on the Survey
Dashboard and download the appropriate CPT Code Workbook. Instructions for downloading the CPT
Code Workbook are available in the Volume of Procedures Measure Specifications.
Each hospital must
complete these steps even if they are part of a hospital system.
Note 2: This subsection will not be scored but will be used in public reporting.
Note 3: The term “hospital outpatient department” is used to refer to all hospital outpatient departments or
areas of the hospital, including areas that are used for both inpatient and outpatient procedures, that
perform the outpatient procedures listed in Section 9C and that share your hospital’s license or CMS
Certification Number (CCN). This would include, but is not limited to surgery centers, free-standing
hospital outpatient departments, as well as outpatient departments that are located in your hospital or are
co-located
30
with your hospital. Hospitals should only include hospital outpatient departments or areas of
the hospital that perform the procedures listed in Section 9C in an operating room
51
or procedure room
52
.
1) 12-month reporting period used:
01/01/2022 – 12/31/2022
07/01/2022 – 06/30/2023
2) Does your hospital perform any of the procedures listed in Section 9C
on an outpatient basis in the hospital or at a hospital outpatient
department co-located
30
with the hospital?
Yes
No
3) Does your hospital perform any of the procedures listed in Section 9C
on an outpatient basis at a surgery center or free-standing hospital
outpatient department that shares your hospital’s license or CMS
Certification Number?
Yes
No
If “no” to question #2 and question #3, skip the remaining questions in Section 9, including all
subsections, and go to the Affirmation of Accuracy.
Reporting Period: 12 months
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o 07/01/2022 – 06/30/2023
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
259 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
4) Total number of operating rooms
51
used to perform the outpatient
procedures listed in Section 9C:
_____
5) Total number of endoscopic procedure rooms
52
used to perform the
outpatient procedures listed in Section 9C:
_____

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
260 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
9B: Medical, Surgical, and Clinical Staff
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Note: The term “hospital outpatient department” is used to refer to all hospital outpatient
departments or areas of the hospital, including areas that are used for both inpatient and outpatient
procedures, that perform the outpatient procedures listed in Section 9C and that share your hospital’s
license or CMS Certification Number (CCN). This would include, but is not limited to surgery centers, free-
standing hospital outpatient departments, as well as outpatient departments that are located in your
hospital or are co-located
30
with your hospital. Hospitals should only include hospital outpatient
departments or areas of the hospital that perform the procedures listed in Section 9C in an operating
room
51
or procedure room
52
.
1) Is there an Advanced Cardiovascular Life Support (ACLS) trained
clinician
53
, as well as a second clinician
53
(regardless of ACLS
training), present at all times and immediately available in the building
while an adult patient (13 years and older) is present in the hospital
outpatient department?
Hospitals with more than one hospital outpatient department, including
a surgery center or free-standing hospital outpatient department,
should respond based on the least intensively staffed location.
Hospitals should report on all hospital outpatient departments or areas
of the hospital that perform the outpatient procedures listed in Section
9C and that share the hospital’s license or CCN.
Hospitals that did not perform any applicable procedures on patients
13 years and older during the reporting period should select “not
applicable; pediatric patients only,” skip question #2 and continue to
question #3. The hospital will be scored as “Does Not Apply.”
Yes
No
Not applicable; pediatric
patients only
2) Is there a physician or CRNA present at all times and immediately
available in the building until all adult patients (13 years and older) are
physically discharged from the hospital outpatient department?
Hospital outpatient departments who have a physician or CRNA
serving as their ACLS trained clinician in question #1 may respond
“yes” to question #2 if the physician/CRNA is present until all adult
patients are physically discharged from the hospital outpatient
department.
Yes
No
Reporting Period: 3 months
Answer questions #1-4 based on the staffing structure currently in place at the time that you submit
this section of the Survey. The staffing structure should have been in place for at least the past 3
months and reflect the ordinary staffing structure for each applicable hospital outpatient department.
Note: As a reminder, the Corrections Period
(December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
261 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
3) Is there a Pediatric Advanced Life Support (PALS) trained clinician
53
,
as well as a second clinician
53
(regardless of PALS training), present
at all times and immediately available in the building while a pediatric
patient (infant through 12 years) is present in the hospital outpatient
department?
Hospitals with more than one hospital outpatient department, including
a surgery center or free-standing hospital outpatient department,
should respond based on the least intensively staffed location.
Hospitals should report on all hospital outpatient departments or areas
of the hospital that perform the outpatient procedures listed in Section
9C and that share the hospital’s license or CCN.
Hospitals that did not perform any applicable procedures on pediatric
patients (infant through 12 years) during the reporting period should
select “not applicable; adult patients only,” skip question #4 and
continue to the next subsection. The hospital will be scored as “Does
Not Apply.”
Yes
No
Not applicable; adult
patients only
4) Is there a physician or CRNA present at all times and immediately
available in the building until all pediatric patients (infant through 12
years) are physically discharged from the hospital outpatient
department?
Hospital outpatient departments who have a physician or CRNA
serving as their PALS trained clinician in question #3 may respond
“yes” to question #4 if the physician/CRNA is present until all pediatric
patients are physically discharged from the hospital outpatient
department.
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
262 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
9C: Volume of Procedures
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Notes:
Note 1: CPT Codes are provided in downloadable Excel files on the Survey Dashboard. To access the
files, click the CPT Code Workbook button next to Section 9. You will be required to complete the
American Medical Association’s Terms of Use before downloading the Excel file and using the individual
CPT codes to query your EHR or billing system. You are only required to complete the Terms of Use
once per Survey Cycle (April 1 – November 30).
The CPT Code Workbooks (Excel files) are labeled for Section 3A: Total Hip Replacement, Total Knee
Replacement, and Bariatric Surgery for Weight Loss and Section 9C: Volume of Procedures. Please note,
if you are part of a hospital system, each hospital will need to complete the Terms of Use. This is a
requirement of the American Medical Association.
Note 2: This subsection will not be scored but will be used in public reporting to inform purchasers and
consumers about the hospital’s experience with the procedure. Additionally, this information will be used
to facilitate the search functionality on Leapfrog’s public reporting website
(i.e., allowing users to search
for hospitals that perform the procedure they need on an outpatient basis).
Note 3: The term “hospital outpatient department” is used to refer to all hospital outpatient departments or
areas of the hospital, including areas that are used for both inpatient and outpatient procedures, that
perform the outpatient procedures listed in Section 9C and that share your hospital’s license or CMS
Certification Number (CCN). This would include, but is not limited to surgery centers, free-standing
hospital outpatient departments, as well as outpatient departments that are located in your hospital or are
co-located
30
with your hospital. Hospitals should only include hospital outpatient departments or areas of
the hospital that perform the procedures listed in Section 9C in an operating room
51
or procedure room
52
.
Note 4: Hospitals should only report on procedures they electively perform. If your hospital does not
perform the procedure on an outpatient basis or ONLY does so when a procedure is urgent, you should
answer “no” and not report on those procedures.
Specifications: See Volume of Procedures Measure Specifications
in the Reference Information
beginning on page 287.
1) 12-month reporting period used:
01/01/2022 – 12/31/2022
07/01/2022 – 06/30/2023
Reporting Period: 12 months
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o 07/01/2022 – 06/30/2023
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
263 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
2) During the reporting period, were one or more of the following
gastroenterology procedures performed at your hospital
outpatient department(s) on adult or pediatric patients:
• Upper GI endoscopy
• Other upper GI procedures
• Lower GI endoscopy
If “no” or “yes, but no longer performs these procedures,” skip
questions #11-13 below.
Yes
Yes, but no longer performs
these procedures
No
3) During the reporting period, were one or more of the following
general surgery procedures performed at your hospital outpatient
department(s) on adult or pediatric patients:
• Cholecystectomy and common duct exploration
• Hemorrhoid procedures
• Inguinal and femoral hernia repair
• Other hernia repair
• Laparoscopy
• Lumpectomy or quadrantectomy of breast
• Mastectomy
If “no” or “yes, but no longer performs these procedures,” skip
questions #14-16 below.
Yes
Yes, but no longer performs
these procedures
No
4) During the reporting period, were one or more of the following
ophthalmology procedures performed at your hospital outpatient
department(s) on adult or pediatric patients:
• Anterior segment eye procedures
• Posterior segment eye procedures
• Ocular adnexa and other eye procedures
If “no” or “yes, but no longer performs these procedures,” skip
questions #17-19 below.
Yes
Yes, but no longer performs
these procedures
No
5) During the reporting period, were one or more of the following
orthopedic procedures performed at your hospital outpatient
department(s) on adult or pediatric patients:
• Finger, hand, wrist, forearm, and elbow procedures
• Shoulder procedures
• Spine procedures
• Hip procedures
• Knee procedures
• Toe, foot, ankle, and leg procedures
• General orthopedic procedures
If “no” or “yes, but no longer performs these procedures,” skip
questions #20-22 below.
Yes
Yes, but no longer performs
these procedures
No

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
264 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
6) During the reporting period, were one or more of the following
otolaryngology procedures performed at your hospital outpatient
department(s) on adult or pediatric patients:
• Ear procedures
• Mouth procedures
• Nasal/sinus procedures
• Pharynx/adenoid/tonsil procedures
If “no” or “yes, but no longer performs these procedures,” skip
questions #23-25 below.
Yes
Yes, but no longer performs
these procedures
No
7) During the reporting period, were one or more of the following
urology procedures performed at your hospital outpatient
department(s) on adult patients:
• Circumcision
• Cystourethroscopy
• Male genital procedures
• Urethra procedures
• Vaginal repair procedures
If “no” or “yes, but no longer performs these procedures,” skip
questions #26-28 below.
Yes
Yes, but no longer performs
these procedures
No
8) During the reporting period, was the following neurological
surgery procedure performed at your hospital outpatient
department(s) on adult patients:
• Spinal fusion procedures
If “no” or “yes, but no longer performs this procedure,” skip
questions #29-31 below.
Yes
Yes, but no longer performs
this procedure
No
9) During the reporting period, were one or more of the following
obstetrics and gynecology procedures performed at your
hospital outpatient department(s) on adult patients:
• Cervix procedures
• Hysteroscopy
• Uterus and adnexa laparoscopies
If “no” or “yes, but no longer performs these procedures,” skip
questions #32-34 below.
Yes
Yes, but no longer performs
these procedures
No
10) During the reporting period, were one or more of the following
plastic and reconstructive surgery procedures performed at
your hospital outpatient department(s) on adult patients:
• Breast repair or reconstruction
• Skin graft/reconstruction procedures
If “no” or “yes, but no longer performs these procedures,” skip
questions #35-37 below.
Yes
Yes, but no longer performs
these procedures
No

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
265 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Gastroenterology
11) Total adult and/or pediatric volume for each of the following applicable procedures performed at
your hospital outpatient department(s) during the reporting period.
You cannot leave any blank. If you did not perform one or more of the procedures listed below
enter 0 (zero). If you had zero volume for all procedures, go back to question #2 and update your
response from “yes” to “no.”
a) Adult Volume
b) Pediatric Volume
Upper GI endoscopies
_____
_____
Other upper GI procedures
_____
_____
Lower GI endoscopies
_____
_____
13) Please provide the name and city location of each surgery center or free-standing hospital
outpatient department performing gastroenterology procedures:
Name
City
Surgery Center/Free-Standing Hospital
Outpatient Department 1
Surgery Center/Free-Standing Hospital
Outpatient Department 2
Surgery Center/Free-Standing Hospital
Outpatient Department 3
Surgery Center/Free-Standing Hospital
Outpatient Department 4
Surgery Center/Free-Standing Hospital
Outpatient Department 5
12) Where were these gastroenterology procedures performed?
Select all that apply.
If only “hospital” is selected, skip question #13 and continue to
question #14.
Hospital
Surgery center or free-
standing hospital outpatient
department

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
266 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
General Surgery
14) Total adult and/or pediatric volume for each of the following applicable procedures performed at
your hospital outpatient department(s) during the reporting period.
You cannot leave any blank. If you did not perform one or more of the procedures listed below
enter 0 (zero). If you had zero volume for all procedures, go back to question #3 and update your
response from “yes” to “no.”
(a) Adult Volume (b) Pediatric Volume
Cholecystectomies and common duct
explorations
_____
Hemorrhoid procedures
_____
Inguinal and femoral hernia repairs
_____
_____
Other hernia repairs
_____
_____
Laparoscopies
_____
Lumpectomies or quadrantectomy of breast
procedures
_____
Mastectomies
_____
15) Where were these general surgery procedures performed?
Select all that apply.
If only “hospital” is selected, skip question #16 and continue to
question #17.
Hospital
Surgery center or free-
standing hospital outpatient
department
16) Please provide the name and city location of each surgery center or free-standing hospital
outpatient department performing general surgery procedures:
Name
City
Surgery Center/Free-Standing Hospital
Outpatient Department 1
Surgery Center/Free-Standing Hospital
Outpatient Department 2
Surgery Center/Free-Standing Hospital
Outpatient Department 3
Surgery Center/Free-Standing Hospital
Outpatient Department 4

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
267 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Surgery Center/Free-Standing Hospital
Outpatient Department 5
Ophthalmology
17) Total adult and/or pediatric volume for each of the following applicable procedures performed at
your hospital outpatient department(s) during the reporting period.
You cannot leave any blank. If you did not perform one or more of the procedures listed below
enter 0 (zero). If you had zero volume for all procedures, go back to question #4 and update your
response from “yes” to “no.”
(a) Adult Volume
(b) Pediatric Volume
Anterior segment eye procedures
_____
_____
Posterior segment eye procedures
_____
_____
Ocular adnexa and other eye procedures
_____
_____
18) Where were these ophthalmology procedures performed?
Select all that apply.
If only “hospital” is selected, skip question #19 and continue to
question #20.
Hospital
Surgery center or free-
standing hospital outpatient
department
19) Please provide the name and city location of each surgery center or free-standing hospital
outpatient department performing
ophthalmology
procedures:
Name
City
Surgery Center/Free-Standing
Hospital Outpatient Department 1
Surgery Center/Free-Standing
Hospital Outpatient Department 2
Surgery Center/Free-Standing
Hospital Outpatient Department 3
Surgery Center/Free-Standing
Hospital Outpatient Department 4
Surgery Center/Free-Standing
Hospital Outpatient Department 5

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
268 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Orthopedics
20) Total adult and/or pediatric volume for each of the following applicable procedures performed at
your hospital outpatient department(s) during the reporting period.
You cannot leave any blank. If you did not perform one or more of the procedures listed below
enter 0 (zero). If you had zero volume for all procedures, go back to question #5 and update your
response from “yes” to “no.”
(a) Adult Volume
(b) Pediatric Volume
Finger, hand, wrist, forearm, and elbow
procedures
_____ _____
Shoulder procedures
_____
_____
Spine procedures
_____
Hip procedures
_____
Knee procedures
_____
_____
Toe, foot, ankle, and leg procedures
_____
_____
General orthopedic procedures
_____
_____
21) Where were these orthopedic procedures performed?
Select all that apply.
If only “hospital” is selected, skip question #22 and continue to
question #23.
Hospital
Surgery center or free-
standing hospital outpatient
department
22) Please provide the name and city location of each surgery center or free-standing hospital
outpatient department performing orthopedic procedures:
Name
City
Surgery Center/Free-Standing
Hospital Outpatient Department 1
Surgery Center/Free-Standing
Hospital Outpatient Department 2
Surgery Center/Free-Standing
Hospital Outpatient Department 3
Surgery Center/Free-Standing
Hospital Outpatient Department 4
Surgery Center/Free-Standing
Hospital Outpatient Department 5

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
269 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Otolaryngology
23) Total adult and/or pediatric volume for each of the following applicable procedures performed at
your hospital outpatient department(s) during the reporting period.
You cannot leave any blank. If you did not perform one or more of the procedures listed below
enter 0 (zero). If you had zero volume for all procedures, go back to question #6 and update your
response from “yes” to “no.”
(a) Adult Volume
(b) Pediatric Volume
Ear procedures
_____
_____
Mouth procedures
_____
_____
Nasal/sinus procedures
_____
_____
Pharynx/adenoid/tonsil procedures
_____
24) Where were these otolaryngology procedures performed?
Select all that apply.
If only “hospital” is selected, skip question #25 and continue to
question #26.
Hospital
Surgery center or free-
standing hospital outpatient
department
25) Please provide the name and city location of each surgery center or free-standing hospital
outpatient department performing otolaryngology procedures:
Name
City
Surgery Center/Free-Standing
Hospital Outpatient Department 1
Surgery Center/Free-Standing
Hospital Outpatient Department 2
Surgery Center/Free-Standing
Hospital Outpatient Department 3
Surgery Center/Free-Standing
Hospital Outpatient Department 4
Surgery Center/Free-Standing
Hospital Outpatient Department 5

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
270 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Urology
26) Total adult volume for each of the following applicable procedures performed at your hospital
outpatient department(s) during the reporting period.
You cannot leave any blank. If you did not perform one or more of the procedures listed below
enter 0 (zero). If you had zero volume for all procedures, go back to question #7 and update your
response from “yes” to “no.”
(a) Adult Volume
(b) Pediatric Volume
Circumcisions
_____
Cystourethroscopies
_____
Male genital procedures
_____
Urethra procedures
_____
Vaginal repair procedures
_____
27) Where were these urology procedures performed?
Select all that apply.
If only “hospital” is selected, skip question #28 and continue to
question #29.
Hospital
Surgery center or free-
standing hospital outpatient
department
28) Please provide the name and city location of each surgery center or free-standing hospital
outpatient department performing urology procedures:
Name
City
Surgery Center/Free-Standing
Hospital Outpatient Department 1
Surgery Center/Free-Standing
Hospital Outpatient Department 2
Surgery Center/Free-Standing
Hospital Outpatient Department 3
Surgery Center/Free-Standing
Hospital Outpatient Department 4
Surgery Center/Free-Standing
Hospital Outpatient Department 5

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
271 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Neurological Surgery
29) Total adult volume for the following procedure performed at your hospital outpatient department(s)
during the reporting period.
You cannot leave any blank. If you did not perform the procedure listed below, go back to question
#8 and update your response from “yes” to “no.”
(a) Adult Volume
(b) Pediatric Volume
Spinal fusion procedures
_____
30) Where were these neurological surgery procedures
performed?
Select all that apply.
If only “hospital” is selected, skip question #31 and continue to
question #32.
Hospital
Surgery center or free-
standing hospital outpatient
department
31) Please provide the name and city location of each surgery center or free-standing hospital
outpatient department performing
neurological surgery
procedures:
Name
City
Surgery Center/Free-Standing
Hospital Outpatient Department 1
Surgery Center/Free-Standing
Hospital Outpatient Department 2
Surgery Center/Free-Standing
Hospital Outpatient Department 3
Surgery Center/Free-Standing
Hospital Outpatient Department 4
Surgery Center/Free-Standing
Hospital Outpatient Department 5
Obstetrics and Gynecology
32) Total adult volume for each of the following applicable procedures performed at your hospital
outpatient department(s) during the reporting period.
You cannot leave any blank. If you did not perform one or more of the procedures listed below
enter 0 (zero). If you had zero volume for all procedures, go back to question #9 and update your
response from “yes” to “no.”
(a) Adult Volume
(b) Pediatric Volume
Cervix procedures
_____
Hysteroscopies
_____

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
272 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Uterus and adnexa laparoscopies
_____
33) Where were these obstetrics and gynecology procedures
performed?
Select all that apply.
If only “hospital” is selected, skip question #34 and continue to
question #35.
Hospital
Surgery center or free-
standing hospital outpatient
department
34) Please provide the name and city location of each surgery center or free-standing hospital
outpatient department performing obstetrics and gynecology procedures:
Name
City
Surgery Center/Free-Standing
Hospital Outpatient Department 1
Surgery Center/Free-Standing
Hospital Outpatient Department 2
Surgery Center/Free-Standing
Hospital Outpatient Department 3
Surgery Center/Free-Standing
Hospital Outpatient Department 4
Surgery Center/Free-Standing
Hospital Outpatient Department 5
Plastic and Reconstructive Surgery
35) Total adult volume for each of the following applicable procedures performed at your hospital
outpatient department(s) during the reporting period.
You cannot leave any blank. If you did not perform one or more of the procedures listed below
enter 0 (zero). If you had zero volume for all procedures, go back to question #10 and update your
response from “yes” to “no.”
(a) Adult Volume
(b) Pediatric Volume
Breast repair or reconstructive procedures
_____
Skin graft/reconstruction procedures
_____
36) Where were these plastic and reconstructive surgery
procedures performed?
Select all that apply.
If only “hospital” is selected, skip question #37 and continue to
the next subsection.
Hospital
Surgery center or free-
standing hospital outpatient
department

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
273 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
37) Please provide the name and city location of each surgery center or free-standing hospital
outpatient department performing
plastic and reconstructive surgery
procedures:
Name
City
Surgery Center/Free-Standing
Hospital Outpatient Department 1
Surgery Center/Free-Standing
Hospital Outpatient Department 2
Surgery Center/Free-Standing
Hospital Outpatient Department 3
Surgery Center/Free-Standing
Hospital Outpatient Department 4
Surgery Center/Free-Standing
Hospital Outpatient Department 5

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
274 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
9D: Safety of Procedures
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Note: The term “hospital outpatient department” is used to refer to all hospital outpatient
departments or areas of the hospital, including areas that are used for both inpatient and outpatient
procedures, that perform the outpatient procedures listed in Section 9C and that share your hospital’s
license or CMS Certification Number (CCN). This would include, but is not limited to surgery centers, free-
standing hospital outpatient departments, as well as outpatient departments that are located in your
hospital or are co-located
30
with your hospital. Hospitals should only include hospital outpatient
departments or areas of the hospital that perform the procedures listed in Section 9C in an operating
room
51
or procedure room
52
.
Patient Follow-up
This section is not applicable to pediatric hospitals.
Specifications: See the Patient Follow-up Measure Specifications
in the Reference Information
beginning on page 294.
Leapfrog obtains data for one CMS Hospital Quality Star Rating Program measure directly from CMS’
website
:
• OP-32: Rate of Unplanned Hospital Visits After an Outpatient Colonoscopy
In order for Leapfrog to obtain the data for this measure, hospitals must provide a valid CMS Certification
Number (CCN) in the Hospital Profile and submit Section 9 of the Leapfrog Hospital Survey.
Hospitals that do not perform adult colonoscopy procedures will be scored and publicly reported as “Does
Not Apply.” Hospitals that do not provide an accurate CCN in the Hospital Profile or do not report data to
CMS will be scored and publicly reported as “Unable to Calculate Score.” Hospitals that do not respond to
questions in Section 9 or do not submit a Leapfrog Hospital Survey will be scored and publicly reported
as “Declined to Respond.”
Please refer to the Deadlines and Reporting Periods table in the
Patient Follow-up Measure
Specifications.
Reporting Period: 24 months
Most recent 24-month reporting period published by CMS.
Leapfrog will update data three times per Survey Cycle for hospitals that have provided an accurate CCN in the Hospital Profile
and submitted Section 9 of the Leapfrog Hospital Survey.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
275 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Patient Selection
Important Note: Patient Selection (questions #1-4) will not be scored but will be used in public reporting.
1) Do your hospital outpatient department(s) have a standard,
written screening protocol to determine whether a patient’s
procedure can safely be performed on an outpatient basis?
Hospitals with more than one hospital outpatient
department, including a surgery center or free-standing
hospital outpatient department, should respond based on
the location with the fewest processes in place.
If “no” to question #1, skip questions #2-4 and continue to
question #5.
Yes
No
2) Which of the following components are included in your
hospital outpatient department(s)’ standard, written
screening protocol:
Select all that apply.
History of difficult intubation
Difficult airway/aspiration risk
Body Mass Index (BMI)
American Society of
Anesthesiologists (ASA)
Physical Status Classification
Recent Medical History (within
30 days of scheduled procedure)
Cognitive Assessment
Sleep Apnea Assessment
Availability of transportation
following discharge
Availability of a caregiver
following discharge
3) Who completes the standard, written screening protocol to
determine whether a patient’s procedure can safely be
performed on an outpatient basis?
Select all that apply.
Anesthesiologist
Certified Registered Nurse
Anesthetist (CRNA)
Physician
Nurse (RN or MSN)
Physician Assistant (PA)
Nurse Practitioner (NP)
Other
4) When patients are identified through your hospital
outpatient department(s)' screening protocol as high-risk,
does an anesthesiologist, certified registered nurse
anesthetist, or Medical Director complete an additional
medical review to determine whether a patient’s procedure
can safely be performed on an outpatient basis?
Yes
No
Reporting Period:
Answer questions #1-4 based on the practices currently in place at the time you submit this section of
the Survey.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
276 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Safe Surgery Checklist for Adult and Pediatric Outpatient Procedures
Important Notes:
Note 1: The elements required for each stage of the safe surgery checklist in questions #5-12 are
adapted from the WHO Surgical Safety Checklist and the AHRQ Endoscopy Checklist
.
Note 2: Hyperlinks throughout these questions refer to the
Safe Surgery Checklist for Adult and Pediatric
Outpatient Procedures FAQs beginning on page 297, not to endnotes. These hyperlinks are not included
in the Online Survey Tool.
Specifications: See the
Safe Surgery Checklist for Adult and Pediatric Outpatient Procedures
Measure Specifications in the Safety of Procedures Reference Information beginning on page 295.
Sufficient Sample: See
Safe Surgery Checklist for Adult and Pediatric Outpatient Procedures
Measure Specifications for instructions on identifying a sufficient sample for question #10.
5) What is the latest 6-month reporting period for which your hospital
is submitting responses to questions #5-12? 6-month reporting
period ending:
______
Format: Month/Year
6) Does your hospital utilize a safe surgery checklist on every
patient, every time one of the applicable procedures in Section 9C
is performed?
Hospitals with more than one hospital outpatient department,
including a surgery center or free-standing hospital outpatient
department, should respond based on the location with the fewest
processes in place.
If “no” to question #6, skip the remaining questions in Section 9D
and go to the next subsection. The hospital will be scored as
“Limited Achievement.”
Yes
No
7) Before the induction of anesthesia, is a safe surgery checklist
that includes all the following elements read aloud in the presence
of the anesthesia professional and nursing personnel:
• Patient ID
• Confirmation of procedure
• Patient consent
• Site marked, if applicable
• Anesthesia/medication check
• Allergies assessed
• Difficult airway/aspiration risk
• Risk of blood loss (only applicable if risk of blood loss is
>500ml for adults or > 7ml/kg for children)
• Availability of devices on-site, if applicable?
Yes
No
Reporting Period: 6 months
Answer questions #5-12 for the latest 6-month period prior to submission of this section of the Survey.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
277 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
8) Before the skin incision and/or before the procedure begins,
is a safe surgery checklist that includes all the following elements
read aloud in the presence of the whole surgical team:
• Clinical team introduction
• Confirmation of patient name, procedure, and, if applicable,
surgical/incision site
• Antibiotic prophylaxis, if applicable
• Anticipated Critical Events (i.e., non-routine steps, length of
procedure, blood loss, patient-specific concerns, sterility)
• Equipment check/concerns
• Essential imaging available, if applicable?
Yes
No
9) Before the patient leaves the operating room and/or
procedure room, is a safe surgery checklist that includes all the
following elements read aloud in the presence of the whole
surgical team:
• Confirmation of procedure performed
• Instrument/supply counts, if applicable
• Specimen labeling, if applicable
• Equipment concerns
• Patient recovery/management concerns?
Yes
No
If “no” to question #7, #8, or #9, skip the remaining questions in Section 9D, and go to the next
subsection. The hospital will be scored as “Limited Achievement.”
Hospitals performing the audit in Section 3C question #6 and the audit in Section 9D question #10 should
audit 15 cases who underwent a procedure included in Section 3A and 15 cases who underwent a
procedure included in Section 9C. Hospitals only performing the audit in Section 9D question #10 and not
in Section 3C question #6 should audit 30 cases who underwent a procedure included in Section 9C.
10) Did your hospital perform an audit (either in-person or via the
medical record or other EHR data) on a sufficient sample of
patients who underwent a procedure included in Section 9C and
measure adherence to the safe surgery checklist?
Hospitals with more than one hospital outpatient department,
including a surgery center or free-standing hospital outpatient
department, should select at least one case from each location.
If “no” to question #10, skip the remaining questions in Section 9D,
and go to the next subsection. The hospital will be scored as
“Limited Achievement.”
Yes
No
11) Which method was used to perform the audit on a sufficient
sample in question #10?
In-person observational audit
Retrospective audit of
medical records or EHR data
Both
12) Based on your hospital’s audit (either in-person or via the medical
record or other EHR data) on a sufficient sample of patients who
underwent a procedure included in Section 9C, what was your
hospitals documented rate of adherence to the safe surgery
checklist (e.g., what percentage of the sampled cases had all
elements in questions #7, #8, and #9 completed)?
90%-100%
75%-89%
50-74%
Less than 50%

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
278 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
9E: Medication Safety for Outpatient Procedures
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Note: The term “hospital outpatient department” is used to refer to all hospital outpatient
departments or areas of the hospital, including areas that are used for both inpatient and outpatient
procedures, that perform the outpatient procedures listed in Section 9C and that share your hospital’s
license or CMS Certification Number (CCN). This would include, but is not limited to surgery centers, free-
standing hospital outpatient departments, as well as outpatient departments that are located in your
hospital or are co-located
30
with your hospital. Hospitals should only include hospital outpatient
departments or areas of the hospital that perform the procedures listed in Section 9C in an operating
room
51
or procedure room
52
.
Specifications: See Medication Safety for Outpatient Procedures Measure Specifications
in the
Reference Information beginning on page 299.
Sufficient Sample: See Medication Safety for Outpatient Procedures Measure Specifications
for
instructions on identifying a sufficient sample for questions #2-7.
1) 12-month reporting period used:
01/01/2022 – 12/31/2022
07/01/2022 – 06/30/2023
2) Did your hospital perform an audit of clinical records for all
patients undergoing those procedures included in Section 9C (or a
sufficient sample of them) who were discharged from a hospital
outpatient department for the reporting period selected and
measure adherence to medication documentation guidelines
regarding home medications, medications administered during the
visit or prescribed at discharge, and medication allergies?
If “no” to question #2, skip questions #3-7 and continue to the next
subsection. The hospital will be scored as “Limited Achievement.”
If “yes, but there were fewer than 30 outpatients discharged for
the reporting period,” skip questions #3-7 and continue to the next
subsection. The hospital will be scored as “Unable to Calculate
Score.”
Yes
No
Yes, but there were fewer
than 30 outpatients
discharged for the reporting
period
3) Number of cases measured (either all cases or a sufficient sample
of them):
_____
4) Number of cases in question #3 with a list of all home
medication(s), including dose, route, and frequency, documented
in the clinical record:
_____
Reporting Period: 12 months
Answer questions #1-7 based on all cases (or a sufficient sample of them),
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o 07/01/2022 – 06/30/2023
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
279 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
5) Number of cases in question #3 with a list of all medication(s)
administered during the visit and new medications
prescribed at discharge, including the strength, dose, route,
date, and time of administration, documented in the clinical record:
_____
6) Number of cases in question #3 with a list of all medication
allergies and adverse reaction(s) documented in the clinical
record:
_____
7) Do the responses in questions #3-6 represent a sample of cases?
Yes
No

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
280 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
9F: Patient Experience (OAS CAHPS)
This section is not applicable to pediatric hospitals.
Hospitals that share a CMS Certification Number are required to report by facility. Please carefully review
Leapfrog’s Multi-Campus Reporting Policy
.
Important Note: The term “hospital outpatient department” is used to refer to all hospital outpatient
departments or areas of the hospital, including areas that are used for both inpatient and outpatient
procedures, that perform the outpatient procedures listed in Section 9C and that share your hospital’s
license or CMS Certification Number (CCN). This would include, but is not limited to surgery centers, free-
standing hospital outpatient departments, as well as outpatient departments that are located in your
hospital or are co-located
30
with your hospital. Hospitals should only include hospital outpatient
departments or areas of the hospital that perform the procedures listed in Section 9C in an operating
room
51
or procedure room
52
.
Specifications: See Patient Experience (OAS CAHPS) Measure Specifications
in the Reference
Information beginning on page 304.
1) What is the latest 12-month reporting period for which your
hospital is submitting responses to this section? 12-month
reporting period ending:
_____
Format: Month/Year
2) Did your hospital have at least 300 eligible discharges
54
during
the 12-month period referenced above?
If “no” to question #2, skip the remaining questions in Section 9F
and go to the Affirmation of Accuracy. The hospital will be scored
as “Does Not Apply.”
Yes
No
3) Has your hospital administered, or started to administer, the entire
OAS CAHPS Survey during the reporting period?
Hospitals not currently administering the OAS CAHPS Survey
should select “no,” skip the remaining questions in Section 9F,
and go to the Affirmation of Accuracy. The hospital will be scored
as “Limited Achievement.”
Yes
No
4) Total number of months in which your hospital administered the
OAS CAHPS Survey during the reporting period.
_____
5) Total number of returned surveys during the reporting period.
If less than 100, skip the remaining questions in Section 9F, and
go to the Affirmation of Accuracy. The hospital will be scored as
“Unable to Calculate Score.”
_____
Reporting Period: 12 months
Answer questions #1-10 for the latest 12-month period prior to the submission of this section of the
Survey.
Note: As a reminder, the Corrections Period (December 1-January 31) is reserved for corrections to previously submitted
Surveys only. Any updates made to reflect a change in performance must be made prior to the November 30 Late Submission
and Performance Update Deadline. Updates made to reflect a change in performance after November 30 will not be scored or
publicly reported.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
281 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
6) Do the responses to the questions in this subsection include
discharges from more than one location (e.g., hospital and
surgery center or free-standing hospital outpatient department,
hospital and multiple free-standing hospital outpatient
departments, etc.)?
Yes
No
In questions #7-10, report your hospital’s Top Box Score
50
(rounded to the nearest whole number) from
each of the following patient experience domains from your vendor report that matches the reporting
period that you selected in question #1.
7) Facilities and Staff
______
8) Communication About Your Procedure
______
9) Patients’ Rating of the Facility
______
10) Patients Recommending the Facility
______

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
282 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Affirmation of Accuracy
As the hospital CEO or as an employee of the hospital to whom the hospital CEO has delegated
responsibility, I have reviewed this information pertaining to the Outpatient Procedure Section at our
hospital, and I hereby certify that this information is true, accurate, and reflects the current, normal
operating circumstances at our hospital. I am authorized to make this certification on behalf of our
hospital.
The hospital and I understand that The Leapfrog Group, its members, the public and entities and persons
who contract or have other business dealings with The Leapfrog Group are relying on the truth and
accuracy of this information. The hospital and I also understand that The Leapfrog Group will make this
information and/or analyses of this information public through the Survey Results public reporting website,
The Leapfrog Group’s Hospital Safety Grade, and/or other Leapfrog Group products and services. This
information and/or analyses and all intellectual property rights therein shall be and remain the sole and
exclusive property of The Leapfrog Group in which The Leapfrog Group retains exclusive ownership. This
information does not infringe upon any third-party intellectual property rights or any other third-party rights
whatsoever and is free and clear of all encumbrances and liens of any kind. The hospital and I
acknowledge that The Leapfrog Group may use this information in a commercial manner for profit. The
hospital shall be liable for and shall hold harmless and indemnify The Leapfrog Group from any and all
damages, demands, costs, or causes of action resulting from any inaccuracies in the information or any
misrepresentations in this Affirmation of Accuracy. The Leapfrog Group and its members and entities and
persons who contract or have other business dealings with The Leapfrog Group reserve the right to omit
or disclaim information that is not current, accurate or truthful.
Affirmed by _____________________, the hospital’s ___________________________,
(first name and last name) (title)
on _______________________.
(date)

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
283 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Section 9: 2023 Outpatient Procedures Reference Information
What’s New in the 2023 Survey
Section 9D: Safety of Procedures
Patient Follow-up
Leapfrog is removing the CMS outcome measure OP-31 Improvement in Patient’s Visual Function within
90 Days Following Cataract Surgery since CMS has made this measure voluntary through the 2023
Hospital Outpatient Reporting (OQR) Final Rule.
There are no changes to the scoring algorithm for OP-32 Rate of Unplanned Hospital Visits After
Colonoscopy.
Safe Surgery Checklist for Adult and Pediatric Outpatient Procedures
Leapfrog is making two updates to the Safe Surgery Checklist for Adult and Pediatric Outpatient
Procedures question set. First, Leapfrog is increasing the audit requirement from 15 sampled cases to 30
sampled cases for hospitals only submitting Section 9 and not also submitting Section 3: Adult and
Pediatric Complex Surgery. Hospitals that submit both Sections 3 and 9 should continue to audit 15
complex surgical cases and 15 outpatient surgical cases.
Second, for hospitals conducting the audit to assess compliance with the Safe Surgery Checklist,
Leapfrog is adding a question asking if the hospital performed an in-person observational audit, a
retrospective audit of medical records or EHR data, or both.
There are no changes to the scoring algorithm for Safe Surgery Checklist for Adult and Pediatric
Outpatient Procedures.
Section 9E: Medication Safety for Outpatient Procedures
Leapfrog is updating the specifications to clarify that only medications newly prescribed at discharge
should be counted as medications prescribed at discharge and/or administered during the visit. We are
also adding intra-op irrigation solutions to the list of excluded medications and updating the measure
specifications to exclude the dose requirement for lidocaine jelly.
There are no changes to the scoring algorithm for Section 9E: Mediation Safety for Outpatient
Procedures.
Section 9F: Patient Experience (OAS CAHPS)
Leapfrog is clarifying that hospitals must currently be administering the OAS CAHPS Survey to respond
“yes” to question #3, which asks if the hospital administers, or has started to administer, the OAS CAHPS
Survey.
There are no changes to the scoring algorithm for Section 9F: Patient Experience (OAS CAHPS).
Change Summary Since Release
June 13, 2023 - Section 9C Volume of Procedures: Added 14 adult and 18 pediatric CPT Codes used to
identify Other Hernia Repairs and added one adult and one pediatric CPT Code used to identify
Nasal/Sinus Procedures that were previously noted as removed. Updates are reflected in the Outpatient
Procedure CPT Code workbook for Adult and Pediatric General Surgery under Other Hernia Repair and
for Otolaryngology under Nasal/Sinus Procedure.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
284 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Please download an updated copy of the Outpatient Procedure CPT Code workbook from the Survey
Dashboard. Instructions for downloading the workbook are available in the Volume of Procedures
Measure Specifications.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
285 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Basic Outpatient Department Information Frequently Asked Questions
(FAQs)
1. Our hospital shares a CMS Certification Number (CCN) with other hospitals, and we have
many hospital outpatient department locations (e.g., surgery centers, endoscopy centers,
free-standing hospital outpatient departments, etc.) all under the same CCN. How should we
report on Section 9: Outpatient Procedures?
As per Leapfrog's Multi-Campus Reporting Policy
, all hospital campuses will need to complete their
own Leapfrog Hospital Survey unless they are co-located.
For Section 9: Outpatient Procedures, each hospital campus will need to indicate in Section 9A if they
are performing the outpatient procedures listed in Section 9C at their hospital and/or at a surgery
center or free-standing hospital outpatient department that shares their hospital's CMS Certification
Number (CCN). You should only include those hospital outpatient departments that your hospital
refers patients to. The remainder of the questions in Section 9 will need to be answered based on:
• all hospital outpatient departments in your hospital, including any areas such as inpatient
units, where outpatient procedures are performed (as reported in Section 9A question #2);
and,
• all surgery centers and free-standing hospital outpatient departments that share your
hospital's CCN (as reported in Section 9A question #3)
In Section 9C, you will be able to provide total volume across all hospital outpatient department
locations (as reported in Section 9A questions #2 and #3) and information for the different outpatient
locations where the specific outpatient procedures are performed. All other questions should be
answered based on all outpatient locations. If you have different adherence across locations, you
should answer based on the least adherent location.
If all hospital campuses are referring patients to the same surgery centers and free-standing hospital
outpatient departments, you would include the information and volume for these locations when
responding to Section 9 on all their Leapfrog Hospital Surveys.
2. How should our hospital report on the number of operating rooms and endoscopic procedure
rooms in questions #4-5 if our operating/procedure rooms are used for both inpatient and
outpatients? How should we report if we have multiple hospital outpatient departments?
Hospitals should report on the total number of adult and pediatric operating rooms or procedure
rooms if these rooms are used for both inpatient and outpatient procedures. Otherwise, hospitals
should only report on the number of operating/procedure rooms that are used for outpatient
procedures. If your hospital has multiple hospital outpatient departments, report the total number of
adult and pediatric outpatient operating rooms or procedure rooms across all locations. You should
only be reporting on operating rooms and procedure rooms that are used to perform the outpatient
procedures listed in Section 9C Volume of Procedures.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
286 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Medical, Surgical, and Clinical Staff Frequently Asked Questions
(FAQs)
1. How does Leapfrog define “immediately available” and “in the building” as it pertains to ACLS
and/or PALS trained clinicians?
“Immediately available” is defined as being physically present in the building and not engaged in an
activity or procedure that cannot be interrupted if hands-on intervention is needed for a patient.
Leapfrog defines "in the building" as within the hospital outpatient department or surgery center or in
an area co-located with the hospital outpatient department or surgery center. Leapfrog defines co-
located as a location within "immediate physical proximity,” meaning the two locations are physically
connected, either by a tunnel, an enclosed bridge, or the locations abut each other so that hallways
readily connect.
2. If a hospital did not perform any outpatient pediatric procedures during the reporting period
selected in Section 9C, should they still report on PALS trained clinicians in Section 9B
questions #3-4?
No. If your hospital did not report any pediatric discharges for the procedures listed in Section 9C
during the reporting period, then you should select “Not applicable; adult patients only” in question #3.
3. Is the PEARS certification the equivalent of PALS certification for the purposes of responding
to question #3?
No. PEARS is not the equivalent of or substitution for PALS certification. The PEARS curriculum is
focused on recognition of and steps to mitigate pediatric respiratory emergencies, whereas the PALS
curriculum informs clinicians how to manage these emergencies, with emphasis on leadership of the
care team, and how to perform key air management techniques. Additionally, the PALS curriculum
instructs providers on multiple ways to obtain IV access to improve circulatory issues.
4. How should hospitals report on ACLS/PALS certification if they have more than one hospital
outpatient department performing the procedures listed in Section 9C Volume of Procedures?
Hospitals with more than one hospital outpatient department are instructed to respond to questions
#1-4 based on the location with the least intensive staffing. For example, if one hospital outpatient
department has an ACLS trained clinician, as well as a second clinician present at all times and
immediately available in the building when adult patients are in the hospital outpatient department,
but another hospital outpatient department does not, you should answer “no” to question #1.
5. If a free-standing pediatric hospital has clinicians trained in PALS, but a small percentage of
the patient population is over 13, should these clinicians also have ACLS training, or would
the PALS training be sufficient?
If your hospital outpatient departments are performing procedures on both adult and pediatric
patients, there should be at least one clinician with ACLS training when adult patients (13 years and
older) are recovering and one clinician with PALS training when pediatric patients (infant to 12 years)
are recovering. This could mean that some clinicians maintain both certifications or some maintain
ACLS, and others maintain PALS.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
287 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Volume of Procedures Measure Specifications
Important Notes:
Note 1: Hospitals that share a CMS Certification Number are required to report by facility. Please carefully
review Leapfrog’s Multi-Campus Reporting Policy
.
Note 2: CPT Codes are provided in downloadable Excel files on the Survey Dashboard. To access the
files, click the CPT Code Workbook button next to Section 9. You will be required to complete the
American Medical Association’s Terms of Use before downloading the Excel file and using the individual
CPT codes to query your EHR or billing system. You are only required to complete the Terms of Use
once per Survey Cycle (April 1 – November 30).
The CPT Code Workbooks (Excel files) are labeled for Section 3A: Total Hip Replacement, Total Knee
Replacement, and Bariatric Surgery for Weight Loss and Section 9C: Volume of Procedures. Please note,
if you are part of a hospital system, each hospital will need to complete the Terms of Use. This is a
requirement of the American Medical Association.
Note 3: When counting patients for the purposes of identifying total volume in Section 9C, you should only
include scheduled outpatient (i.e., admitted and discharged on the same day) procedures performed at
your hospital outpatient departments within the reporting period. Include scheduled outpatient procedures
with a planned observation or extended recovery stay. Exclude scheduled outpatient procedures with a
planned inpatient admission. Exclude emergent/urgent cases, such as procedures for patients that come
through the emergency department. To count patient discharges, use the Volume of Procedures Measure
Specifications below and the CPT codes available in the Library on the Survey Dashboard.
Source: The Leapfrog Group, American Medical Association, The Health Care Cost Institute
Reporting Period: 12 months
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o
07/01/2022 – 06/30/2023
Questions #2-10: Respond “yes” or “no” based on whether your hospital outpatient department(s)
performed any of the procedures during the reporting period on adult and/or pediatric patients. The
procedures fall within 9 specialty areas:
Adult Procedures
Gastroenterology procedures:
upper GI endoscopy; other upper GI procedures; and lower GI
endoscopy
General surgery procedures:
cholecystectomy and common duct exploration; hemorrhoid
procedures; inguinal and femoral hernia repairs; other hernia repairs; laparoscopy;
lumpectomy or quadrantectomy of breast; and mastectomy
Ophthalmology procedures:
anterior segment eye procedures; posterior segment eye
procedures; and ocular adnexa and other eye procedures
Orthopedic procedures
: finger, hand, wrist, forearm, and elbow procedures; shoulder
procedures; spine procedures; hip procedures; knee procedures; toe, foot, ankle, and leg
procedures; and general orthopedic procedures
Otolaryngology procedures:
ear procedures; mouth procedures; and nasal/sinus procedures
Urology procedures: circumcision; cystourethroscopy; male genital procedures; urethra
procedures; and vaginal repair procedures
Neurological surgery procedures: spinal fusion procedures

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
288 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Obstetrics and gynecology procedures: cervix procedures; hysteroscopy; and uterus and
adnexa laparoscopies
Plastic and reconstructive surgery procedures:
breast repair or reconstructive procedures; and
skin graft/reconstruction procedures
Pediatric Procedures
Gastroenterology procedures:
upper GI endoscopy; other upper GI procedures; and lower GI
endoscopy
General surgery procedures:
inguinal and femoral hernia repairs; and other hernia repairs
Ophthalmology procedures:
anterior segment eye procedures; posterior segment eye
procedures; and ocular adnexa and other eye procedures
Orthopedic procedures:
finger, hand, wrist, forearm, and elbow procedures; shoulder
procedures; knee procedures; toe, foot, ankle, and leg procedures; and general orthopedic
procedures
Otolaryngology procedures:
ear procedures; mouth procedures; nasal/sinus procedures;
pharynx/adenoid/tonsil procedures
Respond “yes” if:
• One or more of your hospital outpatient departments performed the procedure on an outpatient
basis for the entire reporting period (12 months) and continues to do so
• One or more of your hospital outpatient departments performed the procedure on an outpatient
basis during part of the reporting period (less than 12 months) and continues to perform the
procedure
Respond “yes, but no longer perform these procedures” if:
• Your hospital outpatient departments performed the procedure for all or some of the reporting
period, but NO longer performs the procedure
Respond “no” if:
• Your hospital outpatient departments do not perform the procedure
Questions #11-37: Based on your responses to questions #2-10, report on the total (a) adult and/or (b)
pediatric volume for each procedure (from questions #2-10) during the reporting period:
Adult Procedures
Gastroenterology procedures:
upper GI endoscopy; other upper GI procedures; and lower GI
endoscopy
General surgery procedures:
cholecystectomy and common duct exploration; hemorrhoid
procedures; inguinal and femoral hernia repair; other hernia repair; laparoscopy; lumpectomy
or quadrantectomy of breast; and mastectomy
Ophthalmology procedures:
anterior segment eye procedures; posterior segment eye
procedures; and ocular adnexa and other eye procedures
Orthopedic procedures:
finger, hand, wrist, forearm, and elbow procedures; shoulder
procedures; spine procedures; hip procedures; knee procedures; toe, foot, ankle, and leg
procedures; and general orthopedic procedures
Otolaryngology procedures:
ear procedures; mouth procedures; and nasal/sinus procedures
Urology procedures:
circumcisions; cystourethroscopy; male genital procedures; urethra
procedures; and vaginal repair procedures

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
289 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Neurological surgery procedures: spinal fusion procedures
Obstetrics and gynecology procedures:
cervix procedures; hysteroscopy; and uterus and
adnexa laparoscopies
Plastic and reconstructive surgery procedures: breast repair or reconstructive procedures; and
skin graft/reconstruction procedures
Pediatric Procedures
Gastroenterology procedures:
upper GI endoscopy; other upper GI procedures; and lower GI
endoscopy
General surgery procedures:
inguinal and femoral hernia repair; and other hernia repair
Ophthalmology procedures:
anterior segment eye procedures; posterior segment eye
procedures; and ocular adnexa and other eye procedures
Orthopedic procedures:
finger, hand, wrist, forearm, and elbow procedures; shoulder
procedures; knee procedures; toe, foot, ankle, and leg procedures; and general orthopedic
procedures
Otolaryngology procedures:
ear procedures; mouth procedures; nasal/sinus procedures; and
pharynx/adenoid/tonsil procedures
When calculating total hospital outpatient department volume for (a) adult and/or (b) pediatric
patients:
• Count the number of patients discharged from your hospital outpatient department(s) within
the reporting period with any one or more of the codes specified for each procedure, subject to
the criteria below:
o Only include scheduled outpatient (i.e., admitted and discharged on the same day)
procedures performed at your hospital outpatient departments within the reporting
period. Include scheduled outpatient procedures with a planned observation or
extended recovery stay.
o Exclude scheduled outpatient procedures with a planned inpatient admission. Exclude
emergent/urgent cases, such as procedures for patients that come through the
emergency department.
o Only the procedure codes provided by Leapfrog should be used to report on the
questions in Section 9C.
o If a patient had more than one of the listed procedures performed on the same visit
(i.e., repair of dislocating knee cap (CPT: 27422) and repair of superior labrum
anterior/posterior (SLAP) lesion (CPT: 29807)), include the patient in the total volume
for both procedures.
See FAQs
for additional information about responding to questions in this section.
2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
290 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Gastroenterology Measure Specifications
For gastroenterology procedures, use the CPT codes available in the Library on the Survey Dashboard to
count patients discharged from your hospital outpatient departments who have undergone any of the 3
procedures during the reporting period.
All three procedures apply to both adult and pediatric patients:
• Upper GI endoscopy
• Other upper GI procedure
• Lower GI endoscopy
Using the “Gastroenterology_adult” sheet, count the total number of adult (18 years of age or older)
patients discharged for each procedure with any of the CPT codes listed. The CPT code can be in any
procedure field.
Using the “Gastroenterology_peds” sheet, count the total number of pediatric (17 years of age and
younger) patients discharged for each procedure with any of the CPT codes listed. The CPT code can be
in any procedure field.
General Surgery Measure Specifications
For general surgery procedures, use the CPT codes available in the Library on the Survey Dashboard to
count patients discharged from your hospital outpatient departments who have undergone any of the 7
procedures during the reporting period.
Five procedures apply to adult patients only:
• Cholecystectomy and common duct exploration
• Hemorrhoid procedure
• Laparoscopy
• Lumpectomy or quadrantectomy of breast
• Mastectomy
Two procedures apply to both adult and pediatric patients:
• Inguinal and femoral hernia repair
• Other hernia repair
Using the “General surgery_adult” sheet, count the total number of adult (18 years of age or older)
patients discharged for each procedure with any of the CPT codes listed. The CPT code can be in any
procedure field.
Using the “General surgery_ped” sheet, count the total number of pediatric (17 years of age and younger)
patients discharged for each procedure with any of the CPT codes listed. The CPT code can be in any
procedure field.
Ophthalmology Measure Specifications
For ophthalmology procedures, use the CPT codes available in the Library on the Survey Dashboard to
count patients discharged from your hospital outpatient departments who have undergone either of the 3
procedures during the reporting period.
All three procedures apply to both adult and pediatric patients:
• Anterior segment eye procedures
• Posterior segment eye procedures
• Ocular adnexa and other eye procedures

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
291 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Using the “Ophthalmology_adult” sheet, count the total number of adult (18 years of age or older) patients
discharged for each procedure with any of the CPT codes listed. The CPT code can be in any procedure
field.
Using the “Ophthalmology_ped” sheet, count the total number of pediatric (17 years of age and younger)
patients discharged for each procedure with any of the CPT codes listed. The CPT code can be in any
procedure field.
Orthopedic Measure Specifications
For orthopedic procedures, use the CPT codes available in the Library on the Survey Dashboard to count
patients discharged from your hospital outpatient departments who have undergone any of the 7
procedures during the reporting period.
Five procedures apply to both adult and pediatric patients:
• Finger, hand, wrist, forearm, and elbow procedures
• Shoulder procedures
• Knee procedures
• Toe, foot, ankle, and leg procedures
• General orthopedic procedures
Two procedures apply to adult patients only:
• Spine procedures
• Hip procedures
Using the “Orthopedic_adult” sheet, count the total number of adult (18 years of age or older) patients
discharged for each procedure with any of the CPT codes listed. The CPT code can be in any procedure
field.
Using the “Orthopedic_ped” sheet, count the total number of pediatric (17 years of age and younger)
patients discharged for each procedure with any of the CPT codes listed. The CPT code can be in any
procedure field.
Otolaryngology Measure Specifications
For otolaryngology procedures, use the CPT codes available in the Library on the Survey Dashboard to
count patients discharged from your hospital outpatient departments who have undergone any of the 4
procedures during the reporting period.
Three procedures apply to both adult and pediatric patients:
• Ear procedures
• Mouth procedures
• Nasal/sinus procedures
One procedure applies to pediatric patients only:
• Pharynx/adenoid/tonsil procedures
Using the “Otolaryngology_adult” sheet, count the total number of adult (18 years of age or older) patients
discharged for each procedure with any of the CPT codes listed. The CPT code can be in any procedure
field.
Using the “Otolaryngology_ped” sheet, count the total number of pediatric (17 years of age and younger)
patients discharged for each procedure with any of the CPT codes listed. The CPT code can be in any
procedure field.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
292 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Urology Measure Specifications
For urology procedures, use the CPT codes available in the Library on the Survey Dashboard to count
patients discharged from your hospital outpatient departments who have undergone any of the 5
procedures during the reporting period.
All five procedures apply to adult patients only:
• Circumcision
• Cystourethroscopy
• Male genital procedures
• Urethra procedures
• Vaginal repair procedures
Using the “Urology_adult” sheet, count the total number of adult (18 years of age or older) patients
discharged for each procedure with any of the CPT codes listed. The CPT code can be in any procedure
field.
Neurological Surgery Measure Specifications
For neurological surgery procedures, use the CPT codes available in the Library on the Survey
Dashboard to count patients discharged from your hospital outpatient departments who have undergone
the procedure during the reporting period.
One procedure applies to adult patients only:
• Spinal fusion procedures
Using the “Neurological surgery_adult” sheet, count the total number of adult (18 years of age or older)
patients discharged with any of the CPT codes listed. The CPT code can be in any procedure field.
Obstetrics and Gynecology Measure Specifications
For obstetrics and gynecology procedures, use the CPT codes available in the Library on the Survey
Dashboard to count patients discharged from your hospital outpatient departments who have undergone
any of the 3 procedures during the reporting period.
Three procedures apply to adult patients only:
• Cervix procedures
• Hysteroscopy
• Uterus and adnexa laparoscopies
Using the “Obstetrics and gynecology_adult” sheet, count the total number of adult (18 years of age or
older) patients discharged for each procedure with any of the CPT codes listed. The CPT code can be in
any procedure field.
Plastic and Reconstructive Surgery Measure Specifications
For plastic and reconstructive surgery procedures, use the CPT codes available in the Library on the
Survey Dashboard
to count patients discharged from your hospital outpatient departments who have
undergone either of the 2 procedures during the reporting period.
Two procedures apply to adult patients only:
• Breast repair or reconstruction
• Skin graft/reconstruction procedures
Using the “Plastic_reconstruct surg_adult” sheet, count the total number of adult (18 years of age or
older) patients discharged for each procedure with any of the CPT codes listed. The CPT code can be in
any procedure field.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
293 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Volume of Procedures Frequently Asked Questions (FAQs)
1. How did Leapfrog select these 9 specialties and the procedures in this section of the Survey?
Leapfrog worked with the Healthcare Cost Institute (HCCI) to identify the most commonly billed
surgical procedures in ambulatory surgery centers and hospital outpatient departments for
commercially insured adult and pediatric patients. Leapfrog’s technical experts then assessed the list
of procedures based on their frequency and type of anesthesia used during the procedure. Those
selected for the Survey represent the highest volume procedures nationally requiring moderate to
general anesthesia (including nerve blocks).
Please reach out to the Leapfrog Help Desk
if you believe additional CPT Codes should be added to
the Survey; Leapfrog will take these suggestions to our technical experts.
2. Should we count patients discharged with a G code for colonoscopy?
No. A “G” (HCPCS II) code is used to differentiate between colonoscopies performed for screening
purposes rather than for a diagnostic or therapeutic procedure. The Survey only includes major
diagnostic and therapeutic procedures.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
294 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Safety of Procedures Measure Specifications
Patient Follow-up
Leapfrog obtains data for one CMS Hospital Outpatient measures directly from CMS’ website
:
• OP-32: Rate of Unplanned Hospital Visits After an Outpatient Colonoscopy
In order for Leapfrog to obtain the data for this measure, hospitals must provide a valid CMS Certification
Number (CCN) in the Hospital Profile and submit Section 9 of the Leapfrog Hospital Survey.
Deadlines and Reporting Periods
Data downloaded from
CMS will be scored
and publicly reported
for Hospitals that have
submitted Section 9 by
CMS Reporting Period
Available on Hospital Details Page
and Public Reporting Website on
June 30, 2023
Most recent 24-month reporting
period
July 12, 2023 Details Page
July 25, 2023 Public Reporting Website
August 31, 2023
Most recent 24-month reporting
period
September 8, 2023
November 30, 2023
Most recent 24-month reporting
period
December 7, 2023
Review your CMS data for OP-32
• Once Leapfrog has published your hospital’s Survey Results on the Hospital Details Page,
hospitals are urged to compare the CMS data Leapfrog has obtained with the data published on
CMS’
website.
• Dates on which the Survey Results will be available on the Hospital Details page are listed in the
“Deadlines and Reporting Periods” table above.
If you find a discrepancy while comparing your facility’s data published on the CMS website
to the data
published on the Hospital Details page, contact Leapfrog’s Help Desk immediately.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
295 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Safe Surgery Checklist for Adult and Pediatric Outpatient Procedures
Important Note: Hospitals that share a CMS Certification Number are required to report by facility.
Please carefully review Leapfrog’s Multi-Campus Reporting Policy
.
Source: The Leapfrog Group, WHO Surgical Safety Checklist, AHRQ Endoscopy Checklist
Reporting Period: The latest 6-month period prior to submission of this section of the Survey.
Safe Surgery Checklist Workbook (Excel)
To complete the data collection and respond to questions #10-12, hospitals should download the Safe
Surgery Checklist Workbook (Excel). This workbook includes five tabs: Instructions, Section 3C
(Complex Surgery), Section 3C – Data Entry, Section 9D (Outpatient Procedures), and Section 9D –
Data Entry. The Section 9D tabs can be used to complete the safe surgery checklist audits for this
subsection and to calculate the response to select in the Online Survey Tool for question #12.
The workbook is available on the Survey Materials Webpage
.
Sampling:
For hospitals with one hospital outpatient department that performs the procedures listed in
Section 9C and Section 3A and submit both Section 9 and 3: Hospitals can randomly sample 15
patients (who had one of the procedures included in Section 9C) and measure and report adherence to
the safe surgery checklist based on that sample.
When sampling from a larger population of cases, hospitals that perform procedures across multiple
surgical specialties (e.g., general surgery, orthopedics, urology, etc.) included in Section 9C and
facilities that perform procedures for both adult and pediatric patients should obtain a representative
sample (i.e., include patients who underwent procedures in different surgical specialties and include
both adult and pediatric patients, if applicable).
For hospitals with multiple hospital outpatient departments that perform the procedures listed
in Section 9C and Section 3A and submit both Section 9 and 3: Hospitals can randomly sample 15
patients (who had one of the procedures included in Section 9C) and measure and report adherence to
the safe surgery checklist based on that sample. However, hospital must sample across the various
locations where the procedures are being performed, including freestanding hospital outpatient
departments or surgery centers, in addition to ensuring that the sample includes patients who
underwent procedures in different surgical specialties and both adult and pediatric patients, if
applicable.
For hospitals with one or multiple hospital outpatient departments that ONLY perform the
procedures in Section 9C or that ONLY submit Section 9: Hospitals must randomly sample 30
patients who had one of the procedures included in Section 9C and report adherence to the safe
surgery checklist on that sample. Hospitals that perform the procedures listed in Section 9C and 3A
should follow the guidance above.
Question #10: Did your hospital perform an audit (either in-person or via the medical record or
other EHR data) on a sufficient sample of patients who underwent a procedure included in
Section 9C and measure adherence to the safe surgery checklist?
To respond “yes” to question #10, hospitals must measure and document whether all the elements in
questions #7, #8, and #9 were verbalized in the presence of the appropriate personnel for each
sampled case. Hospitals that completed the audit should respond “yes” to this question regardless of
whether the adherence to the checklist was 100%. Hospitals will report on adherence to the checklist in
question #12.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
296 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Question #11: What method was used to perform the audit on a sufficient sample in question
#10?
Hospitals can only perform a retrospective audit by reviewing medical records or EHR data if
adherence to the safe surgery checklist is clearly documented in the medical record or EHR and the
documentation clearly demonstrates that each element was verbalized in the presence of the
appropriate personnel. If hospitals cannot clearly determine whether (a) the checklist element was
verbalized and (b) that the appropriate personnel were present via a retrospective audit, an in-person
observational audit must be completed.
Hospitals can perform the in-person audits up to six months prior to and during the reporting period.
Question #12: Based on your hospital’s audit (either in-person or via the medical record or
other EHR data) on a sufficient sample of patients who underwent a procedure included in
Section 9C, what was your hospitals documented rate of adherence to the safe surgery
checklist (e.g., what percentage of the sampled cases had all elements in questions #7, #8, and
#9 completed)?
Based on the audit completed for question #10, determine the total number of patient audits where all
elements of the safe surgery checklist (included in questions #7, #8, and #9) were read aloud in the
presence of the appropriate personnel 1) before the induction of anesthesia, 2) before the skin incision
and/or the procedure began, and 3) before the patient left the operating and/or procedure room.
Included cases:
• The patient audit shows that all elements of the safe surgery checklist, at all three time points,
were read aloud in the presence of the appropriate personnel.
Excluded cases:
• The patient audit does not demonstrate that all elements of the safe surgery checklist were
read aloud in the presence of the appropriate personnel (i.e., one or more elements of the
checklist were not read aloud or the appropriate personnel were not present during the
checklist).
• T
he patient audit does not demonstrate that the safe surgery checklist was read aloud in the
presence of the appropriate personnel at all three time points (i.e., before anesthesia, before
skin incision, and before the patient leaves).
Important Note: If a Safe Surgery Checklist element includes the qualifier “if applicable”, hospitals
should determine if that element of the checklist was applicable to the patient and procedure being
performed. If the element was determined to be “not applicable” to the patient and procedure, it does
not count against the total adherence. For example, if your hospital performed a hernia repair, the
availability of a device on-site would not be applicable. Therefore, when completing the Safe Surgery
Checklist Workbook for this patient, indicate N/A for that element (device on-site) and your hospital
would be able to count that case as adherent.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
297 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Safety of Procedures Frequently Asked Questions (FAQs)
General Questions
1. How should hospitals report on the processes in Section 9D Safety of Procedures if they have
more than one hospital outpatient department performing the procedures listed in Section 9C
Volume of Procedures?
Hospitals with more than one hospital outpatient department are instructed to respond to Section 9D
based on the location with the fewest processes in place. For example, if one hospital outpatient
department uses a safe surgery checklist on all patients, but another hospital outpatient department
does not, you should answer “no” to question #6.
Patient Follow-up
1. Do hospitals need to do anything for Leapfrog to obtain data for the CMS measures?
Leapfrog will automatically use your hospital’s data that are reported to CMS for OP-32 if you provide
an accurate CCN in the Hospital Profile and submit Section 9 of the 2023 Leapfrog Hospital Survey.
For OP-32, if your hospital does not perform adult lower GI endoscopy procedures, you will be scored
as “Does Not Apply.” More information is available in the Leapfrog Hospital Survey
Scoring
Algorithms.
Patient Selection
2. What are examples of appropriate tools for assessing cognition as part of patient screening
and selection?
Examples of tools that may be used to assess cognition include the Montreal Cognitive Assessment
(MOCA), Mini-Mental State Exam (MMSE), and Mini-Cog. More information on these cognitive
assessments, as well as other commonly used tools, may be found here:
https://www.americangeriatrics.org/sites/default/files/inline-files/kkaycee_sink.pdf
, as well as here:
https://www.aafp.org/family-physician/patient-care/care-resources/cognitive-care.html.
3. Why does a Medical Director need to perform a second screening of high-risk patients?
If an anesthesiologist and/or CRNA performs the initial screening for high-risk patients, then the
second screening should be conducted by a Medical Director, as the Medical Director should take
ownership of how the hospital outpatient department screens patients. The Medical Director should
also have the clinical expertise to determine whether it is safe and appropriate for a patient to have an
invasive procedure or surgery performed on an outpatient basis.
Safe Surgery Checklist for Adult and Pediatric Outpatient Procedures
4. Does the safe surgery checklist referenced in Section 9D questions #5-12 apply to all
procedures, including colonoscopies, endoscopies, etc.?
Yes, it applies to all procedures in Section 9C questions #2-10. If your hospital outpatient
department(s) do not utilize a safe surgery checklist for colonoscopy and/or endoscopy, respond “No”
to question #6.
5. Can different elements of the “Before the induction of anesthesia” checklist (question #7) be
performed in different places?
Yes, hospitals may perform some elements of the pre-anesthesia checklist outside of the operating
room. Hospitals may read aloud the following elements in a pre-procedure (preop) area: Patient ID,
Confirmation of procedure, Patient consent, Site marked, if applicable, and Allergies assessed.
Hospitals should not remove conversation prompts as it is harmful to the purpose of the checklist and
should continue debriefing and requiring participation of the surgical team members in key moments.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
298 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
6. Do the Safe Surgery Checklist components included in questions #7, #8, and #9 need to be in
one document that would align with the WHO example
or can they be in different documents
(i.e., pre-anesthesia notes, surgeon H&P, and pre-surgical checklists, etc.)?
It is possible to have separate checklists for each phase of the surgical procedure (i.e., before the
induction of anesthesia, before the skin incision and/or before the procedure begins, and before the
patient leaves the operating or procedure room). However, each individual component included in
questions #7, #8, and #9 should be listed on the checklist(s) and hospitals should document whether
the components were read aloud at the appropriate time with the appropriate members of the surgical
team present.
7. How is “whole surgical team” defined?
“Whole surgical team” is comprised of the surgeons, anesthesia professionals, nurses, technicians,
and other operating room personnel involved in surgery. Based off the World Alliance for Patient
Safety “Implementation Manual Surgical Safety Checklist (First Edition)”:
https://www.who.int/patientsafety/safesurgery/tools_resources/SSSL_Manual_finalJun08.pdf
).

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
299 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Medication Safety for Outpatient Procedures Measure Specifications
Important Note: Hospitals that share a CMS Certification Number are required to report by facility.
Please carefully review Leapfrog’s Multi-Campus Reporting Policy
.
Reporting Period: 12 months
• Surveys submitted prior to September 1:
o 01/01/2022 – 12/31/2022
• Surveys (re)submitted on or after September 1:
o 07/01/2022 – 06/30/2023
Medication Safety Documentation Workbook (Excel)
To complete the data collection for this subsection and respond to questions #3-7, hospitals should
download the Medication Safety Documentation Workbook (Excel). This workbook includes seven tabs:
Instructions, 2022 Sampling, 2023 Sampling, Home Meds, Visit Meds, Allergies, and Data Entry and can
be used to identify patients to sample in order to complete the three clinical record audits, as well as
calculate the responses to enter into the Online Hospital Survey Tool for each of the audits.
This workbook is available on the Survey Materials webpage
and should be used when completing this
subsection.
Sampling:
For hospitals with one hospital outpatient department that performs the procedures listed in
Section 9C:
If you have more than 30
cases that meet the criteria for inclusion in the denominator of the process
measures during the time period of the clinical record audit, you may randomly sample 30 of them for the
denominator of each documentation guideline, and measure and report adherence based on that sample.
When sampling from a larger population of cases, this is the minimum number of cases needed to make
a statistically reliable statement of percent adherence to the process guidelines.
For hospitals with multiple hospital outpatient departments that perform the procedures listed in
Section 9C:
If you have more than 30
cases total (across all hospital outpatient departments) that meet the criteria for
inclusion in the denominator of the process measure during the time period of the clinical record audit,
you may randomly sample a minimum of 10 cases from the hospital (including any co-located hospital
outpatient departments or inpatient areas performing outpatient procedures) and each free-standing
hospital outpatient department or surgery center. Hospitals will need to report on a total minimum sample
size of at least 30, and measure and report adherence based on that sample. When sampling from a
larger population of cases, this is the minimum number of cases needed to make a statistically reliable
statement of percent adherence to the process guidelines.
For example, if you are reporting on your hospital (including multiple areas where outpatient procedures
are performed), as well as another hospital outpatient department that is free-standing, you could sample
15 cases from each for a total sample size of 30. If you are reporting on seven locations, you could
sample 10 cases from each for a total sample size of 70.
Note: Hospitals that share a CMS Certification Number (CCN) with other hospitals may use the same
sample for reporting on a shared hospital outpatient department location (e.g., surgery center, endoscopy
center, free-standing hospital outpatient department, etc.). For example, Hospital A and Hospital B share
a CCN and both refer patients to the same gastroenterology center. Hospital A samples 15 cases from
their hospital and 15 cases from the gastroenterology center. Hospital B can use the sample of 15 cases
from the gastroenterology center in addition to sampling from their hospital when reporting on their own
Leapfrog Hospital Survey.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
300 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Medications: For the purposes of this measure, a medication is a substance that is taken into, or placed
onto, the body of a person for one or more of the following reasons:
• as a placebo
• to prevent a disease (e.g., flu vaccine)
• to make a diagnosis (e.g., contrast dye)
• to test for the possibility of an adverse effect
• to modify a physiological, biochemical or anatomical function or abnormality (e.g.,
heparin/heparin flushes, statins, antihypertensives, etc.)
• to replace a missing factor (e.g., blood product)
• to ameliorate a symptom (e.g., aspirin)
• to treat a disease or condition (including topicals, nasal sprays, eye drops, compounds, inhalants,
injectables, patches, etc.)
• to induce anesthesia
• to stabilize or hydrate during medical treatment or procedures (e.g., IV fluids, normal saline,
lactated ringers, etc.)
• to provide nutrition (e.g., enteral nutrition products, parenteral nutrition products, breast milk
[breast milk applies to BCMA measure only])
• as a supplement (e.g., iron for a patient with iron deficiency anemia or calcium/vitamin D for a
patient with osteoporosis)
For the purposes of this measure, the following are not considered medications:
• Pre-filled saline flushes and pre-filled heparin flushes
• Chlorhexidine and alcohol preparation pads
• Intra-op irrigation solutions
Question #3 (denominator): Number of cases measured (either all cases or a sufficient sample of
them).
Your hospital should perform a clinical record audit of either all adult and/or pediatric patients undergoing
those procedures included in Section 9C discharged during the reporting period or a sufficient sample of
those patients discharged during the reporting period as described above.
This audit of clinical records can be done retrospectively (anytime during the Survey Cycle of April 1 –
November 30).
The total number of clinical records included in your audit is reported in question #3.
Excluded cases: Patients discharged from the hospital outpatient departments without having one of the
procedures included in Section 9C performed during the reporting period.
Question #4 (numerator): Number of cases in question #3 with a list of all home medication(s),
including dose, route, and frequency, documented in the clinical record.
Determine the total number of clinical records included in the audit (in question #3), where a list of all
home medication(s), including dose, route, and frequency, was documented in the clinical record either
on the day of the procedure or after a pre-screening phone call (i.e., 1-2 days in advance of the
procedure). Records that include documentation that the patient had no home medications should be
included.
“Home medications” are defined as medications that the patient was taking prior to admission.
The following home medications may be excluded from the clinical record unless they are clinically
relevant* (e.g., herbal supplement that is known to interact with anesthesia):
• as needed (PRN) medications
• topical lotions/creams
• saline nasal spray and artificial tear eye drops
• herbals and supplements and vitamins

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
301 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Home medications that must be included in the clinical record include clinically relevant* PRN
medications such as inhalers, nitroglycerin, analgesics (opioid and non-opioid), muscle relaxants, and
sedatives.
*Clinically relevant is defined as any PRN medication that treats a medical condition on the patient’s
problem list and/or condition they are being treated for during the visit (i.e., reason for the hospital stay).
Included cases:
• The clinical record includes documentation that the patient has no home medications.
Excluded cases:
• The clinical record is missing a list of all home medication(s).
• The clinical record is missing dose, route, or frequency for a home medication
Question #5 (numerator): Number of cases in question #3 with a list of all medication(s) administered
during the visit and/or new medications prescribed at discharge, including the strength, dose, route,
date, and time of administration, documented in the clinical record.
Determine the total number of clinical records included in the audit (question #3), where a list of all
medication(s) administered during the visit and/or new medications prescribed at discharge, including the
strength, dose, route, date, and time of administration, was documented in the clinical record on the day
of the procedure.
Local, regional, and general anesthesia medications must only have total dose, date, and time of
administration documented in the clinical record to be considered complete.
IV solutions must have strength, dose, route, date, and time of administration documented in the
clinical record to be considered complete. However, if the IV solution only comes in one
concentration/strength (such as the LR Injection), the strength can be entered as N/A and the start time
and volume of the bag can be documented for dose in order to be considered complete.
New medications prescribed at discharge, but not administered at the facility must only have strength,
dose, route, and date the medication was prescribed documented in the clinical record to be
considered complete.
Excluded cases:
• The clinical record is missing a medication that was administered or prescribed during the visit or
at discharge.
• The clinical record is missing strength, dose, route, date, or time of administration for a
medication administered or prescribed during the visit or at discharge except as described above
for anesthesia medications, IV solutions, and prescribed medications.
• The clinical record is missing dose for lidocaine jelly.
Question #6 (numerator): Number of cases in question #3 with a list of all medication allergies and
adverse reaction(s) documented in the clinical record.
Determine the total number of clinical records included in the audit (question #3), where a list of all
medication allergies and adverse reaction(s) was documented in the clinical record. Hospitals should only
assess medication allergies (i.e., hospitals do not need to assess food or environmental allergies).
Included cases:
• The clinical record includes documentation that the patient reported no known allergies.
Excluded cases:
• The clinical record does not include either a list of allergies and adverse reaction(s) nor
documentation of no known allergies.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
302 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
• The clinical record does include a list of allergies but does not include documentation of the
adverse reaction(s) for each allergy.
Important Note: In addressing allergies and adverse reaction statuses noted as “unknown” in the clinical
record, hospitals should assess if:
1. “unknown” is used to indicate that the patient (or patient’s family) was asked for the adverse
reaction status, but they indicated it was not known, in which situation the case should be
included in the numerator (question #6); or
2. “unknown” is used in the clinical record to indicate that the information is not available because it
was not requested or documented by the clinician, in which situation the case should be excluded
from the numerator (question #6)
See FAQs
for additional information about responding to questions in this section.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
303 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Medication Safety for Outpatient Procedures Frequently Asked
Questions (FAQs)
1. How often do home medications need to be updated in the clinical record for Section 9E
question #4?
Home medications must be recorded in a pre-screening phone call 1-2 days in advance of the
procedure or updated on the day of the clinical procedure (for all procedures included in Section 9C
of the 2023 Leapfrog Hospital Survey).
Patients who are returning for a second or follow-up procedure within 12 months of the initial
procedure are not required to have an updated home medication list in their clinical record in order for
the record to be counted in the numerator of the home medication audit (i.e., included in the count in
question #4). However, in cases of frequent repeated clinical visits, the home medications list should
be updated at least once every 12 months.
2. For medications that only ever have a single route of administration (e.g., orally only), does
route have to be documented in the clinical record for questions #4 and #5?
No. If there is a medication that can only ever be given via a single route, route does not have to be
documented to count the case when responding to questions #4 and #5.
3. We do medication reconciliation on patients for certain outpatient procedures. Will the
medication reconciliation on the procedure day suffice for the "medication audits"
requirement in question #4? Or does it have to be a retrospective audit after the procedure
day?
Leapfrog specifies that, unless all medical records are audited, the clinical record audit should be
conducted on a random sample of discharged patients. If your facility does not perform medication
reconciliation before all patients are discharged to ensure that the correct dose, route, and frequency
are documented in the clinical record for all home medications this would not meet the requirements
of Section 9E.
Instead, your facility should randomly sample from all clinical records of discharged outpatients
(adults and children) that had the procedures included in Section 9C during the reporting period, not
just those records that had medication reconciliation conducted during the same day visit. This audit
may occur on the same day as the patient visit following patient discharge.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
304 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Patient Experience (OAS CAHPS) Measure Specifications
Important Note: Hospitals that share a CMS Certification Number are required to report by facility.
Please carefully review Leapfrog’s Multi-Campus Reporting Policy
Source: Developed by Centers for Medicare and Medicaid Services (CMS) using Agency for
Healthcare Quality and Research (AHRQ) guidelines. More information available at
https://oascahps.org/General-Information/About-OAS-CAHPS-Survey
.
Reporting Period: The latest 12-month period prior to the submission of this section of the Survey.
Hospitals can elect to use either survey return date or discharge date to pull reports for this measure.
This section of the Survey asks hospitals about their results from the OAS CAHPS Survey. The first
several questions are designed to learn more about the current administration of the survey. The last
four questions collect the
Top Box Score
50
for each of the following four domains:
• Facilities and Staff
• Communication About Your Procedure
• Patient’s Rating of the Facility
• Patients Recommending the Facility
Hospitals using Press Ganey, NRC, or PRC to administer the OAS CAHPS Survey
can use the CMS
OAS CAHPS crosswalk below to ensure they are reporting on the correct domains. The crosswalk is
designed to help translate the standard vendor report to the CMS domains used in questions #7-10.
CMS OAS CAHPS Crosswalk
CMS Domain Name
Press Ganey Domain
Name
NRC Domain Name
PRC Domain Name
Facilities and Staff
Facility/Personal
Treatment
About Facilities and
Staff
Facility and Staff
Communication About
Your Procedure
Communication
Communications
About Your
Procedure
Communication
Patient’s Rating of the
Facility
Facility Rating 0-10
Overall Rating of
Facility
Overall Facility
Rating
Patients
Recommending the
Facility
Recommend the
Facility
Would Recommend
Facility
Would Recommend
Question #2: Did your hospital have at least 300 eligible discharges
54
during the 12-month reporting
period?
This section of the Survey is designed for hospitals that discharged at least 300 eligible patients during
the reporting period. Hospitals that discharged fewer than 300 eligible patients should respond “no,”
skip the rest of the questions, and move on to the Affirmation of Accuracy.
Eligible discharges include discharges for adult patients (ages 18 years and older) who had both
medically and non-medically necessary outpatient surgeries and/or procedures. A detailed description
of patient sampling criteria, including a list of OAS CAHPS-eligible surgeries and procedures, is
available in the Protocols and Guidelines Manual, version 7.0 at https://oascahps.org/Survey-Materials
.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
305 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Question #3: Has your hospital administered, or started to administer, the entire OAS CAHPS Survey,
during the reporting period?
The OAS CAHPS survey includes questions about patients’ experiences with their preparation for the
surgery or procedure, check-in processes, cleanliness of the facility, communications with the facility
staff, discharge from the facility, and preparation for recovering at home. The survey also includes
questions about whether patients received information about what to do if they had possible side
effects during their recovery. OAS CAHPS is designed to be national in scope and requires
standardized administration protocols.
If your hospital is not currently administering the OAS CAHPS Survey, a list of approved vendors is
available at https://oascahps.org/General-Information/Approved-Survey-Vendors
.
Question #4: Total number of months in which your hospital administered the OAS CAHPS Survey
during the reporting period.
It is recommended that hospitals (or their survey vendor) sample over a 12-month period and ensure
an even distribution of patients is sampled over the 12-month period. However, Leapfrog will accept
OAS CAHPS results from hospitals that have administered the survey over a period of time less than
12 months if they have at least 100 returned surveys.
Question #5: Total number of returned surveys during the reporting period.
It is recommended that hospitals (or their survey vendor) administer the survey to a large enough
sample in order to achieve 300 returned surveys in a 12-month reporting period. However, Leapfrog
will accept OAS CAHPS results from hospitals that have at least 100 returned surveys.
Question #6: Do the responses to the questions in this subsection include discharges from more than
one location (e.g., hospital and surgery center or free-standing hospital outpatient department, hospital
and multiple free-standing hospital outpatient departments, etc.)?
Indicate “yes” if your OAS CAHPS results include eligible patient discharges from multiple locations
(e.g., hospital and surgery center or free-standing hospital outpatient department, hospital and multiple
free-standing hospital outpatient departments, etc.). Otherwise indicate “no.”
Questions #7-10: In questions #7-10, report your hospital’s Top Box Score
50
(rounded to the nearest
whole number) from each of the following patient experience domains from your 12-month vendor
report that matches the reporting period selected in question #1. Hospitals should not use domain
scores that are publicly reported on the CMS Hospital Compare
website as these scores have been
risk adjusted. Hospitals should report results from all hospital outpatient departments.
These four questions capture the Top Box Score for each of the four domains of patient experience:
facilities and staff, communication about your procedure, patients’ rating of the facility, and patients
recommending the facility.
The following questions from the OAS CAHPS Survey are included in each domain:
Facilities and Staff
Q3: Did the check-in process run smoothly?
Q4: Was the facility clean?
Q5: Were the clerks and receptionists at the facility as helpful as you thought they should be?
Q6: Did the clerks and receptionists at the facility treat you with courtesy and respect?
Q7: Did the doctors and nurses treat you with courtesy and respect?
Q8: Did the doctors and nurses make sure you were as comfortable as possible?
Communication About Your Procedure
Q1: Before your procedure, did your doctor or anyone from the facility give you all the information you
needed about your procedure?
Q2: Before your procedure, did your doctor or anyone from the facility give you easy to understand
instructions about getting ready for your procedure?

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
306 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Q9: Did the doctors and nurses explain your procedure in a way that was easy to understand?
Q10: Anesthesia is something that would make you feel sleepy or go to sleep during your procedure.
Were you given anesthesia?
Q11: (If ‘Yes’ to Q10) Did your doctor or anyone from the facility explain the process of giving
anesthesia in a way that was easy to understand?
Q12: (If ‘Yes’ to Q10) Did your doctor or anyone from the facility explain the possible side effects of the
anesthesia in a way that was easy to understand?
Patients’ Rating of the Facility
Q23: Using any number from 0 to 10, where 0 is the worst facility possible and 10 is the best facility
possible, what number would you use to rate this facility?
Patients Recommending the Facility
Q24: Would you recommend this facility to your friends and family?
Please note that question numbers are taken from the OAS CAHPS Survey, which you can download
at https://oascahps.org/Survey-Materials
.
See FAQs
for additional information about responding to questions in this section.

2023 Leapfrog Hospital Survey – Hard Copy Section 9: Outpatient Procedures
307 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Patient Experience (OAS CAHPS) Frequently Asked Questions (FAQs)
1) Why is Leapfrog asking for results of the OAS CAHPS Survey, given that it is not required by
CMS and many facilities are not currently administering it?
While we understand that the OAS CAHPS Survey is still a voluntary component of the CMS Quality
Reporting Program, this survey is the only nationally standardized instrument designed to compare
patient experience in both HOPDs and ASCs. No other survey has been tested and validated for this
purpose. All measures included in Leapfrog’s programs are predicated on the latest evidence and
recommended by Leapfrog’s panels of experts. They are also selected because of their importance to
consumers, employers, and other purchasers.
Leapfrog will continue to include these questions on the Leapfrog Hospital Survey/Leapfrog ASC
Survey and would welcome additional feedback from participating facilities.
2) Can we report OAS CAHPS results to Leapfrog if we don’t currently report our results to CMS?
Yes, hospitals can report OAS CAHPS results to Leapfrog even if they are not reporting OAS CAHPS
results to CMS.
3) Isn’t 300 returned surveys the minimum sample size recommended by CMS?
Yes, however, Leapfrog has received feedback that many hospitals and ambulatory surgery centers
have only recently started to administer the survey. In order to ensure as many hospitals and
ambulatory surgery centers as possible are able to report on this subsection, we have reduced the
minimum sample size for reporting results to the Leapfrog Hospital and ASC Surveys to 100 returned
surveys. This will help ensure that hospitals and ASCs that have made the investment to administer
the Survey are able to earn credit for doing so. Additionally, this minimum sample size aligns with
Section 8A of the Leapfrog Hospital Survey, which asks about the CAHPS Child Hospital Survey, and
with the CMS requirement for the CAHPS Child Hospital Survey.
If possible, however, it is recommended that hospitals (or their survey vendor) administer the survey
to a large enough sample in order to achieve 300 returned surveys in a 12-month reporting period.
4) We administer our own patient experience survey to collect specific information about our
patient’s experience. Can we report the results from our hospital’s patient experience survey?
No, hospitals can only report the results of the official OAS CAHPS Survey on Section 9F of the
Leapfrog Hospital Survey.
However, according to the OAS CAHPS Protocols and Guidelines Manual, survey vendors and
ASCs/HOPDs may choose to add up to 15 supplemental questions after the ‘core’ OAS CAHPS
Survey questions that are personalized to the hospital/vendor. More information on these
supplemental questions, including restrictions and required approval, may be reviewed on pages 18-
20 of the CMS OAS CAHPS Survey Protocols and Guidelines Manual, which is available for
download here: https://oascahps.org/Survey-Materials
. Please note, the responses to these
supplemental questions will not be reported on the Leapfrog Hospital Survey.

2023 Leapfrog Hospital Survey – Hard Copy Endnotes
308 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Endnotes
1
CMS Certification Number (CCN)
A CMS Certification Number (CCN) is issued by the Centers for Medicare and Medicaid Services (CMS)
to financial reporting entities, which may be individual hospitals or a group of hospitals, for the purpose of
reimbursement. While Leapfrog does ask each campus of a multi-hospital system to submit an individual
Survey, hospitals within the system may be assigned the same CMS Certification Number and therefore
should have the same CCN reported in this field. CCNs are six digits; with the first two digits representing
the state in which the hospital is located. Hospitals that do not receive Medicare reimbursement may not
have a CMS Certification Number and should not have a CCN reported in this field. Leapfrog pre-
populates this field in the Online Hospital Survey Tool. If the hospital’s CCN is different from the one
shown online, please contact the Help Desk
.
2
National Health Safety Network (NHSN) ID
A NHSN ID is issued by the Centers for Disease Control and Prevention and is used as a unique identifier
for facilities participating in NHSN surveillance activities. Each hospital within a system, even if they share
a CCN, should report separately to NHSN and should have their own NHSN ID if they are located
separately. Please see the NHSN instructions available at
http://www.leapfroggroup.org/survey-
materials/join-nhsn. NHSN IDs are five digits. Leapfrog pre-populates this field in the Online Hospital
Survey Tool for hospitals that provided a valid NHSN ID, joined our NHSN Group, and submitted the 2022
Leapfrog Hospital Survey. If the hospital NHSN ID is different from the one shown online, please update
accordingly.
In order to be scored and publicly reported for any of the five applicable infection measures and to have
the hospital’s teaching status represented, hospitals must: (1) provide an accurate NHSN ID in the Profile,
(2) join Leapfrog’s NHSN Group by the appropriate deadline
, and (3) submit the 2023 Leapfrog Hospital
Survey by the appropriate deadline.
3
Vermont Oxford Network (VON) Transfer Code
A Vermont Oxford Network (VON) Transfer Code is issued by the Vermont Oxford Network and is used
as a unique identifier for hospitals reporting data through the VON Nightingale Internet Reporting System.
Each hospital has their own VON Transfer Code, which can be found at
https://public.vtoxford.org/transfer-codes/
. VON Transfer Codes are four, five, or seven digits. Leapfrog
pre-populates this field in the Online Hospital Survey Tool Profile for hospitals that provided a valid VON
Transfer Code in 2022. If the hospital’s VON Transfer Code is different from the one shown online, please
update accordingly.
4
Federal Tax Identification Number (TIN)
Enter the TIN that your hospital uses for billing purposes. The number is a nine-digit number (e.g.,
098765432) and must conform precisely to this format – be sure to enter any leading 0. If your hospital
has more than one TIN, use the one that would most typically be used for UB-04 claims filed with
commercial health insurance plans for inpatient hospital stays.
5
National Provider Identifier (NPI)
The NPI is a Health Insurance Portability and Accountability Act (HIPAA) Administrative Simplification
Standard. The NPI is a unique identification number of covered health care providers. The NPI is a 10-
position, intelligence-free numeric identifier (10-digit number). This means that the numbers do not carry
other information about healthcare providers, such as the state in which they live or medical specialty.
Leapfrog pre-populates this field in the Online Hospital Survey Tool. If the hospital’s NPI is different from
the one shown online, please contact the Help Desk
.

2023 Leapfrog Hospital Survey – Hard Copy Endnotes
309 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
6
State
Your hospital is assigned to a state based on the CMS Certification Number assigned (or identifier
specially issued by the Leapfrog Help Desk) to your hospital. If your hospital is incorrectly assigned to a
state, contact the Help Desk
to resolve the discrepancy.
7
Tips for entering Web addresses
This address becomes the link attached to your hospital’s name in the public release of Survey Results.
Enter it exactly as you wish it to be and test it.
• Do not exit out of the Online Hospital Survey Tool to go to the Web page of interest while you are
entering data into the Survey or some of your Survey entries may be lost.
• Instead, minimize (but don’t close) the Survey window and any other windows that are open, then
open your internet browser in a separate window. Find the Web page whose address you wish to
enter and Copy/Paste the entire address into the Survey entry. The http:// prefix needs to be
included.
• If entering the Web page address manually, be careful to type it correctly, without embedded
spaces. Forward (/) or backward (\) slashes may be used. Don’t forget the “www.” if that is part of
the address. The http:// prefix needs to be included.
• Make sure to use .org, rather than .com, if that’s the domain for your hospital’s website.
• Although many hospitals elect to enter the address for the home page of their hospital website,
consider pointing it to a page specific to patient safety, the Leapfrog safety practices, or other
quality improvement activities that you want to communicate to your community.
8
Healthcare System
A hospital is considered part of a system if it is owned, leased, or sponsored by a central organization that
owns, leases, or sponsors two or more hospitals. If your hospital is submitting a Survey for only one
facility in the health care system, you would mark "yes" to this question and indicate the name of the
health care system.
9
Licensed Acute-Care Beds
If your state does not designate and license bed types, enter the number of staffed beds from question
#3. Include short-term, acute-care medical, surgical, obstetrical, and ICU beds, as licensed by your state.
Exclude beds licensed or used for psychiatric care, rehabilitation, or sub-acute care (e.g., skilled nursing
facility, hospice extended care, sub-acute eating disorder treatment, extended care facility, or residential
substance abuse treatment). If the number of licensed beds has changed in the last year, indicate the
most recent number for which it is licensed.
10
Staffed Acute-Care Beds
Include licensed beds regularly in operation, whether currently occupied by a patient or not. If the number
has changed over the last year, indicate the average or other number most representative of your
operating bed capacity over the last year.
11
Total Adult Acute-Care General Admissions
Include adult (aged 18 years and older) acute-care medical, surgical, obstetrical, and ICU admissions to
any inpatient unit. Include transfers from other hospitals as admissions to your hospital. Include any
admissions directly to an ICU in your hospital, even if counted in question #9. Include admissions to
telemetry/step-down/progressive units.
Exclude rehabilitation, observation, short and long-term psychiatric, or sub-acute care (e.g., skilled
nursing facility, hospice extended care, sub-acute eating disorder treatment, extended care facility, or
residential substance abuse treatment) admissions.

2023 Leapfrog Hospital Survey – Hard Copy Endnotes
310 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
12
Total Pediatric Acute-Care General Admissions
Include pediatric (ages 17 years and younger) acute-care medical, surgical, and ICU admissions to any
inpatient unit. Include transfers from other hospitals as admissions to your hospital. Include any
admissions directly to an ICU or neonatal ICU (NICU) in your hospital, even if counted in question #9 or
#11. Include admissions to telemetry/step-down/progressive units.
Exclude newborn admissions to the nursery and pediatric patients admitted for maternity care,
rehabilitation, observation, short and long-term psychiatric, or sub-acute care (e.g., skilled nursing facility,
hospice extended care, sub-acute eating disorder treatment, extended care facility, or residential
substance abuse treatment). Exclude admissions for patients that were transferred to another facility.
13
Licensed ICU Beds
If your state separately designates ICU beds, indicate the number of licensed beds in adult and pediatric
general medical and/or surgical ICUs and neuro ICUs (medical and surgical). If your state does not
designate and license ICU beds, enter the number of staffed beds from question #8. See endnote #34 for
more information.
Exclude beds “dedicated exclusively” to patients with specialized conditions (e.g., cardiac, burn, trauma,
neonatal) that are distinct and separate from other adult or pediatric general medical and/or surgical ICUs
or neuro ICUs unless the same ICU is used for both specialized intensive care patients as well as general
medical and/or surgical or neuro intensive care patients. “Dedicated exclusively” means that general
medical and/or surgical or neuro patients are not also cared for in these specialized units (except in rare
overflow situations).
14
Staffed ICU Beds
Indicate the number of ICU beds from question #7 that are regularly in operation, whether currently
occupied by a patient or not. If the number has changed over the last year, indicate the average or other
number most representative of your operating ICU capacity over the last year. See endnote #34 for more
information.
15
ICU Admissions to Adult and Pediatric General Medical and/or Surgical or Neuro ICUs
Include admissions to adult and pediatric general medical and/or surgical ICUs and neuro ICUs (medical
and surgical) from question #8, whether directly admitted to the unit or transferred to the unit from another
area of your hospital (e.g., post-operatively). Include transfers from other hospitals as admissions to your
hospital. Count the number of hospitalizations that include an ICU stay, not the number of patient trips to
the ICU.
Exclude admissions to units “dedicated exclusively” to patients with specialized conditions (e.g., cardiac,
burn, trauma, neonatal, etc.) that are distinct and separate from other adult or pediatric general medical
and/or surgical ICUs or neuro ICUs unless the same ICU is used for both specialized intensive care
patients as well as general medical and/or surgical or neuro intensive care patients. “Dedicated
exclusively” means that general medical and/or surgical or neuro patients are not also cared for in these
specialized units (except in rare overflow situations). Ignore admissions or transfers to intermediate care
or telemetry/step-down/progressive units for this question.
16
Neonatal ICU Admissions
Include admissions to any level neonatal ICU (NICU), even if counted in question #5. Include transfers
from other hospitals as admissions to your hospital. Exclude admissions for patients that were transferred
to another facility.
17
CPOE Linked to Pharmacy, Laboratory, ADT Information
The ability of a CPOE system to catch the majority of common, serious prescribing errors depends on
proper identification of patients (ADT information), current and recent pharmacy orders and drug
dispensing history, and access and integration of key laboratory results for the patient. CPOE systems

2023 Leapfrog Hospital Survey – Hard Copy Endnotes
311 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
that are not linked to those other systems or do not reflect that current information accurately about the
patient are not likely to catch serious prescribing errors.
18
Intensive Care Units
For the purposes of reporting on Section 2C: BCMA, all adult, pediatric, and/or neonatal ICUs should be
included, such as general medical/surgical ICUs, all specialty ICUs, and mixed-acuity units that include
ICU patients.
19
Medical and/or Surgical Units
An exact definition on which units would be included in general medical, surgical, or medical/surgical
cannot be provided because each hospital is laid out differently. For information about what is considered
a general medical, surgical, or medical/surgical unit, please refer to the CDC’s definitions of Medical
Ward, Medical/Surgical Ward, and Surgical Ward on p. 15-17 to 15-20 of the following link:
http://www.cdc.gov/nhsn/PDFs/pscManual/15LocationsDescriptions_current.pdf
.
The flowchart on p. 15-3 can also be used to help define units in your hospital.
Telemetry/step-down/progressive units are considered medical/surgical units and must be included when
reporting on Section 6D: Hand Hygiene and Section 2C: BCMA. However, step-down/progressive units
should be excluded when reporting on Section 6C: Nurse Staffing and Skill Level. Units for patients from
a specific service type (e.g., burn, cardiac) should not be included. Observation units should be excluded.
20
Labor and Delivery Units
Labor and delivery units should include all antepartum and postpartum units. Nursery units, OR units, and
procedural areas should be excluded.
21
Certified Pharmacy Technician
A certified pharmacy technician would include a pharmacy technician that has earned either the
ASHP
Medication History-Taking Certificate or the Pharmacy Technicians Certification Board’s (PTCB)
Medication History Certificate.
22
Sampling for Medication Reconciliation
The sample should contain at least 30 patients from a 6-month reporting period. Sampling is limited to
medical/surgical units. Patients that were discharged or expired before the Gold Standard Medication
History could be obtained should be excluded from the sample. Patients that do not have discharge
orders written during the reporting period should also be excluded from the sample. A sampling
worksheet is available in the Medication Reconciliation Workbook
for those who would like assistance in
obtaining a random sample of patients.
23
Gold Standard Medication History
The Gold Standard Medication History is the list of medications that the patient was taking prior to
admission. Within 24 hours after admission, a trained pharmacist, pharmacy resident, or certified
pharmacy technician must interview each patient selected for the 6-month sample and obtain the Gold
Standard Medication History. Note that this is in addition to, and separate from, any pre-admission
medication list that was created as part of normal care. Best practices for collecting the Gold Standard
Medication History can be found in the “Other Supporting Materials” for Section 2 on our website
.
24
Discrepancies in Gold Standard Medications
For each Gold Standard Medication, there may be up to two unintentional discrepancies: a discrepancy in
admission orders and a discrepancy in discharge orders. For example, if a medication on the Gold
Standard Medication History is ordered for a patient on admission with the incorrect dose, this counts as
one discrepancy. If this medication is ordered on discharge for the same incorrect dose, this counts as a
second discrepancy. The number of unintentional discrepancies is a count of medication orders where an
unintentional discrepancy occurred. You should not count the number of errors associated with the same

2023 Leapfrog Hospital Survey – Hard Copy Endnotes
312 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
medication order (e.g., a discrepancy in the dose and frequency in the same medication in admission
orders counts as one discrepancy).
25
Unintentionally Ordered Additional Medications
Count medications where a patient was not taking (and was not supposed to be taking) a certain
medication, but the medical team incorrectly thought the patient was taking the medication and therefore
ordered it on admission and/or discharge. Count each medication once, regardless of whether it was
ordered on admission, discharge, or both.
26
Discrepancies due to Unintentionally Ordered Additional Medications
For each unintentionally ordered additional medication, there may be up to two discrepancies:
unintentionally ordered at admission, unintentionally ordered at discharge, or both. For example, if a
medication is unintentionally ordered at admission, then this counts as one discrepancy. If the same
medication is also ordered at discharge, then this counts as a second discrepancy.
27
Data Completeness Requirement for the STS Mitral Valve Repair/Replacement (MVRR)
Composite Score
Participants are excluded from the analysis if they have fewer than 36 isolated MVRR procedures in the
patient population. Please find more information in the NQF-endorsed measure specifications, available
at https://www.sts.org/quality-safety/performance-measures/descriptions#MVRRComposite
.
28
Appropriateness Criteria
A procedure-specific set of criteria developed and implemented at the hospital that surgeons use to
evaluate whether the patient is appropriate for surgery. These criteria should not be surgeon-specific, but
procedure-specific. These criteria should be developed by those performing the procedure at the hospital
and are not solely the product of a payment or reimbursement program.
29
High-Risk Deliveries Electively Admitted
Includes deliveries with:
• expected birth weight <1500 grams; or
• gestational age at least 22 weeks but <32 weeks.
Not all women at risk for delivery of babies with these conditions are known beforehand to be at risk.
Therefore, deliveries in which these high-risk conditions were unknown prior to admission are not
considered electively admitted high-risk deliveries.
If your hospital admits deliveries where these conditions are known prior to admission, then your hospital
electively admits high-risk deliveries and you should answer “yes” to question #1; otherwise, answer “no.”
30
Co-located
A location within “immediate physical proximity” of the hospital would be considered a co-located location
or unit, e.g., a co-located neonatal ICU (NICU) or co-located hospital outpatient location. "Immediate
physical proximity” means the two locations must be physically connected, either by a tunnel, an enclosed
bridge, or the locations abut each other so that the hallways readily connect.
31
Very low birth weight babies
Complicated newborns are those infants with a birth weight <1500 grams. If your hospital has a neonatal
ICU (or is co-located with a hospital that has a neonatal ICU) that admits or accepts transfers of neonates
with these conditions, you should answer “yes” to question #2.
32
VON’s Death or Morbidity Measure
This measure is collected and calculated by the Vermont Oxford Network and includes patients who have
died or are known to have one or more of the following: severe intraventricular hemorrhage (SIVH);

2023 Leapfrog Hospital Survey – Hard Copy Endnotes
313 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
chronic lung disease (CLD); necrotizing enterocolitis (NEC); pneumothorax; any late infection (bacterial,
fungal, or coagulase negative staph); or cystic periventricular leukomalacia (PVL).
33
All Critical Care Patients
“All critical care patients” means all general medical and/or surgical ICU patients and neuro ICU
patients in the ICU.
34
Adult or Pediatric General Medical and/or Surgical ICUs or Neuro ICUs
Section 5: IPS standard applies only to adult and pediatric general medical and/or surgical ICUs and
neuro ICUs (medical and surgical). When responding to Section 5, only include adult and pediatric
general medical and/or surgical ICUs and neuro ICUs (medical and surgical). Ignore units “dedicated
exclusively” to patients with specialized conditions (e.g., cardiac, burn, trauma, neonatal, etc.) that are
distinct and separate from other adult or pediatric general medical and/or surgical ICUs or neuro ICUs
unless the same ICU is used for both specialized intensive care patients as well as general medical
and/or surgical or neuro intensive care patients. “Dedicated exclusively” means that general medical
and/or surgical or neuro patients are not also cared for in these specialized units (except in rare overflow
situations). Ignore admissions or transfers to intermediate care or telemetry/step-down/progressive units
for this question.
Cardiac critical care patients in a general medical or surgical ICU that are in beds dedicated to cardiac
critical care patients and being cared for by dedicated cardiac physicians (e.g., cardiologist or cardiac
surgeon) and nursing staff are not included in Leapfrog's ICU Physician Staffing standard and do not
need to be managed/co-managed by the intensivist. Cardiac critical care patients in a general medical or
surgical ICU bed being cared for by general medical or surgical critical care physicians and nursing staff
are included in the standard and do need to be managed/co-managed by the intensivist.
For hospitals that have more than one type of ICU included in this standard, where the ICU physician
staffing structure may differ among ICU types, hospitals are instructed to report on the ICU with the lowest
level of staffing by physicians certified in critical care medicine when responding to questions #1-15 in
Section 5: ICU Physician Staffing. For example, if the pediatric medical ICU is staffed by intensivists at
least 8 hours/day, 7 days/week, but the adult medical ICU is not, the hospital would respond to questions
#1-15 based on the adult medical ICU.
35
Managed or Co-Managed
The intensivist, when present (whether on-site or via telemedicine), is authorized to diagnose, treat, and
write orders for a patient in the ICU on the intensivist’s own authority. Mandatory consults or daily rounds
by an intensivist are not sufficient to meet the managed/co-managed requirement. However, an ICU need
not be closed to meet this requirement.
36
Certified in Critical Care Medicine
A physician who is “certified in Critical Care Medicine” is a board-certified physician who is additionally
certified in the subspecialty of Critical Care Medicine. Certification in Critical Care Medicine is awarded by
the American Boards of Internal Medicine, Surgery, Anesthesiology, Pediatrics, and Emergency
Medicine.
“Neurointensivists” include any physician that meets at least one of the following paths:
• Physicians who are board-certified in their primary specialty and who are additionally certified in
the subspecialty of Neurocritical Care Medicine. Certification in Neurocritical Care Medicine is
awarded by the United Council for Neurologic Subspecialties (UCNS) or by the American Board
of Psychiatry and Neurology, Inc. (ABPN). Physicians who have not yet passed a certifying exam,
either through UCNS or ABPN, are considered to be equivalent to a physician “certified in
Neurocritical Care Medicine” for up to 3 years after they are eligible to take either: (1) the UCNS
exam (UCNS currently offers a “grandfathering” option for their “Practice Track” for exam
eligibility) or (2) the ABPN exam (ABPN currently offers a “grandfathering” or practice pathway

2023 Leapfrog Hospital Survey – Hard Copy Endnotes
314 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
track for exam eligibility, which will last until 2026). These options provide a 3-year grace period
for clinicians to take and pass the necessary exams. To qualify for the grace period, hospitals
and/or clinicians will need to provide clear documentation of what their eligibility dates were to sit
for one or both of these exams.
• Physicians who are board-certified in their primary specialty and who are additionally credentialed
by the American Board of Neurological Surgery (ABNS) through their Recognition of Focused
Practice in Neurocritical Care. Physicians who have not yet passed the ABNS Neurocritical Care
RFP exam are considered to be equivalent to a physician “certified in Neurocritical Care
Medicine” for up to 3 years after they are eligible to take the exam. This provides a 3-year grace
period for clinicians to take and pass the necessary exam. To qualify for the grace period,
hospitals and/or clinicians will need to provide clear documentation of what their eligibility dates
were to sit for the exam.
• For the 2023 and 2024 Survey Cycles, physicians who are board-certified in their primary
specialty and are additionally certified by the Committee on Advanced Subspecialty Training
(CAST) in Neurocritical Care will need to convert their CAST certificate to the ABNS RFP by
December 31, 2023 (https://sns-cast.org/rfp-and-cast-certificate-conversion/) or take the ABPN
Neurocritical Care exam by December 31, 2024 (https://www.abpn.com/wp-
content/uploads/2023/04/2023-and-2024-Exam-Schedule-4.13.23.pdf). Effective with the 2025
Survey Cycle, this path will be removed and Leapfrog will no longer recognize physicians who
hold a CAST certificate in Neurocritical Care as being a neurointensivist.
Expanded Definition of Certified in Critical Care Medicine
On an interim basis, three other categories of physicians are considered by Leapfrog to be equivalent to a
physician “certified in Critical Care Medicine” for the purpose of meeting the standard:
• Physicians who completed training prior to availability of subspecialty certification in critical care
in their specialty (1987 for Internal Medicine, Surgery, Anesthesiology, Pediatrics and 2013 for
Emergency Medicine), who are board-certified in their specialty, and who have provided at least
six weeks of full-time ICU care annually. (The weeks need not be consecutive weeks.)
• Physicians who have finished their fellowship in Critical Care Medicine, but have not yet passed
an existing board-certifying exam, are considered to be equivalent to a physician “Certified in
Critical Care Medicine” for up to three years after completion of the fellowship. This provides the
physician an adequate window to take her/his boards and re-take if necessary.
• Physicians who are board-certified in their primary specialty and have completed a critical care
fellowship at an ACGME-accredited program, but are ineligible to sit for a board-certifying exam
in Critical Care in either their primary specialty or subspecialty because their training occurred
under two separate certifying boards, are considered to be equivalent to a physician “Certified in
Critical Care Medicine” if they are board-certified in their primary specialty and have provided at
least six weeks of full-time ICU care annually. (The weeks need not be consecutive weeks.)
Physicians who have let their board certification lapse are not considered to be “Certified in Critical Care
Medicine.”
37
Ordinarily and Exclusively Present in the ICU
“Ordinarily present in the ICU” refers to direct on-site presence in the ICU of an intensivist during the 4-
hour or 8-hour period. While it need not be the same intensivist for the entire 4-hour or 8-hour period, it is
expected that the ICU(s) are primarily staffed by dedicated ICU intensivists who are ordinarily and
exclusively present in the ICU(s). "Presence" does not mean staffed part-time by multiple physicians who
are not ordinarily and exclusively dedicated to the ICU, nor does it mean the cumulative time that one or
more intensivists spend in the unit visiting, rounding, consulting, or responding to pages.
The standard allows for normally expected intensivist activities outside of the ICU related to their
responsibilities in the ICU (e.g., evaluating patients proposed for ICU admission), as long as intensivists
are ordinarily present in the ICU and return immediately when paged. An intensivist present in one ICU

2023 Leapfrog Hospital Survey – Hard Copy Endnotes
315 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
immediately adjacent to another can be considered present in both units as long as they can respond to
demands in both units as if they would if both units were one larger unit. For the purposes of this Survey,
“adjacent” units are those units that can be reached within 5 minutes. While tele-intensivists can be used
to meet the presence requirement, some on-site intensivist presence is still necessary to meet the
Leapfrog specifications.
“Exclusively” means that when the physician is in the ICU, they have no concurrent clinical
responsibilities to non-ICU patients.
38
Intensivist Presence via Telemedicine
To meet the Leapfrog ICU requirement for intensivist presence in the ICU via telemedicine, a hospital
must be able to document that it fulfills all the following 10 key features based on a modification of the
approach reported in Critical Care Medicine (Rosenfeld, B. et al. “Intensive care unit telemedicine:
Alternate paradigm for providing continuous intensivist care,” Critical Care Medicine, Vol. 28, No. 1, pp.
3925-3931).
1. A physician certified in critical care medicine (see endnote #36) who is physically present in the ICU
(“on-site intensivist”) performs a comprehensive review of each ICU patient each day and establishes
and/or revises the care plan. The on-site intensivist must be available by phone to answer any
questions from the tele-intensivist related to the established or revised care plan.
The tele-intensivist, who must also be a physician certified in critical care medicine (see endnote
#36), has immediate access to information regarding the on-site intensivist’s care plan at the time the
management responsibility is transferred to the tele-intensivist by the on-site intensivist.
When care is transferred back to the on-site intensivist, the tele-intensivist must communicate any
changes to the care plan to the on-site intensivist. Hospitals relying on electronic hand-offs should
ensure that physician sign-in and sign-out of reports is being recorded. In addition, these reports
should be monitored as one way to audit compliance with the hand-off process described above.
2. When an intensivist is not on-site in the ICU managing or co-managing all ICU patients, a tele-
intensivist is continuously monitoring and able to manage all ICU patients for the remaining 24 hours
per day, 7 days per week. “Continuously monitoring” means the tele-intensivist has no other
concurrent responsibilities, is immediately available to communicate with ICU staff, and is
continuously in the physical presence of the tele-ICU’s patient monitoring and communications
equipment. "Manage" means authorized to diagnose, treat, and write orders for a patient in the ICU
on the intensivist’s own authority.
3. The tele-intensivist’s care is proactive, with routine visualization and physiological review of all
patients at a frequency appropriate to their severity of illness.
4. The tele-intensivist has immediate access to key patient data, including:
a) physiologic bedside monitor data (in real-time);
b) laboratory orders and results;
c) medications ordered and administered; and,
d) notes, radiographs, ECGs, etc. on demand.
5. Bedside cameras are physically available in each ICU. If cameras are not mounted in each patient
room within the unit, then at least one dedicated bedside camera must be available for every five
beds in the ICU, plus a dedicated back-up camera in each ICU. If ICUs are located on different floors
of the hospital, each ICU on each floor must have its own set of dedicated cameras if they are not
mounted in every room. “Dedicated” means that the cameras are on the unit for the sole purpose of
patient care. Personal devices (e.g., personal phones, tablets, etc.) would not meet this requirement.

2023 Leapfrog Hospital Survey – Hard Copy Endnotes
316 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
6. The tele-intensivist must be able to turn on each bedside camera to visualize patients, with sufficient
clarity to assess breathing pattern and communicate with on-site personnel at the bedside in real
time.
7. Within five minutes of a request for assistance being initiated by hospital staff, the tele-intensivist’s
patient workload ordinarily permits the tele-intensivist to complete a comprehensive visual and
physiological assessment of any patient.
8. Data links between the ICU and the tele-intensivist are reliable (>98% up-time) and secure (HIPAA
compliant).
9. Written standards for remote care are established and include, at a minimum:
a) tele-intensivists are certified by a national medical specialty board in critical care medicine;
b) tele-intensivists are licensed to practice in the legal jurisdiction in which the ICU is located;
c) tele-intensivists are credentialed in each hospital to which he/she provides remote care (can be
special telemedicine credentialing);
d) activities of the tele-intensivist are reviewed within the hospital’s quality assurance committee
structure;
e) there are explicit written policies regarding roles and responsibilities of both the on-site
intensivist and the tele-intensivist;
f) ICU staff are educated regarding the function, roles, and responsibilities of the tele-intensivist;
and
g) there is an established written process to ensure effective communication between the ICU staff
and the tele-intensivist.
10. The tele-intensivist documents patient care activities and this documentation is incorporated into the
patient record.
39
Quantified Analysis of Response Times
Hospitals can monitor the response times of intensivists, “effectors,” and clinical pharmacists in multiple
ways, as long as the data collection process is non-biased and scientific.
As an example, hospitals can have the ICU staff maintain an exception log in the ICU(s) on six randomly
sampled days per year. On those days, ICU nurses could record:
• For question #7, the number of calls/pages/texts made to the intensivist when they were not
present in the unit (whether on-site or via telemedicine) and the number of times the intensivist’s
response time exceeded 5 minutes
• For question #8, the number of calls/pages/texts made to a physician, physician assistant, nurse
practitioner, or FCCS-certified nurse or intern “effector” and the number of times these individuals
did not reach the patient at the bedside within 5 minutes
• For question #13, the number of calls/pages/texts made to the clinical pharmacist on days when
they were not rounding on-site in the unit and the number of times the clinical pharmacist’s
response time exceeded 5 minutes
Hospitals can then estimate whether they meet the 95% timely response standards by dividing the total
number of log exceptions by the total number of calls/pages/texts per day.
Hospitals may exclude low-urgency calls/pages/texts, if the notification device system can designate low-
urgency calls/pages/texts, or if the hospital has an alternative scientific method for documenting high-
urgency calls/pages/texts that are not returned within 5 minutes.
Hospitals that use telemedicine to cover ‘call’ for the on-site intensivist should review endnote 41.

2023 Leapfrog Hospital Survey – Hard Copy Endnotes
317 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
Hospitals that have an effector that is dedicated 24/7 to the ICU (as defined as being within a 5 min walk
to the ICU) should review endnote 42.
40
FCCS-Certified Nurse or Intern “Effector”
FCCS certificates are awarded to nurses and doctors upon their successful completion of a brief course
developed by the Society for Critical Care Medicine to improve/confirm critical care knowledge and skills.
For more information visit
https://www.sccm.org/Education-Center/Educational-
Programming/Fundamentals/Fundamental-Critical-Care-Support. At present, this is the only such course
recommended by The Leapfrog Group’s expert advisory panel. Intensivists and any other physicians who
are certified in critical care medicine (or eligible based on residency training or fellowship) need not also
be FCCS certified. Physicians, physician assistants, and nurse practitioners also are not required to be
FCCS certified, but they must meet the criteria specified in endnote 42 to serve as the
responder/”effector.”
41
Use of Tele-intensivists to Cover Calls
Hospitals that use telemedicine to cover ‘call’ for the on-site intensivist are able to answer “yes” to
question #7 if: (1) the telemedicine service meets all ten of the requirements outlined in endnote #38; and
(2) the hospital has an ‘effector’ (physician/PA/NP/FCCS certified nurse or intern) on-site during that time
period to carry out the tele-intensivist’s orders and can reach the ICU patient within 5 minutes, 95% of the
time.
42
Physician/PA/NP serving as the Responder/“Effector”
Physicians/PAs/NPs (includes Clinical nurse Specialists, Nurse Anesthetists, or NPs) serving as the
responder in the ICU should meet the following criteria:
1. Be a graduate with a training license from an ACGME accredited training program or have an
active state license to practice as a physician, nurse practitioner, or physician assistant in the
state in which the patient is located.
2. Have privileges to provide medical services in the unit (i.e., ICU) and for patients of the age range
approved in advance by the hospital’s governing body (e.g., medical staff committee, chief
medical officer, chief nursing officer, etc.), as specified by the institutions internal policies
(bylaws).
3. Carry out the intensivist’s orders and instructions, under the intensivist’s guidance, when they are
serving in a responder role.
If the effector is dedicated 24/7 to the ICU (as defined as being within a 5 min walk to the ICU), then
hospitals can indicate “yes” to meeting the response time requirement (5 min response; 95% of the time)
in question #8, in lieu of conducting a response time audit.
43
Modified Intensivist Presence via Telemedicine
To earn reduced credit on the Leapfrog ICU standard for intensivist presence in the ICU via
telemonitoring, a hospital must affirm that its telemonitoring intensivist presence fulfills the following nine
key features based on a modification of the approach reported in Critical Care Medicine (Rosenfeld, B. et
al. “Intensive care unit telemedicine: Alternate paradigm for providing continuous intensivist care,” Critical
Care Medicine, Vol. 28, No. 1, pp. 3925-3931). Note that, as with other Leapfrog specifications, these
features must be met under ordinary circumstances.
1. When an intensivist is not on-site in the ICU managing or co-managing all ICU patients, a tele-
intensivist is continuously monitoring and able to manage all ICU patients for the remaining 24 hours
per day, 7 days per week. “Continuously monitoring” means the tele-intensivist has no other
concurrent responsibilities, is immediately available to communicate with ICU staff, and is
continuously in the physical presence of the tele-ICU’s patient monitoring and communications
equipment. "Manage" means authorized to diagnose, treat, and write orders for a patient in the ICU

2023 Leapfrog Hospital Survey – Hard Copy Endnotes
318 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
on the intensivist’s own authority.
2. The tele-intensivist’s care is proactive, with routine visualization and physiological review of all
patients at a frequency appropriate to their severity of illness.
3. The tele-intensivist has immediate access to key patient data, including:
a) physiologic bedside monitor data (in real-time);
b) laboratory orders and results;
c) medications ordered and administered; and,
d) notes, radiographs, ECGs, etc. on demand.
4. Bedside cameras are physically available in each ICU. If cameras are not mounted in each patient
room within the unit, then at least one dedicated bedside camera must be available for every five
beds in the ICU, plus a dedicated back-up camera in each ICU. If ICUs are located on different floors
of the hospital, each ICU on each floor must have its own set of dedicated cameras if they are not
mounted in every room. “Dedicated” means that the cameras are on the unit for the sole purpose of
patient care. Personal devices (e.g., personal phones, tablets, etc.) would not meet this requirement.
5. The tele-intensivist must be able to turn on each bedside camera to visualize patients, with sufficient
clarity to assess breathing pattern and communicate with on-site personnel at the bedside in real
time.
6. Within five minutes of a request for assistance being initiated by hospital staff, the tele-intensivist’s
patient workload ordinarily permits the tele-intensivist to complete a comprehensive visual and
physiological assessment of any patient.
7. Data links between the ICU and the tele-intensivist are reliable (>98% up-time) and secure (HIPAA
compliant).
8. Written standards for remote care are established and include, at a minimum:
a) tele-intensivists are certified by a national medical specialty board in critical care medicine;
b) tele-intensivists are licensed to practice in the legal jurisdiction in which the ICU is located;
c) tele-intensivists are credentialed in each hospital to which he/she provides remote care (can be
special telemedicine credentialing);
d) activities of the tele-intensivist are reviewed within the hospital’s quality assurance committee
structure;
e) there are explicit written policies regarding roles and responsibilities of both the on-site
intensivist and the tele-intensivist;
f) ICU staff are educated regarding the function, roles, and responsibilities of the tele-intensivist;
and
g) there is an established written process to ensure effective communication between the ICU staff
and the tele-intensivist.
9. The tele-intensivist documents patient care activities and this documentation is incorporated into the
patient record.
44
Individuals who touch patients or who touch items that will be used by patients
This would include individuals who are formally engaged by the hospital to help support the patient care
process. This would include both direct and indirect care providers that are likely to have contact with
patients in a patient care unit, enter a patient care unit, touch items that will be used by patients in a
patient care unit, or interact with patient fluids (e.g., blood, specimens) in a patient care unit, such as
doctors, mid-levels, nurses, pharmacists, environmental services staff, phlebotomists, laboratory techs,
etc. This would also include students and volunteers. These individuals should be trained to identify and

2023 Leapfrog Hospital Survey – Hard Copy Endnotes
319 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
perform proper hand hygiene for the specific indications/moments (see WHO's 5 Moments for Hand
Hygiene, CDC’s Guideline for Hand Hygiene) that are relevant to their work.
Administrative workers that only perform office duties and do not touch patients or touch items that will be
used by patients in a patient care unit would not be included in this definition. Patients and their visitors
would also not be included in this definition. While patients and their loved ones are important parts of the
patient care process, they are not formally engaged by the hospital for this work. Vendors would also not
be included.
45
Professional with Appropriate Training and Skills
This would include staff formally trained in Infection Control or Infectious Diseases, whose tasks include
dedicated time for staff training. In some settings, this could also be medical or nursing staff involved in
clinical work, with dedicated time to acquire thorough knowledge of the evidence for and correct practice
of hand hygiene.
The minimum required knowledge of the trainer can be found in the WHO Guidelines on Hand Hygiene in
Health Care and the Hand Hygiene Technical Reference Manual.
46
Never Event
In 2011, the National Quality Forum released a list of 29 events that they termed “serious reportable
events,” extremely rare medical errors that should never happen to a patient. Often termed “never
events,” these include errors such as surgery performed on the wrong body part or on the wrong patient,
leaving a foreign object inside a patient after surgery, or discharging an infant to the wrong person. This is
an update of NQF’s original 2002 and 2006 reports. Please see NQF’s “Never Events” list at
https://www.qualityforum.org/topics/sres/serious_reportable_events.aspx
. Hospitals may not earn credit
for this question if they have only implemented a policy that includes the Center for Medicare and
Medicaid (CMS) Never Events.
47
Apology to the Patient
While Leapfrog recognizes that on very rare occasions “never events” can occur that are not the fault of
care systems or clinical care staff, given the high level of trust patients place in health care providers,
Leapfrog feels it is appropriate for caregivers to apologize when a patient within their care setting suffers
a serious event.
As the National Quality Forum identified in their 2002, 2006, and 2011 Serious Reportable Events Report,
given the serious nature of these events, it is reasonable for hospitals to initially assume that the adverse
event was due to the referenced course of care. And while further investigation and/or root cause analysis
of the unplanned event may be needed to confirm or refute the presumed relationship, delaying an
apology to the patient is not treating the patient with compassion and sympathy.
48
Reporting Never Events to External Agencies
If your hospital is not accredited by The Joint Commission or DNV GL Healthcare, is located in a state
without a state-wide reporting program for medical errors, AND there is no available Patient Safety
Organization to which your hospital can report medical errors, the hospital should report the event to the
Board of Trustees. Full implementation of the Never Events policy still requires the hospital to conduct
a root cause analysis of the event.
49
Root Cause Analysis
The National Patient Safety Foundation published a set of best practices and guidelines in its report
“RCA
2
Improving Root Cause Analysis and Action to Prevent Harm.” The report can be found at
http://www.ihi.org/resources/Pages/Tools/RCA2-Improving-Root-Cause-Analyses-and-Actions-to-Prevent-
Harm.aspx.

2023 Leapfrog Hospital Survey – Hard Copy Endnotes
320 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
50
Top Box Score
The percent of survey respondents who chose the most positive score for a given item. Looking at
the top box is an approach to understand the number of responses with a strong sentiment.
For the CAHPS Child Hospital Survey “Global Rating – Recommend hospital” domain, responses of 9
and 10 are included in the top box score. For the “Global Rating – Recommend hospital” domain,
responses of “Definitely yes” are included in the top box score. For all other domains included in Section
8A, the top box score is the percent of survey respondents choosing “Always.”
For the OAS CAHPS Survey “Patients’ Rating of the Facility” domain, responses of 9 or 10 are included in
the top box score. For the “Patients Recommending the Facility” domain, responses of “Definitely yes” are
included in the top box score. For all other domains included in Section 9E, the top box score is the
percent of survey respondents choosing “Yes, definitely.”
51
Operating Rooms
If your state designates and licenses operating rooms, enter the number of operating rooms licensed by
your state that are used to perform the outpatient procedures listed in Section 9C. If your state does not
designate and license operating rooms, enter the number of operating rooms used to perform the
outpatient procedures listed in Section 9C that meet the following definition from the 2018 FGI Guidelines:
a room that meets the requirements of a restricted area, is designated and equipped for performing
surgical or other invasive procedures, and has the environmental controls for an OR as indicated in
ASHRAE 170. An aseptic field is required for all procedures performed in an OR.
More information about the 2018 FGI Guidelines can be found at https://www.fgiguidelines.org/wp-
content/uploads/2017/08/SLS17_FGI_ExamProcedureOperatingImaging_170721.pdf.
Hospitals should include the total number of operating rooms that are used to perform the outpatient
procedures listed in Section 9C, including operating rooms that are used for both inpatient and outpatient
procedures.
52
Endoscopic Procedure Rooms
If your state designates and licenses procedure rooms, enter the number of procedure rooms licensed by
your state that are used for endoscopies listed in Section 9C. If your state does not designate and license
procedure rooms, enter the number of procedure rooms that are used for endoscopies listed in Section
9C that meet the following definition from the 2018 FGI Guidelines: a room designated for the
performance of patient care that requires high-level disinfection or sterile instruments and some
environmental controls but is not required to be performed with the environmental controls of an operating
room.
More information about the 2018 FGI Guidelines can be found at https://www.fgiguidelines.org/wp-
content/uploads/2017/08/SLS17_FGI_ExamProcedureOperatingImaging_170721.pdf.
Hospitals should include the total number of procedure rooms that are used to perform the endoscopies
listed in Section 9C, including procedure rooms that are used for both inpatient and outpatient
procedures.
53
Clinician
A clinician refers to a physician, physician assistant (PA), nurse practitioner (NP), certified registered
nurse anesthetist (CRNA), nurse (RN or MSN), or respiratory therapist.
54
Eligible Discharges
Discharged adult patients (ages 18 years and older) who had both medically and non-medically
necessary outpatient surgeries and/or procedures are eligible to complete the OAS CAHPS Survey. A
detailed description of patient sampling criteria, including a list of OAS CAHPS-eligible surgeries and

2023 Leapfrog Hospital Survey – Hard Copy Endnotes
321 Version 9.0 First Release: April 1, 2023
© 2023 The Leapfrog Group Updated Release: July 21, 2023
Table of Contents
procedures is available in the Protocols and Guidelines Manual, version 7.0 at
https://oascahps.org/Survey-Materials
.
The 2023 Leapfrog Hospital Survey is a publication of The Leapfrog Group. The Survey cannot be
copied, distributed, or used without the written permission of The Leapfrog Group for any purpose other
than an individual hospital’s participation in the Survey.











