
@
Technical Reports SeriEs No. 484
The Environmental
Behaviour of Polonium
F. Carvalho, S. Fernandes, S. Fesenko, E. Holm, B. Howard,
P. Martin, M. Phaneuf, D. Porcelli, G. Pröhl, J. Twining
The Environmental Behaviour of Poloniumtechnical reportS series no. 484
THE ENVIRONMENTAL
BEHAVIOUR OF POLONIUM

AFGHANISTAN
ALBANIA
ALGERIA
ANGOLA
ANTIGUA AND BARBUDA
ARGENTINA
ARMENIA
AUSTRALIA
AUSTRIA
AZERBAIJAN
BAHAMAS
BAHRAIN
BANGLADESH
BARBADOS
BELARUS
BELGIUM
BELIZE
BENIN
BOLIVIA, PLURINATIONAL
STATE OF
BOSNIA AND HERZEGOVINA
BOTSWANA
BRAZIL
BRUNEI DARUSSALAM
BULGARIA
BURKINA FASO
BURUNDI
CAMBODIA
CAMEROON
CANADA
CENTRAL AFRICAN
REPUBLIC
CHAD
CHILE
CHINA
COLOMBIA
CONGO
COSTA RICA
CÔTE D’IVOIRE
CROATIA
CUBA
CYPRUS
CZECH REPUBLIC
DEMOCRATIC REPUBLIC
OF THE CONGO
DENMARK
DJIBOUTI
DOMINICA
DOMINICAN REPUBLIC
ECUADOR
EGYPT
EL SALVADOR
ERITREA
ESTONIA
ETHIOPIA
FIJI
FINLAND
FRANCE
GABON
GEORGIA
GERMANY
GHANA
GREECE
GUATEMALA
GUYANA
HAITI
HOLY SEE
HONDURAS
HUNGARY
ICELAND
INDIA
INDONESIA
IRAN, ISLAMIC REPUBLIC OF
IRAQ
IRELAND
ISRAEL
ITALY
JAMAICA
JAPAN
JORDAN
KAZAKHSTAN
KENYA
KOREA, REPUBLIC OF
KUWAIT
KYRGYZSTAN
LAO PEOPLE’S DEMOCRATIC
REPUBLIC
LATVIA
LEBANON
LESOTHO
LIBERIA
LIBYA
LIECHTENSTEIN
LITHUANIA
LUXEMBOURG
MADAGASCAR
MALAWI
MALAYSIA
MALI
MALTA
MARSHALL ISLANDS
MAURITANIA
MAURITIUS
MEXICO
MONACO
MONGOLIA
MONTENEGRO
MOROCCO
MOZAMBIQUE
MYANMAR
NAMIBIA
NEPAL
NETHERLANDS
NEW ZEALAND
NICARAGUA
NIGER
NIGERIA
NORWAY
OMAN
PAKISTAN
PALAU
PANAMA
PAPUA NEW GUINEA
PARAGUAY
PERU
PHILIPPINES
POLAND
PORTUGAL
QATAR
REPUBLIC OF MOLDOVA
ROMANIA
RUSSIAN FEDERATION
RWANDA
SAN MARINO
SAUDI ARABIA
SENEGAL
SERBIA
SEYCHELLES
SIERRA LEONE
SINGAPORE
SLOVAKIA
SLOVENIA
SOUTH AFRICA
SPAIN
SRI LANKA
SUDAN
SWAZILAND
SWEDEN
SWITZERLAND
SYRIAN ARAB REPUBLIC
TAJIKISTAN
THAILAND
THE FORMER YUGOSLAV
REPUBLIC OF MACEDONIA
TOGO
TRINIDAD AND TOBAGO
TUNISIA
TURKEY
TURKMENISTAN
UGANDA
UKRAINE
UNITED ARAB EMIRATES
UNITED KINGDOM OF
GREAT BRITAIN AND
NORTHERN IRELAND
UNITED REPUBLIC
OF TANZANIA
UNITED STATES OF AMERICA
URUGUAY
UZBEKISTAN
VANUATU
VENEZUELA, BOLIVARIAN
REPUBLIC OF
VIET NAM
YEMEN
ZAMBIA
ZIMBABWE
The following States are Members of the International Atomic Energy Agency:
The Agency’s Statute was approved on 23 October 1956 by the Conference on the Statute of the
IAEA held at United Nations Headquarters, New York; it entered into force on 29 July 1957.
The Headquarters of the Agency are situated in Vienna. Its principal objective is “to accelerate and enlarge
the contribution of atomic energy to peace, health and prosperity throughout the world’’.
TECHNICAL REPORTS SERIES No. 484
THE ENVIRONMENTAL
BEHAVIOUR OF POLONIUM
F. CARVALHO, S. FERNANDES, S. FESENKO,
E. HOLM, B. HOWARD, P. MARTIN, M. PHANEUF,
D. PORCELLI, G. PRÖHL, J. TWINING
INTERNATIONAL ATOMIC ENERGY AGENCY
VIENNA, 2017
COPYRIGHT NOTICE
All IAEA scientific and technical publications are protected by the terms of
the Universal Copyright Convention as adopted in 1952 (Berne) and as revised
in 1972 (Paris). The copyright has since been extended by the World Intellectual
Property Organization (Geneva) to include electronic and virtual intellectual
property. Permission to use whole or parts of texts contained in IAEA publications
in printed or electronic form must be obtained and is usually subject to royalty
agreements. Proposals for non-commercial reproductions and translations are
welcomed and considered on a case-by-case basis. Enquiries should be addressed
to the IAEA Publishing Section at:
Marketing and Sales Unit, Publishing Section
International Atomic Energy Agency
Vienna International Centre
PO Box 100
1400 Vienna, Austria
fax: +43 1 2600 29302
tel.: +43 1 2600 22417
email: [email protected]
http://www.iaea.org/books
© IAEA, 2017
Printed by the IAEA in Austria
November 2017
STI/DOC/010/484
IAEA Library Cataloguing in Publication Data
Names: International Atomic Energy Agency.
Title: The environmental behaviour of polonium / International Atomic Energy
Agency.
Description: Vienna : International Atomic Energy Agency, 2017. | Series: Technical
reports series (International Atomic Energy Agency), ISSN 0074–1914 ; no. 484 |
Includes bibliographical references.
Identifiers: IAEAL 17-01115 | ISBN 978–92–0–112116–5 (paperback : alk. paper)
Subjects: LCSH: Radioecology. | Radioactive pollution. | Polonium.
Classification: UDC 539.163 | STI/DOC/010/484
FOREWORD
Polonium-210 is the main contributor to internal doses due to ingestion
of radionuclides from the uranium and thorium decay series. With the expected
increase in uranium mining and other industries generating naturally occurring
radioactive material residues in the future, it is likely that the radiological impact
of
210
Po will increase in importance.
The IAEA attaches great importance to the dissemination of information
that can assist Member States with the implementation and improvement of
activities relating to radiation safety, including the management of radioactive
residues containing natural radionuclides. This publication outlines the behaviour
of polonium in air, water and soil, and in the human body. The primary objective
is to provide information that can be used in radiological assessments of
accidental releases and routine discharges of polonium to the environment. Case
studies and environmental applications of polonium isotopes are also presented.
The IAEA wishes to express its appreciation of the work of all the
contributors to the drafting and review of this publication. The IAEA officers
responsible for this publication were S. Fesenko and M. Phaneuf of the
IAEA Environment Laboratories.
EDITORIAL NOTE
Although great care has been taken to maintain the accuracy of information contained
in this publication, neither the IAEA nor its Member States assume any responsibility for
consequences which may arise from its use.
This publication does not address questions of responsibility, legal or otherwise, for acts
or omissions on the part of any person.
Guidance provided here, describing good practices, represents expert opinion but does
not constitute recommendations made on the basis of a consensus of Member States.
The use of particular designations of countries or territories does not imply any
judgement by the publisher, the IAEA, as to the legal status of such countries or territories, of
their authorities and institutions or of the delimitation of their boundaries.
The mention of names of specic companies or products (whether or not indicated as
registered) does not imply any intention to infringe proprietary rights, nor should it be construed
as an endorsement or recommendation on the part of the IAEA.
The IAEA has no responsibility for the persistence or accuracy of URLs for external or
third party Internet web sites referred to in this book and does not guarantee that any content
on such web sites is, or will remain, accurate or appropriate.
CONTENTS
CHAPTER 1. INTRODUCTION .............................. 1
1.1. Background ......................................... 1
1.2. Objective ........................................... 2
1.3. Scope .............................................. 2
1.4. Structure............................................ 3
References to Chapter 1 .................................... 3
CHAPTER 2. RADIOECOLOGY: BASIC DEFINITIONS AND
CONCEPTS ................................... 5
2.1. Basic parameters ..................................... 5
2.2. Classifications of plants and animals used for exposure
assessment models.................................... 7
2.2.1. Transfer to human foodstuffs ...................... 7
2.2.2. Transfer to wildlife: Reference organism concept ...... 9
References to Chapter 2 .................................... 13
CHAPTER 3. PROPERTIES OF POLONIUM.................... 15
3.1. Physical properties.................................... 15
3.1.1. Isotopes....................................... 15
3.1.2. Decay series systematics.......................... 15
3.2. Chemical properties................................... 22
3.2.1. Solution chemistry .............................. 23
3.2.2. Adsorption .................................... 25
3.2.3. Biovolatilization ................................ 27
3.3.
210
Po determination ................................... 27
References to Chapter 3 .................................... 31
CHAPTER 4. OCCURRENCE AND CYCLING OF
210
Pb AND
210
Po
IN THE ENVIRONMENT
........................ 35
4.1. Occurrence of
210
Pb and
210
Po in rock and soil . . . . . . . . . . . . . . 35
4.2. Occurrence of
210
Pb and
210
Po in the atmosphere ............ 38
4.3. Occurrence of
210
Pb and
210
Po in terrestrial ecosystems ....... 40
4.3.1.
210
Pb and
210
Po in plants .......................... 40
4.3.2.
210
Pb and
210
Po in animals......................... 42
4.4. Occurrence of
210
Pb and
210
Po in marine ecosystems ......... 43
4.4.1.
210
Pb and
210
Po in marine water .................... 43
4.4.2.
210
Pb and
210
Po in marine organisms................. 44
4.5. Occurrence of
210
Pb and
210
Po in freshwater ecosystems ...... 44
4.5.1.
210
Pb and
210
Po in groundwater..................... 44
4.5.2.
210
Pb and
210
Po in surface water and sediments ........ 45
4.5.3.
210
Pb and
210
Po in freshwater organisms.............. 46
4.6. Occurrence of
210
Pb and
210
Po in food (including seafood)
and drinking water
.................................... 46
4.7. Anthropogenic sources of
210
Po in the environment .......... 51
4.7.1. Industrial uses of human-made
210
Po ................ 51
4.7.2. Environmental release of naturally occurring
radioactive material.............................. 52
4.8. Summary ........................................... 54
References to Chapter 4 .................................... 55
CHAPTER 5.
210
Pb AND
210
Po IN ATMOSPHERIC SYSTEMS...... 61
5.1. Introduction to the atmospheric environment ............... 61
5.2. Sources of
210
Pb and
210
Po in the atmosphere ............... 63
5.2.1. Radon exhalation ............................... 63
5.2.2. Volcanic activity ................................ 65
5.2.3. Resuspended soil dust............................ 65
5.2.4. Biomass burning ................................ 66
5.2.5. Wind blown sea spray and emission of volatile
polonium compounds from water surfaces............ 66
5.2.6. Anthropogenic sources ........................... 66
5.3. Processes for the removal of
210
Pb and
210
Po from the
atmosphere
.......................................... 67
5.3.1. Wet deposition ................................. 67
5.3.2. Dry deposition ................................. 69
5.3.3. Deposition fluxes ............................... 69
5.4. Distribution of
210
Pb and
210
Po in the atmosphere............ 70
5.4.1. Concentrations at the land surface .................. 70
5.4.2. Vertical profiles................................. 72
References to Chapter 5 .................................... 73
CHAPTER 6.
210
Pb AND
210
Po IN TERRESTRIAL SYSTEMS ...... 79
6.1. Description of the terrestrial environment.................. 79
6.2. Major transfer processes and pathways.................... 80
6.3. Atmosphere–plant interactions .......................... 81
6.3.1. Interception.................................... 82
6.3.2. Physical and biological weathering ................. 83
6.3.3. Foliar translocation .............................. 83
6.4. Mobility of polonium in soils ........................... 84
6.4.1. Resuspension .................................. 84
6.4.2. Radon exhalation ............................... 85
6.4.3. Partitioning of the soil Po:Pb ratio into solution ....... 85
6.5.
210
Pb and
210
Po transfer from soil to plants ................. 87
6.5.1. Processes governing
210
Pb and
210
Po transfer to
plants
......................................... 87
6.5.2.
210
Pb and
210
Po transfer to mosses, plants and
lichens
........................................ 87
6.5.3.
210
Pb and
210
Po transfer to berries, mushrooms and
understory species
............................... 88
6.5.4.
210
Pb and
210
Po transfer to agricultural plants.......... 93
6.6
210
Pb and
210
Po transfer to animals ....................... 99
6.6.1. Processes governing distribution of
210
Pb and
210
Po
in animals
..................................... 99
6.6.2. Quantification of
210
Pb and
210
Po transfers to
agricultural animals
.............................. 101
6.6.3. Transfer to terrestrial animals...................... 103
References to Chapter 6 .................................... 106
CHAPTER 7.
210
Pb AND
210
Po IN FRESHWATER AND
GROUNDWATER SYSTEMS
..................... 111
7.1. Description of the hydrological cycle ..................... 111
7.2. Groundwater ........................................ 112
7.2.1. Groundwater environment ........................ 112
7.2.2. Sources of
210
Pb and
210
Po in groundwater............ 113
7.2.3. Groundwater concentrations....................... 115
7.2.4. Particles and colloids in groundwater................ 115
7.2.5. In situ adsorption coefficients...................... 117
7.3. Surface water........................................ 121
7.3.1. Sources in surface fresh water ..................... 122
7.3.2. Levels of
210
Po in the freshwater environment ......... 123
7.3.3. Behaviour of
210
Po in standing water bodies .......... 123
7.3.4. Quantification of water and sediment quality.......... 126
7.3.5. Freshwater aquatic biota .......................... 127
References to Chapter 7 .................................... 132
CHAPTER 8.
210
Pb AND
210
Po IN MARINE SYSTEMS............ 137
8.1. Introduction to the marine environment ................... 137
8.2. Polonium sources in the marine environment ............... 137
8.3. Polonium in the oceans ................................ 138
8.4. Accumulation and turnover of
210
Pb and
210
Po
in marine organisms
................................... 142
8.5. Polonium in marine biota living in several ocean regions...... 147
8.5.1. Intertidal zone .................................. 147
8.5.2. Coastal seas.................................... 153
8.5.3. Epipelagic zone................................. 157
8.5.4. Mesopelagic and bathypelagic zones ................ 160
8.5.5. Abyssal zone................................... 161
8.6. Polonium and lead concentration ratios in marine
organisms
........................................... 163
8.7. Transfer of
210
Pb and
210
Po in marine food chains............ 163
References to Chapter 8 .................................... 167
CHAPTER 9. POLONIUM IN HUMANS ....................... 175
9.1. Introduction ......................................... 175
9.2. Ingestion and inhalation................................ 177
9.3. Distribution in tissues ................................. 177
9.4. Retention and biological half-lives ....................... 181
References to Chapter 9 .................................... 184
CHAPTER 10. RADIOLOGICAL DOSE ASSESSMENT FOR
210
Po
TO HUMANS AND OTHER BIOTA
............... 187
10.1. Models and data for estimating internal exposures to
humans
............................................. 187
10.2. Dose conversion factors for wildlife ...................... 188
10.2.1. General assumptions ............................ 188
10.2.2. Dose conversion factors for flora and fauna .......... 193
10.2.3. Non-homogeneous distribution .................... 200
10.2.4. Application of dose conversion coefficients .......... 201
References to Chapter 10 ................................... 202
APPENDIX I: GEOCHRONOLOGICAL APPLICATIONS OF
POLONIUM ................................... 205
APPENDIX II: RADIOCAESIUM,
210
Po AND
210
Pb IN WOLVES .... 209
APPENDIX III:
210
Po IN MARINE MAMMALS ................... 215
APPENDIX IV: POLONIUM RELEASES FROM VEGETATION
AND FOREST FIRES
........................... 227
APPENDIX V: POLONIUM IN TOBACCO AND DOSES TO
HUMANS..................................... 237
APPENDIX VI: ESTIMATION OF AEROSOL RESIDENCE TIMES
IN THE ATMOSPHERE
......................... 241
APPENDIX VII: USE OF
210
Po AS A RADIOTRACER IN
BIOGENIC PARTICULATE FLUX STUDIES
IN THE OCEANS
.............................. 247
ABBREVIATIONS ............................................ 253
CONTRIBUTORS TO DRAFTING AND REVIEW .................. 255
1
Chapter 1
INTRODUCTION
1.1. BACKGROUND
For many years, the IAEA has supported efforts to develop publications on
the environmental behaviour and use of radionuclides in response to the needs
of its Member States. Since
210
Po is the main contributor to internal doses due to
ingestion of radionuclides from the uranium and thorium decay series
[1.1, 1.2],
it was determined that a resource publication for polonium would be beneficial.
Polonium-210 is an alpha emitting radionuclide with no radioactive progeny and
produces only very-low-intensity gamma rays at very low abundance; thus, the
dose largely arises from internal exposure. The main reasons for its radiological
importance are its relatively high activity concentrations in certain foods and its
relatively high ingestion dose coefficient.
There are seven polonium isotopes naturally present in the environment:
210
Po,
214
Po and
218
Po of the uranium decay series;
212
Po and
216
Po of the thorium
decay series; and
211
Po and
215
Po of the actinium decay series. Polonium-210 has
a half-life of approximately 138
days, which is long enough to play a significant
role in many environmental processes. All of the other naturally occurring
isotopes have half-lives of only 3
minutes or less. The isotopes
214
Po and
218
Po
are short lived progeny of
222
Rn, and are important in dose assessment. However,
they are best examined in relation to the environmental behaviour of radon and
other short lived radon progeny. Hence, the primary focus of this publication is
the environmental behaviour of
210
Po.
Radiation doses from
210
Po arise owing to natural occurrences of the
radionuclide as well as to human activities. As
210
Po is part of the uranium decay
series, it is naturally occurring and is found in varying amounts worldwide. The
anthropogenic sources include uranium mining and milling activities, as well
as the production of naturally occurring radioactive material (NORM), such as
phosphogypsum, and oil and gas scales. With uranium mining and other industries
generating NORM residues expected to grow in the future, it is likely that the
radiological impact of
210
Po will be of increasing importance. The challenges
faced have been addressed in a number of IAEA publications on radionuclide
transfer in the environment, which focus on radionuclide transfers in terrestrial,
freshwater and marine environments, and provide information on key transfer
processes, concepts and models important in radiological assessments for all
radionuclides (see Refs [1.3–1.8]).
2
CHAPTER 1
Since 2004, the IAEA has also organized a series of projects aimed at
improving environmental assessment and remediation. Through these projects,
the environmental behaviour of radionuclides has been presented in other IAEA
publications, which cover the following topics:
— Contaminated site characterization [1.9–1.11];
— Waste discharge, surveillance and management [1.12–1.14];
— Environmental remediation [1.15, 1.16];
— Remediation of uranium mill tailings [1.17];
— Remediation of dispersed contamination [1.18];
— Radiation protection and the management of radioactive waste in the oil
and gas industry
[1.19].
1.2. OBJECTIVE
This publication provides information on the environmental behaviour
of polonium, which can be used in the radiological assessment of polonium
occurring naturally in the environment or present due to routine discharges and
accidental releases. This publication can support environmental investigations,
assessments, and remediation in areas contaminated by polonium, and presents
concepts, models and parameters describing the behaviour of polonium.
1.3. SCOPE
This publication explores the behaviour of polonium in atmospheric,
terrestrial, freshwater and marine environments. The primary focus is the
environmental behaviour of
210
Po, the only polonium isotope with a half-life long
enough to play a significant role in environmental processes but, at 138
days,
also short enough that ingrowth from its immediate progenitors
210
Bi and
210
Pb
can be important when studying polonium’s behaviour and for inferring kinetics
and mechanisms of the processes. Information on the progenitors of
210
Po — in
particular
210
Pb — is also presented to capture
210
Po behaviour in the environment.
In addition to presenting information on the environmental transfer of
radionuclides to humans and non-human biota, this publication provides further
information on the assessment of the impact of radioactive discharges (e.g. see
Refs [1.12, 1.20]. Applications of
210
Po as a tracer of environmental processes
are discussed, but industrial, military and other applications of
210
Po, such as
occupational dose assessment, are outside the scope of this publication.
3
INTRODUCTION
1.4. STRUCTURE
Chapter 2 introduces basic definitions and concepts for radioecology.
The physical, chemical and decay properties of polonium are provided in
Chapter
3, and Chapter 4 addresses sources and cycling of
210
Pb and
210
Po in
the environment. Chapters
5–8 explore the behaviour of
210
Pb and
210
Po in
the atmospheric, terrestrial, freshwater (including groundwater) and marine
environments, respectively. Chapter
9 describes the metabolism of polonium
in the human body. Radiological dose assessment models for humans and
wildlife relating to the occurrence of polonium in the environment are given in
Chapter
10. Appendices I–VII consider various case studies and environmental
applications of polonium.
REFERENCES TO CHAPTER 1
[1.1] UNITED NATIONS SCIENTIFIC COMMITTEE ON THE EFFECTS OF ATOMIC
RADIATION, Sources and Effects of Ionizing Radiation, UNSCEAR 2000 Report to
the General Assembly, with Scientific Annexes, Vol. I: Sources, United Nations,
New York (2000) Annex B.
[1.2] UNITED NATIONS SCIENTIFIC COMMITTEE ON THE EFFECTS OF ATOMIC
RADIATION, Sources and Effects of Ionizing Radiation, UNSCEAR 2008 Report to
the General Assembly, with Scientific Annexes, Vol. II: Effects, United Nations,
New York (2008) Annex E.
[1.3] INTERNATIONAL ATOMIC ENERGY AGENCY, The Environmental Behaviour of
Radium: Revised Edition, Technical Reports Series No.
476, IAEA, Vienna (2014).
[1.4] INTERNATIONAL ATOMIC ENERGY AGENCY, Measurement and Calculation of
Radon Releases from NORM Residues, Technical Reports Series No.
474, IAEA,
Vienna
(2013).
[1.5] INTERNATIONAL ATOMIC ENERGY AGENCY, Sediment Distribution Coefficients
and Concentration Factors for Biota in the Marine Environment, Technical Reports
Series No.
422, IAEA, Vienna (2004).
[1.6] INTERNATIONAL ATOMIC ENERGY AGENCY, Quantification of Radionuclide
Transfer in Terrestrial and Freshwater Environments for Radiological Assessments,
IAEA-TECDOC-1616, IAEA, Vienna
(2009).
[1.7] INTERNATIONAL ATOMIC ENERGY AGENCY, Handbook of Parameter Values
for the Prediction of Radionuclide Transfer in Terrestrial and Freshwater Environments,
Technical Reports Series No.
472, IAEA, Vienna (2010).
[1.8] INTERNATIONAL ATOMIC ENERGY AGENCY, Handbook of Parameter Values
for the Prediction of Radionuclide Transfer to Wildlife, Technical Reports Series
No.
479, IAEA, Vienna (2014).
4
CHAPTER 1
[1.9] INTERNATIONAL ATOMIC ENERGY AGENCY, Characterization of Radioactively
Contaminated Sites for Remediation Purposes, IAEA-TECDOC-1017, IAEA,
Vienna
(1998).
[1.10] INTERNATIONAL ATOMIC ENERGY AGENCY, Site Characterization Techniques
Used in Environmental Restoration Activities, IAEA-TECDOC-1148, IAEA,
Vienna
(2000).
[1.11] INTERNATIONAL ATOMIC ENERGY AGENCY, Strategy and Methodology for
Radioactive Waste Characterization, IAEA-TECDOC-1537, IAEA, Vienna (2007).
[1.12] INTERNATIONAL ATOMIC ENERGY AGENCY, Regulatory Control of Radioactive
Discharges to the Environment, IAEA Safety Standards Series No.
WS-G-2.3, IAEA,
Vienna
(2000).
[1.13] INTERNATIONAL ATOMIC ENERGY AGENCY, Management of Radioactive
Waste from the Mining and Milling of Ores, IAEA Safety Standards Series
No.
WS-G-1.2, IAEA, Vienna (2002).
[1.14] INTERNATIONAL ATOMIC ENERGY AGENCY, Monitoring and Surveillance of
Residues from the Mining and Milling of Uranium and Thorium, Safety Reports Series
No.
27, IAEA, Vienna (2002).
[1.15] INTERNATIONAL ATOMIC ENERGY AGENCY, Factors for Formulating Strategies
for Environmental Restoration, IAEA-TECDOC-1032, IAEA, Vienna
(1998).
[1.16] INTERNATIONAL ATOMIC ENERGY AGENCY, Technologies for Remediation of
Radioactively Contaminated Sites, IAEA-TECDOC-1086, IAEA, Vienna
(1999).
[1.17] INTERNATIONAL ATOMIC ENERGY AGENCY, The Long Term Stabilization of
Uranium Mill Tailings, IAEA-TECDOC-1403, IAEA, Vienna
(2004).
[1.18] INTERNATIONAL ATOMIC ENERGY AGENCY, Remediation of Sites with
Dispersed Radioactive Contamination, Technical Reports Series No.
424, IAEA,
Vienna
(2004).
[1.19] INTERNATIONAL ATOMIC ENERGY AGENCY, Radiation Protection and the
Management of Radioactive Waste in the Oil and Gas Industry, Safety Reports Series
No.
34, IAEA, Vienna (2003).
[1.20] INTERNATIONAL ATOMIC ENERGY AGENCY, Generic Models for Use in
Assessing the Impact of Discharges of Radioactive Substances to the Environment,
Safety Reports Series No.
19, IAEA, Vienna (2001).

5
Chapter 2
RADIOECOLOGY: BASIC DEFINITIONS AND CONCEPTS
Several generic parameters and concepts are used throughout this
publication to characterize the environmental behaviour of polonium in various
media. They are defined here, generally according to the recommendations of
the International Commission on Radiation Units and Measurements
[2.1], and
follow IAEA publications on radionuclide transfers in different environments
(see Refs
[2.2, 2.3]). This publication has the advantage that the relatively
large quantity of data for
210
Pb and
210
Po, covering a wide range of species and
ecosystems, is typically described using these concepts.
2.1. BASIC PARAMETERS
There are several parameters which are commonly used to describe
radionuclide partitioning between environmental compartments. These include
transfer to plants or animals from soil, water or within the food chain, namely
concentration ratios (CRs) or transfer coefficients (e.g. B
v
, F
m
and F
f
), and
between sediments or soil minerals and water, using distribution coefficients (K
d
).
These parameters are defined for equilibrium conditions, and so it is assumed
that an organism or particle is in equilibrium with its surroundings in terms of
its accumulation of the radionuclide of interest. The definitions of other, more
specific parameters are given in Ref.
[2.2]. The parameters described here are
also regularly applied within existing predictive models to estimate the transfer
of radionuclides in the environment and the resulting exposures for humans and
non-human biota.
For terrestrial plants or wildlife, the primary indicator used to characterize
radionuclide behaviour in the soil–plant system is the soil–plant CR, also often
referred to as the transfer factor (TF), and it describes the transfer of radionuclides
from the soil to the plant when uptake by plant roots is the only process affecting
the transfer. The CR is defined as the ratio of the activity concentration of
radionuclide in the plant or animal (Bq/kg, DW) and in soil (Bq/kg, DW), based
on the dry weight (DW):
( )
( )
Activity concentration of radionuclide in edible tissue Bq/kg, DW
CR
Activity concentration of radionuclide in soil Bq/kg, DW
=
(2.1)

6
CHAPTER 2
The soil can be measured to a defined depth (e.g. 10 cm or 20 cm) and can
refer to soil contamination per m
2
as an aggregated concentration ratio (CR
agg
),
particularly for materials deposited via atmospheric transfer (see Chapter 5 for
a more detailed discussion). The CRs for plants are usually given for the edible
parts of the plant.
A similar ratio is applied for aquatic organisms with activity concentration
in water (Bq/L) or sediment (Bq/kg), and often on a fresh weight (FW) basis. The
transfer of radionuclides from water to aquatic organisms includes the intake of
water, sediments and feed, so the assumption is often that all those processes are
aggregated.
The CR for any organism can sometimes be expressed as a bioavailable
ratio (CR
bioav
). This acknowledges that some of any environmental radionuclide
(
210
Po in this case) will be in a chemical or physical form not amenable for
biological uptake. In those cases, geochemical speciation modelling (e.g. based
on pH, Eh, dissolved organics or other water chemistry) or physical processing
(e.g. filtration to remove particulates) will be used to estimate the bioavailable
portion in the soil or water and this, in turn, can be used to estimate the CR
bioav
.
For estimating radionuclide transfer from feeds to domestic animal food
products, a transfer coefficient is widely used for both milk (F
m
) and meat (F
f
).
This coefficient is defined as the equilibrium ratio of the radionuclide activity
concentration in the milk/meat on a FW basis to the daily dietary radionuclide
intake:
( )
( )
m/f
Activity concentration in milk/meat Bq/kg, FW
Daily radionuclide intake Bq/d
F =
(2.2)
CRs for human food chains and biota have some differences. For human
food chains, CRs are always ratios of the radionuclide activity concentrations
in the edible parts of the organisms to that in the surroundings. However, for
non-human species, the CR is calculated for the whole organism. This difference
reflects the purpose in the application of these parameters: in the first case, the
CRs are intended for assessments of radionuclide activity concentrations in food
that may be ingested by humans and are used to provide a radiological dose;
whereas the whole organism CRs are intended for dose assessments of the biota.
Considering abiotic compartments, K
d
is one of the basic parameters used
to characterize the mobility of radionuclides in the environment. The degree to
which
210
Po is bound to solids (soils in the terrestrial environment and sediments
in aquatic systems) is an important factor for determining the concentration of
a radionuclide in solution, and it directly influences the fraction of radionuclide
that can be incorporated by organisms (see above regarding bioavailability).
Dissolved radionuclide ions can bind to solid surfaces by a number of processes

7
RADIOECOLOGY
that are often classified under the broad term of ‘sorption’. Polonium is
particularly affected by sorption owing to its low environmental concentrations
and its variable oxidation state (see Chapter
3). K
d
for the partitioning of a
radionuclide between the particulate phase and the dissolved phase under
equilibrium conditions is similar to the aquatic CR described above and is
defined as:
( )
( )
d
Activity concentration in solid phase Bq/kg, DW
Activity concentration in aqueous phase Bq/L
K =
(2.3)
All of the above approaches have some limitations owing to the inherent
complexity and variability of natural environmental conditions. In particular,
the assumption of equilibrium being established in ecosystems is debatable.
Hence, the application of CRs for radiological conditions undergoing substantial
temporal variations in radionuclide activity concentrations in the environmental
media, for example following any accidental release, might be inappropriate.
Dynamic models are widely used for quantifying transfers of
210
Po in
non-equilibrium situations (see Ref. [2.3]), although the data coverage to populate
these models is not always sufficient for robust assessments.
2.2. CLASSIFICATIONS OF PLANTS AND ANIMALS USED FOR
EXPOSURE ASSESSMENT MODELS
There are many data in the scientific literature on radionuclide transfer to
plants and animals. The data are given for many different species, for whole or
parts of plants and animals, as well as for different environmental conditions.
However, to suit the purpose of dose assessment using predictive models such
as PC-CREAM
[2.4] or the ERICA Tool [2.5], the data need to represent some
widely recognized, standardized conditions. Such classifications for both human
and wildlife food chains can be found in IAEA publications and are discussed
here (see Refs [2.2, 2.6, 2.7]).
2.2.1. Transfer to human foodstuffs
The IAEA plant classification system is based on just 14 plant groups
[2.2].
All plants are categorized as cereals, maize, rice, leafy vegetables, non-leafy
vegetables, leguminous vegetables, root crops, tubers, fruits, grasses (cultivated
species), leguminous fodder (cultivated species), pasture (species mixture —
natural or cultivated), herbs and other crops. This system has been proposed
8
CHAPTER 2
as a basis for estimating the transfer of radionuclides to plant foodstuffs in the
framework of the assessment of exposures to humans through ingestion.
Plant tissues are subdivided into ten compartments: berries, buds, fruits,
grains, heads, leaves, roots, seeds and pods, stems and shoots, and tubers. Not all
of these ten compartments are assigned to each plant group, but only where the
portion represents an edible part of a specific plant.
The transfer of radionuclides to plants greatly depends on soil properties.
Existing international soil classification systems try to capture important
information for plant cultivation. The soil classification system established by the
Food and Agriculture Organization of the United Nations (FAO) and the United
Nations Educational, Scientific and Cultural Organization (UNESCO) has
28
units (or categories) of soil and 125 subunits [2.8]. TF values are not available
for subunits defined on such a detailed basis: the differences between these units
in terms of radionuclide transfer are generally not substantial. Therefore, a much
simpler classification system based on texture and organic matter content was
suggested in Ref.
[2.3], while ensuring that a reasonable amount of data are
available for each category. Four soil groups — sand, loam, clay and organic
soil — are defined for radiological assessments and are mostly based on grain
size (clay
≤ 0.004 mm, sand ≥ 0.06–2 mm, gravel > 2 mm and loams being
intermediate). The soils are grouped according to the percentage of sand and clay
mineral and the organic matter content in the soil
[2.3]:
“For the mineral soils, three groups were created according to the sand
and clay percentages…: ‘Sand group’: sand fraction ≥65%; clay fraction
<18%; ‘Clay group’: clay fraction ≥35%; ‘Loam group’: rest of cases. A
soils was included in the ‘Organic group’ if the organic matter content was
≥20%. Finally, an ‘Unspecified soil group’ was created for soils without
characterization data, or for mineral soils with unknown sand and clay
contents.”
With regard to animals, there are only five categories (cattle, sheep, goats,
pigs and poultry) and three groups (meat, milk and eggs) that are considered
to provide transfer coefficients from daily radionuclide intake of animals and
animal products consumed by humans
[2.3]. The classification systems are
assumed adequate to cover the variability of environmental conditions and can
also be applied for the assessment of the transfer of polonium through the human
consumption of food. A detailed discussion of the data and concepts used to
define these categories of soil, plants and animals are given in Refs [2.2, 2.3],
and many empirical data on
210
Po (and
210
Pb, where appropriate) presented in this
publication use these parameters.
9
RADIOECOLOGY
2.2.2. Transfer to wildlife: Reference organism concept
Since it is impossible to consider all species of plants and animals in
radiological impact assessments, a classification based on a reference organisms
is applied. Larsson [2.9] defines reference organism as “a series of entities that
provide a basis for the estimation of radiation dose rate to a range of organisms
which are typical, or representative, of a contaminated environment”. Such
groups need to be selected on the basis of their representativeness for the selected
environments of interest and should also allow realistic assessments, illustrating
possible exposure pathways. This approach is used in the ERICA project, which
develops a framework for radiation protection of the environment (see Ref.
[2.5]).
A similar concept has been applied by the International Commission on
Radiological Protection (ICRP), which introduced a system of discrete and
clearly defined reference animals and plants for assessing radiation effects on
wildlife. According to the ICRP definition [2.10]:
“A Reference Animal or Plant is a hypothetical entity, with the assumed
basic characteristics of a specific type of animal or plant, as described to
the generality of the taxonomic level of Family, with defined anatomical,
physiological and life-history properties, that can be used for the purposes
of relating exposure to dose, and dose to effects, for that type of living
organism.”
The ICRP approach is based on the consideration of 12 more or less
globally representative reference animals and plants, covering different life stages
(e.g. fish egg, adult fish). This is a basis for systematically relating radionuclide
exposure to radiological dose, and then dose (or dose rate) to different types of
effect, for a number of organisms that are characteristic of different types of
natural environment [2.10].
In 2009, the IAEA, within the international programme Environmental
Modelling for Radiation Safety (EMRAS
II), initiated a working group to
develop an international handbook for estimating the transfer of radionuclides
to wildlife, similar to Ref.
[2.2] for estimating transfer to human foodstuffs.
The approach adopted for classification of wildlife was based on a reference
organism concept
[2.6, 2.7] and was consistent with the ICRP approach [2.11].
However, it was applied more generally, and it defines broader wildlife groups
(e.g. soil invertebrate, predatory fish and terrestrial mammal; see Table
2.1). In
some cases, a consideration of specific subcategories is included. This approach
is also used in this publication, along with the relevant data for
210
Pb and
210
Po
collected within the EMRAS
II programme to harmonize environmental impact
assessments.

10
CHAPTER 2
TABLE 2.1. WILDLIFE GROUPS AND REFERENCE ORGANISMS
Broad group Subcategory Reference organism
Terrestrial environments
Amphibians —
a
Frog
Annelids —
a
Earthworm
Arachnids —
a
—
a
Arthropods
Carnivorous
Detritivorous
Herbivorous
—
a
—
a
Bee
Birds
Carnivorous
Herbivorous
Omnivorous
Duck
Duck
Duck
Duck
Ferns —
a
—
a
Fungi
Mycorrhizal
Parasitic
Saprophytic
—
a
—
a
—
a
Gastropods —
a
—
a
Grasses and herbs
Grasses
Herbs
b
Wild grass
—
a
Lichens/bryophytes —
a
—
a
Mammals
Carnivorous
Herbivorous
c
Marsupial
d
Omnivorous
Rangifer spp.
Rat or deer
Rat
Rat or deer
—
a
Rat
—
a
Reptiles
Carnivorous
Herbivorous
—
a
—
a
Shrubs —
a
—
a
Trees
Coniferous
Broadleaf
Pine tree
—
a

11
RADIOECOLOGY
TABLE 2.1. WILDLIFE GROUPS AND REFERENCE ORGANISMS (cont.)
Broad group Subcategory Reference organism
Freshwater environments
Algae —
a
—
a
Amphibians —
a
Frog
Birds
Carnivorous
Herbivorous
Omnivorous
Duck
Duck
Duck
Duck
Crustaceans —
a
—
a
Fish
Benthic feeding
e
Piscivorous
f
Forage
g
—
a
Salmonid
—
a
Insects —
a
—
a
Insect larvae
h
—
a
—
a
Mammals
Carnivorous
Herbivorous
Omnivorous
—
a
—
a
—
a
Molluscs
Bivalve
Gastropod
—
a
—
a
Phytoplankton —
a
—
a
Reptiles —
a
—
a
Vascular plants —
a
Wild grass
Zooplankton —
a
—
a
Marine environments
Birds
Carnivorous
Herbivorous
Omnivorous
Duck
Duck
Duck
Duck
Crustaceans
Large
Small
Crab
—
a

12
CHAPTER 2
TABLE 2.1. WILDLIFE GROUPS AND REFERENCE ORGANISMS (cont.)
Broad group Subcategory Reference organism
Fish
Benthic feeding
e
Piscivorous
f
Forage
g
Flatfish
Salmonid
—
a
Insects —
a
—
a
Macroalgae —
a
Brown seaweed
Mammals
Carnivorous
Herbivorous
Planktivorous
—
a
—
a
—
a
Molluscs
Bivalve
Cephalopod
i
Gastropod
—
a
—
a
—
a
Phytoplankton —
a
—
a
Polychaete worms —
a
—
a
Reptiles —
a
—
a
Sea anemones/true corals —
a
—
a
Vascular plants —
a
—
a
Zooplankton —
a
—
a
Source: Tables 2–4 of Ref. [2.6]. The list is based on an on-line database available at
www.wildlifetransferdatabase.org
a
—: data not available.
b
Herb refers to any non-woody plant which does not fall into one of the other categories.
c
Does not include Rangifer spp. (reindeer and caribou).
d
All marsupials, regardless of feeding strategy.
e
Fish feeding on benthic dwelling organisms.
f
Fish consuming smaller fish, amphibians or birds.
g
Fish feeding on primary producers and pelagic invertebrates and zooplankton.
h
Insect larvae are included as the aquatic life phase is important for many species which are
terrestrial as an adult.
i
Squid, octopus and cuttlefish.
13
RADIOECOLOGY
REFERENCES TO CHAPTER 2
[2.1] INTERNATIONAL COMMISSION ON RADIATION UNITS AND
MEASUREMENTS, Quantities, Units and Terms in Radioecology, ICRU Report
65,
ICRU, Bethesda, MD (2001).
[2.2] INTERNATIONAL ATOMIC ENERGY AGENCY, Handbook of Parameter Values
for the Prediction of Radionuclide Transfer in Terrestrial and Freshwater Environments,
Technical Reports Series No.
472, IAEA, Vienna (2010).
[2.3] INTERNATIONAL ATOMIC ENERGY AGENCY, Quantification of Radionuclide
Transfer in Terrestrial and Freshwater Environments for Radiological Assessments,
IAEA-TECDOC-1616, IAEA, Vienna
(2009).
[2.4] SMITH, J.G., SIMMONDS, J.R. (Eds), The Methodology for Assessing the
Radiological Consequences of Routine Releases of Radionuclides to the Environment
Used in PC-CREAM
08, HPA-RPD-058, Health Protection Agency, Chilton,
Didcot
(2009).
[2.5] BROWN, J.E., et al., The ERICA Tool, J. Environ. Radioact. 99 (2008) 1371–1383.
[2.6] INTERNATIONAL ATOMIC ENERGY AGENCY, Handbook of Parameter Values
for the Prediction of Radionuclide Transfer to Wildlife, Technical Reports Series
No.
479, IAEA, Vienna (2014).
[2.7] HOWARD B.J., et al., The IAEA handbook on radionuclide transfer to wildlife,
J. Environ. Radioact. 121 (2013) 55–74.
[2.8] FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS,
UNITED NATIONS EDUCATIONAL, SCIENTIFIC AND CULTURAL
ORGANIZATION, Soil Map of the World, 1:5 000 000, UNESCO, Paris (1994).
[2.9] LARSSON, C.-M., The FASSET Framework for assessment of environmental impact
of ionising radiation in European ecosystems: An overview, J. Radiol. Prot.
24
(2004)
A1–A12.
[2.10] INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION, The
2007 Recommendations of the International Commission on Radiological Protection,
Publication
103, Elsevier, Amsterdam (2007).
[2.11] INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION,
Environmental Protection: The Concept and Use of Reference Animals and Plants,
Publication
108, Elsevier, Amsterdam (2008).

15
Chapter 3
PROPERTIES OF POLONIUM
This chapter summarizes the physical and chemical properties of polonium
and acts as a reference for the subsequent chapters. It includes decay data
and series systematics, solution chemistry and adsorption, and methods of
determining
210
Po activity concentrations in samples.
3.1. PHYSICAL PROPERTIES
3.1.1. Isotopes
There are 42 known isotopes of polonium in the National Nuclear Data
Center Chart of Nuclides
1
and none is stable. There are seven naturally occurring
radioactive isotopes of polonium, which are part of the natural decay series of
long lived radionuclides (see Table 3.1 and Fig. 3.1):
— The
238
U series:
210
Po,
214
Po and
218
Po.
— The
235
U series:
211
Po and
215
Po.
— The
232
Th series:
212
Po and
216
Po.
At 138 days, however, only
210
Po has a half-life long enough to play a
significant role in environmental processes. The decay scheme for
210
Po, along
with those of
210
Bi and
210
Pb, which precede
210
Po in the
238
U decay series, is
shown in Fig. 3.2. There are several anthropogenic isotopes with a half-life
longer than a day, which are generally produced by irradiation of bismuth
(see Table 3.1). The longest lived,
209
Po (T
1/2
= 102 a), has been used for
polonium chemistry experiments and as a yield tracer of polonium during sample
processing and radiochemical analyses.
3.1.2. Decay series systematics
The distribution of
210
Po in the environment is to be considered in the
context of
238
U decay series systematics. The concentration of each radionuclide
is controlled by the concentration of its parent, its own half-life and the amount
1
See www.nndc.bnl.gov/chart

16
CHAPTER 3
TABLE 3.1. POLONIUM ISOTOPES
Isotope Half-life
Decay constant
(a
−1
)
Major decay modes
(>1%)
Specific activity
(Bq/g)
Energy of decay
(MeV)
(with % of total decays)
Emission energy
(MeV)
Naturally
occurring
210
Po 138.376 d 1.829 73 α 1.66 × 10
14
5.407 (>99.9%) 5.304
211
Po 0.516 s 4.24 × 10
7
α 3.83 × 10
21
7.594 (98.9%)
7.024 (0.54%)
6.695 (0.52%)
7.450
6.891
6.568
212
Po 0.300 μs 7.29 × 10
13
α 6.56 × 10
27
8.954 (100%) 8.785
214
Po 162 μs 1.35 × 10
11
α 1.20 × 10
25
7.833 (99.99%)
7.034 (0.01%)
7.687
6.903
215
Po 1.781 ms 1.228 × 10
10
α 1.09 × 10
24
7.526 (99.93%)
7.087 (0.06%)
7.386
6.955
216
Po 0.148 s 1.48 × 10
8
α 1.31× 10
22
6.906 (>99.99%) 6.778
218
Po 3.07 m 1.19 × 10
5
α 1.04 × 10
19
6.115 (99.98%) 6.002
Anthropogenic
(T
1/2
> 1 d)
206
Po 8.8 d 29 α (5.45%)
ε (94.55%)
2.7 × 10
15
5.327 (5.45%)
1.846 (94.55%)
5.224
208
Po 2.898 a 0.239 2 α 2.19 × 10
13
5.215 (>99.99%) 5.115
209
Po 115 a 6.03 × 10
−3
α 5.50 × 10
11
4.716 (0.55%)
4.977 (79.2%)
4.979 (19.8%)
4.622
4.883
4.885
Source: Data are from the Decay Data Evaluation Project (www.nucleide.org/DDEP_WG/DDEPdata.htm), where available, otherwise (for
206
Po
and
208
Po) from the National Nuclear Data Center Chart of Nuclides (www.nndc.bnl.gov/chart).
Note: Transformations with intensities less than 0.01% have not been included in this table. Intensities are given as a percentage of total decay.
Where more complete data are required, the original sources should be consulted.

17
PROPERTIES
Note: The only long lived polonium isotope,
210
Po, is circled.
FIG. 3.1. The naturally occurring
238
U,
235
U and
232
Th decay series, all of which contain
polonium isotopes (Z = 84).

18
CHAPTER 3
of time since fractionation between the isotope and its parent occurred. For the
decay series elements within samples that have been closed over long timescales,
the activity of each isotope (decay rate) is the same as that of its parent (i.e. it is
in secular equilibrium with its parent). For the
238
U decay series, which contains
210
Po, this can be represented by:
(
238
U) = (
226
Ra) = (
222
Rn) = … = (
210
Pb) = (
210
Po) (3.1)
where the parentheses denote activity, which is equal to the number of atoms
multiplied by the decay constant.
There are two important points to note:
— In secular equilibrium, the activity concentration (and, from that, the molar
concentration) of a daughter radionuclide is controlled by that of the parent
radionuclide, and so, ultimately, all radionuclides in the series are controlled
by the longest lived parent (here
238
U).
— While the activities are equal, the molar abundances are inversely
proportional to the decay constants, so that the molar concentrations of
very short lived nuclides are very low.
The molar ratio of
210
Po:
238
U is 8.5 × 10
−11
, while the molar ratio of
210
Po:
226
Ra is 2.4 × 10
−4
. Therefore, even where uranium or radium is greatly
enriched, polonium has an extremely low molar concentration — even when it
exhibits high radioactivity. This has important implications for understanding its
behaviour in the environment.
When any series is not in secular equilibrium due to chemical or physical
fractionation, the activity of the nuclides will tend to evolve back towards secular
equilibrium over a timescale determined by the half-life of the daughter isotope.
The section of the
238
U decay series that is most relevant to understanding
210
Po
FIG. 3.2. The decay scheme for
210
Bi,
210
Pb and
210
Po.
19
PROPERTIES
is below
226
Ra. Including only those isotopes with half-lives longer than one day,
this part of the series is:
1602 a 3.82 d 22.3 a 5.01 d 138.4 d
226 222 210 210 210 206
Ra Rn Pb Bi Po Pb¾¾¾® ¾¾¾® ¾¾¾® ¾¾¾® ¾¾¾¾®
(3.2)
In secular equilibrium, the activities of all these radionuclides are the same.
However, the following environmental processes can separate them:
(a) The isotope
222
Rn, a noble gas, can migrate. It is generally unreactive and
forms no chemical bonds within environmental materials. It is sometimes
released from solids by a process known as alpha recoil (see below) and,
despite its relatively low aqueous solubility, it can enter any surrounding
water (e.g. pore water, groundwater and lake water) or vapour phase
(e.g. pore spaces, bubbles and the atmosphere), thereby separating from its
226
Ra parent.
(b) The radionuclides
226
Ra,
222
Rn and
210
Pb, which are produced by alpha
decay, and the very short lived radionuclides
218
Po and
214
Pb, which are
between
222
Rn and
210
Pb, are propelled in the direction opposite to that of
the alpha particle and with sufficient energy to travel approximately 20
nm
in mineral structures. They can therefore escape to the surrounding water
or air or come to rest in an adjacent phase
[3.1, 3.2]. The direction of recoil
is random for each decay, and the rate of ejection is determined only by
the fraction of parent nuclides within recoil distance of mineral surfaces or
channels to the surface. This is sometimes referred to as alpha recoil.
(c) Lead and polonium can volatize at high temperature in volcanic vents,
wildfires (see Appendix IV) and industrial processes, and then condense or
scavenge during subsequent cooling.
(d) There can be contrasting adsorption behaviour of lead, polonium and
radium on particles in the atmosphere, and on surfaces in soils and aquifers,
and into colloids or particulates in surface waters.
(e) Contrasting uptake, excretion and biodistribution of lead, polonium and
radium can occur in biota.
The systematics of
210
Po under these varying circumstances are shown
in Fig. 3.3. In each case, following disequilibrium, the activity of the daughter
nuclide grows into secular equilibrium with the parent over a timescale relating to
its half-life. To understand the evolution of
210
Po and the resulting radiation, the
evolution of
210
Pb and
210
Bi also needs to be considered. When
210
Po is isolated
from the other isotopes in the decay series, such as owing to the migration of
222
Rn and subsequent decay to
210
Po, it will decay unsupported. If it is then not

20
CHAPTER 3
Note: (A)
210
Po is unsupported by decay of the parent
210
Bi (and so by decay of
210
Pb), for
example when
210
Po is separated from
210
Pb and
210
Bi by precipitation or volatilization.
(B) There is
210
Pb present, and the evolution of
210
Po is shown for several different
starting activities. The excess or deficiency of
210
Po will decay according to the
half-life of
210
Po. Such situations are found in both biological systems and abiotic
environments, where
210
Po can be fractionated from
210
Pb to generate either an excess
or a deficiency of
210
Po. The inset shows the ingrowth of
210
Bi, which occurs over
a much shorter time period, and so the amount of initial
210
Bi has little effect on the
overall evolution of
210
Po. (C) The ingrowth of
210
Pb is shown in the presence of
226
Ra,
for example within barite (BaSO
4
), which strongly incorporates radium. The different
timescale for this circumstance should be noted. Over this time period,
226
Ra is almost
constant. The activity of
210
Po will closely follow that of
210
Pb.
FIG. 3.3. The closed-system evolution of
210
Po under various circumstances.

21
PROPERTIES
subject to further additions, and losses only by decay, its activity will evolve over
time according to:
( ) ( )
210
Po
210 210
0
Po Po e
tl-
=
(3.3)
where the activity (
210
Po) decays from the initial value (
210
Po)
0
according to the
decay constant
210
Po
l
. The evolution is shown in Fig. 3.3, where the activity will
drop to 1% of the initial value in 6.6 half-lives or about 2.5 years.
When
210
Pb and
210
Po are separated from the rest of the decay series, the
activity of
210
Pb will evolve according to an equation analogous to Eq. (3.3),
although it will decay with a half-life of 22.3 years. Its daughter,
210
Bi, will grow
according to:
( ) ( )
( )
210
210
210
Po
Bi
210 210
210 210
Bi
0
Bi Pb
Bi Pb e e
t
t
l
l
l
ll
-
-
=-
-
(3.4)
Over the time period in which the activity of
210
Bi approaches that of
210
Pb
(i.e. to within 1% in 33 days), the activity of
210
Pb drops by only 0.3% and so can
be considered constant:
( ) ( )
( )( )
( )( )
( )( )
210 210
210
Pb
210 210 210 210
210 210
210
Bi
210 210 210 210
210 210
210
Po
210 210 210 210
Bi Po
Bi Pb Po Pb
210 210 210
Bi Po
0
Pb Bi Po Bi
Bi Po
Pb Po Bi Po
e
Po Pb e P
e
t
t
t
l
l
l
ll
l ll l
ll
llll
ll
l ll l
-
-
-
éù
êú
êú
êú
--
êú
êú
êú
êú
êú
=+ +
êú
êú
--
êú
êú
êú
êú
êú
êú
+
êú
--
êú
ëû
( )
210
Po
0
oe
tl-
(3.5)
Substituting values for the decay constants gives:
( ) ( ) ( )
210
Pb
210
210
Po
Bi
210
Po
210 210 210
00
1.0179e
Po Pb 0.0376e Po e
1.0555e
t
t
t
t
l
l
l
l
-
-
-
-
éù
êú
êú
êú
=+ +
êú
êú
êú
-
êú
ëû
(3.6)
Regardless of the initial activity of
210
Po relative to that of
210
Pb, the activity
of
210
Po will evolve to within 1% of that of
210
Pb in 2.5 years. Over this time, the
22
CHAPTER 3
activity of
210
Pb drops by only 7.5% (i.e. the term drops to 0.925, and so for much
of the time can be approximated to 1).
3.2. CHEMICAL PROPERTIES
While in some environments the activity concentration of
210
Po can be
of concern, the molar concentrations in the environment are always extremely
low. In the long term, the distribution of
210
Po is controlled by that of the parent,
210
Pb. The behaviour of
210
Po in aqueous systems is generally dominated by
adsorption onto surfaces, although incorporation into colloids, biovolatilization
and precipitation in sulphides can be important in some circumstances.
There have been several reviews on polonium chemistry and its behaviour
in the environment
[3.3, 3.4]. However, the data have been limited and some
reasons for this include the following:
(a) The very high specific activity of
210
Po (1.66 × 10
14
Bq/g; see Table 3.1)
causes practical difficulties in the safe handling of even small amounts of
natural polonium. The specific activities of artificially produced
208
Po and
209
Po are lower but still high.
(b) The very high specific activity also readily damages crystal lattices and
materials in experiments, so it is difficult to obtain undamaged materials
(e.g. precipitated minerals) for characterization.
(c) Naturally occurring
210
Po is very scarce. One gram of
238
U is generally
accompanied by 75 pg of
210
Po. Hence, in crustal rocks with an average
concentration of 2.7 ppm uranium, there is only 0.20 ng of
210
Po per
tonne. Production of
208
Po and
209
Po requires bombardment of bismuth by
neutrons in a reactor.
(d) In the environment,
210
Po is present at such low concentrations that it clearly
does not form separate phases (see Sections
3.2.1–3.2.3 and Chapter 6 for
more details). For example, groundwater typically has less than 40 mBq/L,
with a maximum value of 19 Bq/L (see Section 4.5.1), which corresponds to
typical groundwater concentrations of less than 0.001 fM and a maximum
of only 0.45 fM.
(e) In many circumstances,
210
Po will decay or approach secular equilibrium
with its parent,
210
Pb. Its distribution is therefore strictly controlled by
210
Pb.
23
PROPERTIES
3.2.1. Solution chemistry
Polonium is in Group 16 of the periodic table, which is known as the
chalcogens and includes oxygen, sulphur, selenium and tellurium (see Fig. 3.4).
It is generally considered to be a metalloid, with physical and chemical
characteristics that are intermediate between those of metals and non-metals
(along with boron, silicon, germanium, arsenic, antimony, tellurium and astatine),
although it can behave more like the metals bismuth and lead (a review of the
early analytical chemistry can be found in Refs [3.5–3.7]). Based on analogy with
selenium and tellurium, Figgins [3.6] predicts that polonium would have stable
oxidation states of −2, +2, +4 and +6, with the +4 oxidation state being the stable
state in solution under oxic freshwater conditions; although Po
2+
has also been
predicted to be most stable in sea water and under reducing conditions [3.8, 3.9].
Polonium hydrolyses, forming PoO(OH)
+
, PoO(OH)
2
and PoO
2
in slightly acidic
to neutral pH regions and PoO
2
−3
in alkaline solutions [3.10].
There are limited solubility data available for polonium, but it is generally
assumed to be highly insoluble. Work was conducted on the precipitation
of polonium from laboratory solutions in the 1950s, in particular during the
development of separation procedures, and polonium was found to precipitate
with various compounds of antimony, bismuth, tellurium and rare earth
elements [3.5]. The solubility product for Po(OH)
4
is 10
−37
[3.6]; hence, in
natural waters, equilibrium activities are highly sensitive to pH [3.11]. In acidic
solutions, trace polonium is precipitated by hydrogen sulphide (H
2
S), along
with other insoluble sulphides. Under these conditions, polonium is also highly
insoluble, sinwith ce the solubility product for PoS at around 5 × 10
−29
[3.6].
The concentration of dissolved polonium can therefore be strictly controlled by
sulphur recycling, where the dissolution and reprecipitation of sulphur minerals
occurs [3.12]. However, away from very dynamic conditions, adsorption onto
particles, mineral surfaces and colloids provides the stronger control on dissolved
polonium concentrations.
Polonium is present in precipitates containing radium owing to ingrowth
after mineral formation. Radium is commonly precipitated in barite (BaSO
4
).
Since polonium is not readily accommodated within a crystal structure like
radium, it might be released during recystallization, although laboratory
experiments indicate that recrystallization does not expel a significant fraction
of polonium [3.13]. In a study of the bacterial mobilization of polonium from
waste gypsum, LaRock et al. [3.14] demonstrate that sulphate reducing bacteria
are effective at mediating polonium release from gypsum, provided the sulphide
levels resulting from their metabolism does not rise above 10 μmol/L, in which
case polonium is evidently co-precipitated as a metal sulphide.

24
CHAPTER 3
FIG. 3.4. The location in the periodic table of polonium and the elements with isotopes within the
238
U decay series.
25
PROPERTIES
Polonium forms colloids in solution [3.15], and while this may be a
significant factor when there are high concentrations of polonium, or in pure
solutions in laboratory experiments (see Ref. [3.10]), it is more likely that in
natural environments polonium is adsorbed onto, or incorporated into, colloids
formed by more abundant elements.
3.2.2. Adsorption
In most natural environments, polonium is highly surface reactive, and
readily adsorbs onto mineral surfaces, particulates and colloids. However, there
are limited data quantifying this. Models for the description of radionuclide
sorption are still mostly based on empirical solid–liquid distribution coefficient
(K
d
) values of bulk materials (see Sections 2.1 and 6.4.3 for more information on
K
d
). This approach is the simplest sorption model available and K
d
is the ratio of
the concentration of radionuclide in a specified solution to the concentration of
the radionuclide sorbed on a specified solid when the solution and solid are at
equilibrium, with units L/kg. However, these values cannot be easily extrapolated
to materials with different compositions.
There are only limited experiments to determine K
d
for polonium on
homogenous materials. Laboratory experiments on montmorillonite clay found
measured adsorption values of around 1.5 × 10
3
L/kg for
210
Po, which were
independent of both ionic strength (up to 0.2 eq/L) and pH (6 and 7) [3.10].
However, polonium that was adsorbed from solution could not be readily
desorbed, with a several orders of magnitude greater proportion of polonium
remaining adsorbed. It was suggested that polonium forms stable covalent surface
complexes. It was also found that
210
Po generated by radioactivity of sorbed
210
Pb
can be more readily desorbed, although a distribution value much higher than the
adsorption experiments, of 2 × 10
5
L/kg, was still obtained. For other materials,
batch partitioning experiments found average values of the following [3.6]:
— 8.4 × 10
4
L/kg for bentonite at pH10.1;
— 3.7 × 10
4
L/kg for a tuff (mainly plagioclase, smectite and the zeolite
clinoptilolite) at pH9.4;
— 2.5 × 10
4
L/kg
at pH10.1 for a granodiorite (largely quartz, plagioclase and
K-feldspar).
These values were obtained by ultrafiltering the solution, and somewhat
lower values were obtained by using 0.45 μm filters. Presumably, a significant
fraction of polonium was not in true solution but rather on colloid sized
particles, and this should be considered when comparing the results of different
studies. Manganese (IV) oxides have often been inferred to significantly

26
CHAPTER 3
adsorb or incorporate substantial amounts of polonium in field studies (see
Refs [3.16–3.18]). Pyrolusite (MnO
2
) has been qualitatively shown to effectively
adsorb polonium in column experiments [3.19]. However, adsorption coefficients
onto manganese minerals has not been quantified in the laboratory.
Some results for partitioning experiments with soils have been published.
An early study [3.20] still provides the basis for most estimates of polonium
adsorption in soil, and is the basis for recent recommended values [3.21, 3.22].
Polonium partitioning has also been determined in aquatic systems,
including rivers, estuaries, coastal seas and the open ocean. In these environments,
210
Po consistently displays K
d
values of approximately 10
5
, and usually slightly
higher than the K
d
of the parent radionuclide,
210
Pb [3.23, 3.24]. Values for a
range of natural environments, determined from measurements of filtered waters
and particulates from filters, are shown in Table 3.2.
TABLE 3.2. AQUATIC K
d
IN SITU DETERMINATIONS
Environment
n
210
Po K
d
210
Pb K
d
Ref.
River 5 (1.0 ± 0.3) × 10
5
(3 ± 1) × 10
4
[3.23]
Estuary 7 (3.3 ± 1.5) × 10
5
(2.4 ± 1.9) × 10
5
[3.24]
Coastal sea, shoreline 17 (2.8 ± 0.9) × 10
5
(1.7 ± 1.0) × 10
5
[3.24]
Surface, open ocean 4 (1–4) × 10
4
(1–4) × 10
4
[3.23]
In rivers,
210
Po is mostly associated with suspended particulate matter and
bottom sediments. In estuaries,
210
Po and lead partitioning may be controlled by
the concentration of dissolved organic matter and other ligands, the nature and
concentration of particulate matter, and by changes in salinity [3.23]. In coastal
sea water,
210
Po is mostly associated with biological particles (bacteria and
microplankton), while
210
Pb is mostly associated with the inorganic particulate
fraction (clay minerals). The enhanced incorporation of
210
Po in particulate
organic material, and also in the first levels of trophic marine chains, has been
linked to metabolic processes and
210
Po binding to proteins [3.25–3.28]. The
same occurs in open ocean waters and the degree of planktonic uptake in the
euphotic zone of oceans may have a strong influence on
210
Po flux in oceanic
water columns [3.29, 3.30].
Overall, the K
d
values that are available can be used as guidelines, but there
is little systematic data exploring the effects of various conditions on polonium
partitioning. Furthermore, K
d
values are generally values for the relative
27
PROPERTIES
concentrations between phases and not partition coefficients for equilibrium
adsorption. Further work is required to determine when polonium incorporation
is reversible, and how it varies with polonium concentration.
3.2.3. Biovolatilization
Hussain et al. [3.31] find that organo-polonium compounds may comprise a
substantial proportion of environmental polonium, based on experiments in which
up to 50% of polonium is lost from Florida groundwater with high polonium
concentrations by bubbling nitrogen. Furthermore, this effect is substantially
reduced when oxidizing agents are added, suggesting that volatile organic species
have been destroyed. By analogy with the behaviour of tellurium, Hussain et
al. [3.31] suggest that dimethyl polonium was present. A similar experiment
with groundwater from Nevada did not find significant polonium volatilization,
indicating that the extent of organic polonium complexation may vary [3.18].
In addition to the implications for volatile loss of polonium to the atmosphere,
these results suggest that there may be organic ligands that can control polonium
adsorption behaviour in soil and groundwater.
Momoshima et al. [3.32] demonstrate that polonium is lost by volatilization
in culture experiments in which microorganism growth has been enhanced
in seawater and coastal sediments, and suggest that methylation of polonium
is responsible. In a further, they show that when methylcobalamin, which is
involved in organic methylation, is added, polonium is also lost. Similar losses
are found from similar culture experiments using fresh waters and increase with
enhanced bacterial growth [3.33]. Volatilization has also been demonstrated in
different pure bacterial cultures [3.34].
3.3.
210
Po DETERMINATION
Only a short summary of
210
Po determination in environmental samples
is presented here, since there are several studies on the topic, including
a comprehensive review
[3.35] and a procedure for analysis of water
samples
[3.36–3.38]. Owing to the volatility of polonium, high temperature
sample preparation and digestion techniques, such as dry ashing and fusion with
molten salts, cannot be used. To avoid polonium losses, drying should be by
freeze drying or oven drying at a relatively low temperature (≤80°C).
Wet ashing is commonly used for sample dissolution, sometimes in
combination with microwave digestion. Soils, sediments and other solid
samples, such as filtered materials, are usually prepared using HCl, HF, HNO
3
and HClO
4
in varying proportions, with ashing in open vessels, pressure vessels
28
CHAPTER 3
or microwave digestion systems. Biological samples are generally treated by
first drying them thoroughly at temperatures of up to 80°C or freeze drying,
followed by treatment with HNO
3
(sometimes also with HCl and H
2
O
2
) to
destroy organic matter. Polonium is pre-concentrated from water samples by
a wide variety of techniques, with the most common involving one of three
procedures: evaporation; co-precipitation, typically on Fe(OH)
3
or MnO
2
; or
chelation, commonly with ammonium pyrrolidine dithiocarbamate (APDC)
species
[3.35, 3.39].
As
210
Po is an alpha emitting radionuclide with a relatively short half-life
and produces only very-low-intensity gamma rays and no radioactive progeny,
the only practical way to determine its presence is to detect its alpha particle.
The most commonly used method is alpha particle spectrometry, which
utilizes a silicon surface barrier or passivated implanted planar silicon (PIPS)
detectors, on the account of their excellent energy resolution, compact size, low
background, excellent stability and low sensitivity to gamma radiation. Alpha
spectrometry can also be performed using gridded ion chambers, which have a
high efficiency and allow the use of large sources. Alternatively, alpha particle
counting may be performed using a ZnS scintillator screen and photomultiplier
tube, gas proportional counter or liquid scintillation counter. This approach has
the disadvantage that a radioisotopic tracer cannot be used, although some liquid
scintillation systems do enable separation of the
209
Po peak.
Whichever detection method is used,
210
Po has first to be chemically
separated from both the sample matrix and from other alpha emitting
radionuclides present in the sample. Polonium separation methods include
those based on solvent extraction, ion exchange chromatography and extraction
chromatography
[3.35]. In addition, polonium spontaneously deposits from mild
acid solutions onto metal surfaces, in particular silver, and this is very often
utilized as a combined chemical separation and source preparation step
[3.40].
The interference of iron during spontaneous deposition is suppressed by the
addition of ascorbic acid or hydroxylamine hydrochloride (to reduce Fe
3+
). Citric
acid may also be added to suppress the effects of other ions present. Metals other
than silver have been used for plating of polonium, including copper and nickel.
However, analyses using these metals are more prone to interference as some
210
Bi or
210
Pb may also be deposited, resulting in an overestimation of
210
Po when
there is a delay between deposition and counting
[3.41, 3.42].
An advantage of the alpha spectrometry detection methods is that an
isotopic yield tracer can be used to allow for losses during sample preparation,
chemical separation and source preparation steps. Historically,
208
Po has been
the preferred tracer, probably on the account of its greater availability. The
advantage of
209
Po over
208
Po is that its E
α
is further separated from that of
210
Po
(see Table
3.1), allowing better peak resolution. In the case of non-spectrometric

29
PROPERTIES
methods, such as total alpha counting using a ZnS scintillator screen or liquid
scintillation counting, the recovery for
210
Po during the separation and source
preparation process needs to be estimated.
Contamination of any alpha spectrometry detector by the tracer (
208
Po or
209
Po) or by
210
Po is a potentially serious problem. This contamination can be
minimized by a period of exposure of the source to air before counting, possibly
due to the formation of a surface oxide film on the silver
[3.5, 3.43]. A delay
period of at least two days has been suggested
[3.39].
A polonium spectrum with
209
Po used as the yield tracer is shown in
Fig.
3.5. The spectrum is a simple one, consisting essentially of two singlet
peaks. The spectrum obtained using
208
Po as a tracer is similar, but with a smaller
separation between the two peaks. As described in Ref. [3.35]:
“Once the count rates in the tracer and
210
Po peaks have been obtained
and tailing allowed for, the contributions due to detector background and
procedure blank need to be subtracted. The ratio of the net count rates in
the two peaks is then used to calculate the activity concentration of
210
Po
in the sample on the date of chemical separation of Po, taking into account
the activity concentration of the tracer solution, the masses of the sample
and the tracer solution used, the decay of
210
Po between separation and
counting, the decay of the tracer between its calibration date and counting,
and the
α emission probabilities in the measured areas in the α-spectrum”.
Finally, the results need to be corrected for decay and for ingrowth from its
progenitors (especially
210
Pb) between the sampling date and the date of chemical
FIG. 3.5. A typical polonium alpha particle spectrum with
209
Po as the yield tracer.

30
CHAPTER 3
separation of polonium from the sample for analysis. This calculation needs to be
carried out separately from the decay correction to separation date because, in the
former, the
210
Po is supported by
210
Pb in the sample, whereas in the latter it is
unsupported (see Fig.
3.6).
In order to make the correction for
210
Po to the sample collection date, the
210
Pb activity concentration needs to be determined. The details of the ingrowth
and decay calculation are dependent on the procedure followed
[3.35]:
“One common approach is that when Po is chemically separated, the solution
containing the
210
Pb is retained and kept for an ingrowth period (usually
several months). A subsequent determination of
210
Po from this solution is
used to calculate the activity concentration of
210
Pb in the sample”.
An alternative approach is to use a separate determination of
210
Pb in the
sample by another method, such as gamma spectrometry or beta counting. In
general, the time delay between sample collection and
210
Po determination should
be kept as short as practicable to avoid errors due to this decay and ingrowth
correction, particularly for sample types for which the
210
Po:
210
Pb activity ratio
may be low (e.g. for rainwater) [3.37].
FIG. 3.6. Decay and ingrowth corrections for
210
Po determination, taking into account the
time intervals t
1
(separation date − sampling date) and t
2
(count date − separation date).
31
PROPERTIES
REFERENCES TO CHAPTER 3
[3.1] KIGOSHI, K., Alpha-recoil thorium-234: Dissolution into water and the uranium-234/
uranium-238 disequilibrium in nature, Science
173 (1971) 47–48.
[3.2] FLEISCHER, R.L., RAABE, O.G., Recoiling alpha-emitting nuclei: Mechanisms for
uranium-series disequilibrium, Geochim. Cosmochim. Acta
42 (1978) 973–978.
[3.3] COPPIN, F., ROUSSEL-DEBET, S., Comportement du
210
Po en milieu terrestre: revue
bibliographique, Radioprot. 39 (2004) 39–58.
[3.4] PERSSON, B.R.R., HOLM, E., Polonium-210 and lead-210 in the terrestrial
environment: A historical review, J. Environ. Radioact.
102 (2011) 420–429.
[3.5] BAGNALL, K.W., The chemistry of polonium, Q. Rev. Chem. Soc. 11 (1957) 30–48.
[3.6] FIGGINS, P.E., The Radiochemistry of Polonium, NAS-NS 3037, National Academy
of Sciences, Washington, DC (1961).
[3.7] BAGNALL, K.W., The chemistry of polonium, Radiochim. Acta 32 (1983) 153–161.
[3.8] BROOKINS, D.G., Eh-pH Diagrams for Geochemistry, Springer-Verlag, Berlin (1988).
[3.9] SWARZENSKI, P.W., McKEE, B.A., SØRESEN, K., TODD, J.F.,
210
Pb and
210
Po,
manganese and iron cycling acoss the O
2
/H
2
S interface of a permanently anoxic fjord:
Framvaren, Norway, Mar. Chem.
67 (1999) 199–217.
[3.10] ULRICH, H.J., DEGUELDRE, C., The sorption of
210
Pb,
210
Bi, and
210
Po on
montmorillonit: A study with emphasis on reversibility aspects and on the effect of the
radioactive decay of adsorbed nuclides, Radiochim. Acta
62 (1993) 81–90.
[3.11] UPCHURCH, S.B., OURAL, C.R., FOSS, D.W., BROOKER, H.R., Radiochemistry
of Uranium-Series Isotopes in Groundwater, Technical Report
PB-98-126253/XAB,
Univ. South Florida, Tampa, FL
(1991).
[3.12] SEILER, R.L., STILLINGS, L.L., CUTLER, N., SALONEN, L., OUTOLA, I.,
Biogeochemical factors affecting the presence of
210
Po in groundwater, Appl.
Geochem.
26 (2011) 526–539.
[3.13] BERWANGER, D.J., DEJONG, G.I., WILES, D.R., The release of polonium-210
upon the recrystallization of Ba(Ra)SO
4
, Water, Air, Soil Pollut. 27 (1986) 77–84.
[3.14] LaROCK, P., HYUN, J.-H., BOUTELLE, S., BURNETT, W.C., HULL, C.D., Bacterial
mobilization of polonium, Geochim. Cosmochim. Acta
60 (1996) 4321–4328.
[3.15] MORROW, P.E., DELLA ROSA, R.J., CASARETT, L.J., MILLER, G.J.,
Investigations of the colloidal properties of polonium-210 solutions by using molecular
filters, Radiat. Res. Suppl.
5 (1964) 1–15.
[3.16] BACON, M.P., BREWER, P.G., SPENCER, D.W., MURRAY, J.W., GODDARD, J.,
Lead-210, polonium-210, manganese and iron in the Cariaco Trench, Deep Sea Res.
Part
A 27 (1980) 119–135.
[3.17] BALISTRIERI, L.S., MURRAY, J.W., PAUL, B., The geochemical cycling of stable
Pb,
210
Pb, and
210
Po in seasonally anoxic Lake Sammamish, Washington, USA,
Geochim. Cosmochim. Acta
59 (1995) 4845–4861.
[3.18] SEILER, R.L.,
210
Po in Nevada groundwater and its relation to gross alpha radioactivity,
Ground Water
49 (2011) 160–171.
[3.19] MARKOSE, P.M., Adsorption of natural radionuclides on pyrolusite, Water, Air, Soil
Pollut.
35 (1987) 381–386.
32
CHAPTER 3
[3.20] HANSEN, W.R., WATTERS, R.L., Unsupported
210
PoO
2
in soil: Soil adsorption and
characterization of soil solution species, Soil Sci.
112 (1971) 145–155.
[3.21] VANDENHOVE, H., GIL-GARCIA, C., RIGOL, A., VIDAL, M., New best estimates
for radionuclide solid–liquid distribution coefficients in soils — Part
2: Naturally
occurring radionuclides, J. Environ. Radioact.
100 (2009) 697–703.
[3.22] INTERNATIONAL ATOMIC ENERGY AGENCY, Handbook of Parameter Values
for the Prediction of Radionuclide Transfer in Terrestrial and Freshwater Environments,
Technical Reports Series No.
472, IAEA, Vienna (2010).
[3.23] CARVALHO, F.P., Distribution, cycling and mean residence time of
226
Ra,
210
Pb and
210
Po in the Tagus estuary, Sci. Total Environ. 196 (1997) 151–161.
[3.24] CARVALHO, F.P., OLIVEIRA, J.M., ALBERTO, G., Factors affecting
210
Po and
210
Pb
activity concentrations in mussels and implications for environmental bio-monitoring
programmes, J. Environ. Radioact.
102 (2011) 128–137.
[3.25] DURAND, J.P., et al.,
210
Po binding to metallothioneins and ferritin in the liver of
teleost marine fish, Mar. Ecol. Prog. Ser.
177 (1999) 189–196.
[3.26] STEWART, G.M., FISHER, N.S., Experimental studies on the accumulation of
polonium-210 by marine phytoplankton, Limnol. Oceanogr. 48 (2003) 1193–1201.
[3.27] WILDGUST, M.A., McDONALD, P., WHITE, K.N., Temporal changes of
210
Po in
temperate coastal waters, Sci. Total Environ.
214 (1998) 1–10.
[3.28] WILDGUST, M.A., McDONALD, P., WHITE, K.N., Assimilation of
210
Po by the
mussel Mytilus edulis from the alga Isochrysis galbana, Mar. Biol.
136 (2000) 49–53.
[3.29] STEWART, G., et al., Exploring the connection between
210
Po and organic matter in
the northwestern Mediterranean, Deep Sea Res. Part
I 54 (2007) 415–427.
[3.30] JEFFREE, R.A., SZYMCZAK, R., Enhancing effect of marine oligotrophy on
environmental concentrations of particle-reactive trace elements, Environ. Sci.
Technol.
34 (2000) 1966–1969.
[3.31] HUSSAIN, N., FERDELMAN, T.G., CHURCH, T.M., LUTHER G.W., III,
Bio-volatilization of polonium: Results from laboratory analyses, Aquat. Geochem.
1
(1995)
175–188.
[3.32] MOMOSHIMA, N., SONG, L.-X., OSAKI, S., MAEDA, Y., Formation and emission
of volatile polonium compound by microbial activity and polonium methylation with
methylcobalamin, Environ. Sci. Tech.
35 (2001) 2956–2960.
[3.33] MOMOSHIMA, N., SONG, L.-X., OSAKI, S., MAEDA, Y., Biologically induced Po
emission from fresh water, J. Environ. Radioact.
63 (2002) 187–197.
[3.34] MOMOSHIMA, N., FUKUDA, A., ISHIDA, A., YOSHINAGA, C., Impact of
microorganism on polonium volatilization, J. Radioanal. Nucl. Chem.
272
(2007)
413–417.
[3.35] MATTHEWS, K.M., KIM, C.-K., MARTIN, P., Determination of
210
Po in
environmental materials: A review of analytical methodology, Appl. Radiat. Isot.
65
(2007)
267–279.
[3.36] INTERNATIONAL ATOMIC ENERGY AGENCY, A Procedure for the Determination
of Po-210 in Water Samples by Alpha Spectrometry, IAEA Analytical Quality in
Nuclear Applications Series No.
12, IAEA, Vienna (2009).
33
PROPERTIES
[3.37] KIM, C.-K., MARTIN, P., FAJGELJ, A., Quantification of measurement uncertainty in
the sequential determination of
210
Pb and
210
Po by liquid scintillation counting and
alpha-particle spectrometry, Accredit. Qual. Assur.
13 (2008) 691–702.
[3.38] KIM, C.-K., LEE, M.H., MARTIN, P., Method validation of a procedure for
determination of
210
Po in water using DDTC solvent extraction and Sr resin, J.
Radioanal. Nucl. Chem.
279 (2009) 639–646.
[3.39] MARTIN, P., HANCOCK, G.J., Routine Analysis of Naturally Occurring
Radionuclides in Environmental Samples by Alpha-Particle Spectrometry, Supervising
Scientist Report 180, Supervising Scientist, Darwin (2004).
[3.40] FLYNN, W.W., The determination of low levels of polonium-210 in the environmental
materials, Anal. Chim. Acta
43 (1968) 221–227.
[3.41] EHINGER, S.C., PACER, R.A., ROMINES, F.L., Separation of the radioelements
210
Pb–
210
Bi–
210
Po by spontaneous deposition onto noble metals and verification by
Cherenkov and liquid scintillation counting, J. Radioanal. Nucl. Chem.
98
(1986) 39–48.
[3.42] HENRICSSON, F., RANEBO, Y., HOLM, E., ROOS, P., Aspects on the analysis of
210
Po, J. Environ. Radioact. 102 (2011) 415–419.
[3.43] BAGNALL, K.W., The Chemistry of Selenium, Tellurium and Polonium, Elsevier,
Amsterdam
(1966).

35
Chapter 4
OCCURRENCE AND CYCLING OF
210
Pb AND
210
Po
IN THE ENVIRONMENT
This chapter provides a general overview of the occurrence, behaviour and
cycling of
210
Pb and
210
Po in the environment and covers some major concepts
and pathways (see Fig.
4.1). The data presented give a feeling for the orders of
magnitude of activity concentrations which can be expected in environmental
media and the relative importance of various pathways. However, the
information is not comprehensive, nor does it provide details of the mechanisms
involved. Environmental media and pathways are discussed in more detail in
Chapters 5–10, including data on activity concentrations, transfer parameters and
fluxes.
4.1. OCCURRENCE OF
210
Pb AND
210
Po IN ROCK AND SOIL
The source of virtually all
210
Pb and
210
Po in the environment is
238
U in the
Earth’s crust. In unweathered rocks,
210
Pb and
210
Po are expected to be in secular
equilibrium with
238
U. A measure of typical natural concentrations is given by the
composition of the upper continental crust (see Table 4.1), which has an average
concentration of uranium of 2.7 ppm or 33 Bq/kg [4.2].
TABLE 4.1. TYPICAL URANIUM CONCENTRATIONS
IN VARIOUS ROCKS
Rock type U (ppm)
238
U (Bq/kg)
Basalts 0.1–1 1–12
Black shale 3–1 250 37–15 000
Gneiss 2 25
Limestone 2 25
Phosphates 50–300 620–3 700
Schist 2–5 25–62
Silicic rocks (granites, dacites) 2.2–6.1 27–75
Source: See Ref. [4.1].

36
CHAPTER 4
FIG. 4.1. Occurrence and cycling of
210
Pb and
210
Po in the environment.
37
OCCURRENCE AND CYCLING IN THE ENVIRONMENT
Three major processes which transfers
210
Pb and
210
Po out of primary
sources in the crust include the following:
(a) Weathering of exposed rocks at the Earth’s surface;
(b) Leaching or dissolution of rocks by groundwater;
(c) Volcanic eruptions.
Surface weathering and subsequent geomorphological and geochemical
processes result in the formation of soils and sediments. These processes can
modify the concentration of uranium and separate the radionuclides within
the uranium series decay chain. The result can be disequilibrium between the
radionuclides (see Section 3.1.2). Surface soils are the interface between the
atmosphere and the subsurface hydrological system, and there are radionuclide
fluxes between these environmental compartments. Secular equilibrium for the
238
U decay chain of radionuclides in soils cannot be assumed, although the degree
of disequilibrium is not large in the majority of cases.
One mechanism which causes disequilibrium in the uranium decay chain
is exhalation of
222
Rn from surface soils to the atmosphere. This subsequently
decays to
210
Pb, which is then deposited to the Earth’s surface. Exhalation of
222
Rn results in a deficiency of
210
Pb relative to
226
Ra in the topsoil (primarily
the top 1 m), and deposition results in an excess. Approximately 90% of
210
Pb
deposition is by wet deposition and greatly depends on the rainfall [4.3, 4.4]. A
confounding factor is
210
Pb in leaf litter, which can lead to excess
210
Pb in the
surface layer [4.5, 4.6]. Since exhalation flux densities of
222
Rn from soils are
about a hundred times greater than those from oceans, which cover more than
70% of the planet,
210
Pb to oceans will result in deficiency of
210
Pb relative to
226
Ra in soils.
Polonium-210 is at, or very close to, secular equilibrium with
210
Pb in
almost all soils [4.7]. Its concentrations in surface soil vary considerably, and
Table 4.2 presents data from studies over the last forty years [4.8]. In areas of
normal radiation background, the concentrations most commonly lie within the
range of 10–200 Bq/kg in the absence of anthropogenic influences [4.7, 4.23].
The upper ranges are mainly due to areas of uranium mining.
The concentrations of
210
Po in surface soils vary across a wide range,
depending on the uranium concentration in the bedrock of the area, the depth
of the soil sampled, climatic conditions and soil properties
[4.10, 4.15, 4.22]. In
addition, phosphate fertilizers commonly contain significant concentrations of
210
Pb and
210
Po [4.24, 4.25].

38
CHAPTER 4
4.2. OCCURRENCE OF
210
Pb AND
210
Po IN THE ATMOSPHERE
The primary source of
210
Pb in the atmosphere is
222
Rn exhalation from soil
and its subsequent decay via short lived radon progeny. This has been estimated
to give rise to a
210
Pb flux to the atmosphere of 3.5 × 10
16
Bq/a, while fluxes
from other sources are more than one order of magnitude lower [4.4]. Much of
this
210
Pb is removed from the atmosphere by wet and dry deposition, but that
fraction which does not deposit decays via
210
Bi to
210
Po, giving a flux from this
source of 1.05 × 10
15
Bq/a.
Volcanic eruptions are a major source of
210
Po in the atmosphere due to
the volatility of polonium at elevated temperatures. An early study estimates
the average flux to be 2.4 × 10
15
Bq/a for
210
Po, but only 6.0 × 10
13
Bq/a for
210
Pb [4.26]. Compared to
222
Rn exhalation from soil, volcanic eruptions are a
highly variable source, with relatively large year to year variations.
Other sources include
222
Rn exhalation from the oceans (see Section 5.2.1),
resuspension of soils (see Section 5.4.1), forest and savanna fires (see
TABLE 4.2. CONCENTRATIONS OF
210
Po IN TOPSOIL
Origin
210
Po
(Bq/kg, DW)
Ref.
Brazil 27–74 [4.9
a
]
Canada 20–22 000 [4.7, 4.10
a
]
Germany 11–210 [4.11–4.13
b
]
India 4–220 [4.14–4.16]
Malaysia 8 [4.17]
Spain 16–780 [4.18
c
]
United Kingdom 51 [4.19]
USA 10–60
70–15 000
[4.14, 4.20]
[4.21
a
]
Worldwide 8–220 [4.22]
Source:
See Ref. [4.8].
a
Near a uranium mine.
b
Near a coal plant.
c
Near a phosphate factory.
39
OCCURRENCE AND CYCLING IN THE ENVIRONMENT
Section IV.2) and the combustion of fossil fuels. Globally, these contribute less
than 10% of the total fluxes of
210
Pb and
210
Po in the atmosphere [4.4], although
they are sometimes important on a local or regional scale.
Both
210
Pb and
210
Po are attached to aerosol particles in the atmosphere.
Radioactive decay is only a minor contributor to their removal from the
atmosphere due to their relatively short residence times. The primary removal
pathway is wet deposition to the Earth’s surface (see Section 5.3). A secondary
pathway is dry deposition to the surface, which accounts for about 10–15%
of total deposition for
210
Pb [4.3, 4.4]. The annual, total deposition flux of
210
Pb ranges from several Bq·m
−2
·a
−1
(as observed in the Antarctic) to several
hundred Bq·m
−2
·a
−1
.
The distribution of
210
Pb and
210
Po in the atmosphere is complex due to the
spatial and temporal variability in the sources, in wet and dry deposition fluxes,
and in atmospheric dispersion processes. The highest
210
Pb concentrations in
surface air are observed in the subtropical and temperate latitudes of the northern
hemisphere owing to the relatively large land masses there. Average annual
concentrations in this region mainly lie in the range of 0.2–1 mBq/m
3
[4.27].
Between the tropics, values are generally in the range of 0.1–0.5 mBq/m
3
,
while south of 30°S, they quickly trend down to less than 0.1 mBq/m
3
, which
is consistent with the radon exhalation flux density trends discussed above.
These data are predominantly for continental locations, where higher surface air
concentrations are on the account of greater
222
Rn exhalation fluxes from soils
than oceans.
There is also clear evidence of a seasonality effect in
210
Pb and
210
Po
concentrations in surface air [4.28–4.31]. Concentrations are normally higher
during dry seasons due to higher exhalation of
222
Rn from the soil, greater
resuspension of soil dust and lower wet deposition rates, which can vary locally.
The vertical distribution of
210
Pb in the troposphere is highly variable.
Although mean vertical profiles of
222
Rn generally show a strong reduction in
concentration from the surface to the upper troposphere of a factor of 100 over
continental areas, for
210
Pb this reduction is generally less than a factor of 5,
and the concentration at the upper troposphere can be higher than in the lower
troposphere [4.32, 4.33]. The reason for this is scavenging of
210
Pb by wet and
dry deposition in the lower troposphere and by precipitation from the mid-layers.
The mean residence time for
210
Pb in the troposphere is about five days [4.28],
but it is significantly longer than this in the upper troposphere, resulting in higher
210
Po:
210
Pb ratios than in the lower and mid-level troposphere.
The geographical distributions of
210
Pb and
210
Po concentrations in
rainwater mostly follow those in surface air. The main factors governing
radionuclide concentrations in rainwater are the size of the aerosols containing
210
Pb and
210
Po, and the type and duration of rainfall [4.34]. Values for
210
Pb
40
CHAPTER 4
concentration in rainwater vary considerably at different locations, being most
commonly in the range of 10–1000
mBq/m
3
with
210
Po:
210
Pb activity ratios in the
range of 0.05–0.5.
In the stratosphere,
210
Pb and
210
Po are present due to the input of
210
Pb
and
222
Rn from stratosphere–troposphere exchanges, and the relatively long
(approximately a year) stratospheric residence times.
4.3. OCCURRENCE OF
210
Pb AND
210
Po IN TERRESTRIAL
ECOSYSTEMS
4.3.1.
210
Pb and
210
Po in plants
Terrestrial plants receive
210
Pb and
210
Po from three principal sources:
(a) Radioactive decay from
222
Rn already accumulated by the plant;
(b) Root uptake from the soil;
(c) Foliar uptake of
210
Pb and
210
Po deposited on the leaves.
It is generally believed that foliar uptake dominates for
210
Po, which is thus
accumulated primarily by absorption and further translocation within the plant
(see Ref.
[4.35]). However, the relative importance of the root and foliar uptake
pathways depends on the concentration of the radionuclides in the soil, the soil–
plant concentration ratio (CR), and the rate of deposition onto plant parts above
ground.
Another important consideration is that in crops such as roots, tubers,
cereals, nuts and legumes whose edible portion is protected by inedible plant parts,
activity concentrations should not be significantly affected by direct deposition.
In contrast, observed concentrations for leafy vegetables may be up to 3–5
times
higher due to deposition effects. Therefore, there can be a large variation in the
radionuclide concentrations in plants, resulting from differences in radionuclide
accumulation in different plant species, variable tissue distributions, climate
variations and soil properties.
Sheppard et al. [4.7] report activity concentrations in plants in Canada in
the range of 1–730
Bq/kg (DW) for
210
Pb and 0.3–460 Bq/kg (DW) for
210
Po.
Data of the same order of magnitude were reported in 1976 for
210
Pb and
210
Po
concentrations in Russian plants by Ladinskaya et
al. [4.36].
Based on a wide review in 1990 of the data available for terrestrial
plants
[4.22, 4.36–4.39], Taskayev and Testov [4.40] report a mean value
of 11 Bq/kg
(DW) with a range of 0.04–111 Bq/kg (DW) for
210
Po activity
concentrations in grassy plants sampled in areas of normal background radiation.

41
OCCURRENCE AND CYCLING IN THE ENVIRONMENT
Activity concentrations of
210
Pb and
210
Po in areas of high natural background are
found to be typically two to three orders of magnitude higher compared to normal
areas. Overall, these estimates are in agreement with assessments provided in
the 2004 review on
210
Po environmental behaviour performed by Coppin and
Roussel-Debet
[4.8] (see Table 4.3).
TABLE 4.3. CONCENTRATIONS OF
210
Po IN TERRESTRIAL PLANTS
Country Plant
Activity conc. of
210
Po
(Bq/kg, DW)
Ref.
Areas undisturbed by humans
Germany Grass and hay 1.1–29.6 [4.12]
Grass
Heather
Juniper
Blueberry
22–160
20–88
20–38
34
[4.11]
India Tobacco 0.1–3.3 [4.14]
Portugal Vegetables
Fruits
Trees
0.084
0.06
0.37
[4.41]
United Kingdom Grass
Lichens
6.5–29
290–370
[4.37]
World data Grass 2.0–35 [4.42]
Tobacco 5.6–57 [4.22]
Areas disturbed by humans
Brazil, uranium mine at 1 km Vegetables 1–8 [4.9]
Canada, uranium mine at Key Lake Spruce needles
Labrador tea
84
1300
[4.10]
Spain, near phosphoric acid factory
Spartina spp.
5.1–40.6 [4.18]
United Kingdom, Cotswolds Grass 4–25 [4.43]
Source: See Ref. [4.8].
Note: The activity concentrations for Portugal are based on fresh weight.

42
CHAPTER 4
4.3.2.
210
Pb and
210
Po in animals
Overall, the distributions of
210
Pb and
210
Po in animals, and their activity
concentrations in different animal tissues reflect their intake with feed. The
activity concentrations of
210
Po in animals vary across four orders of magnitude
depending on the animal and organs (see Table
4.4). Lead-210 is largely retained
in bones, while
210
Po is distributed mainly in soft tissue. Target tissues for
210
Po
are the spleen, liver and kidneys. The
210
Po:
210
Pb ratio typically exceeds unity
in soft tissue; for example, ratios greater than 100 have been reported for the
muscle tissue of wild boar
[4.51]. However, observed
210
Po:
210
Pb ratios are time
dependent and can be substantially modified by continuing radioactive decay
from
210
Pb, resulting in support for
210
Po in older animals.
TABLE 4.4. CONCENTRATIONS OF
210
Po IN ANIMAL TISSUES
Tissue
210
Po activity conc.
(Bq/kg, FW)
Ref.
Meat
Mutton
Beef and pork
0.11–0.43
0.037–2.0
[4.44, 4.45]
[4.46]
Liver
Beef, lamb and mutton
Chicken
Caribou
0.15–120
0.21–1.03
332
[4.11, 4.37, 4.42, 4.47]
[4.48]
[4.10]
Kidney
Beef, lamb and mutton
0.74–67
[4.37, 4.42, 4.47, 4.49]
Gizzards
Chicken
0.48
[4.48]
Offal 0.19–37
6.2 × 10
−2*
[4.22, 4.50]
Milk (0.33–6.7) × 10
−2
[4.12, 4.22]
Eggs 0.11–37 [4.46, 4.48, 4.50]
Source: See Ref. [4.8].
*
Prepared for consumption.
43
OCCURRENCE AND CYCLING IN THE ENVIRONMENT
4.4. OCCURRENCE OF
210
Pb AND
210
Po IN MARINE ECOSYSTEMS
4.4.1.
210
Pb and
210
Po in marine water
Polonium enters the marine environment by deposition from the atmosphere
at the ocean surface, from the in
situ radioactive decay of soluble
226
Ra and from
the decay of
222
Rn gas exhaled from the sea-floor. It also enters the coastal seas
with river water and sediment discharges, which includes a component from
anthropogenic discharges. The resulting
210
Po concentrations in sea water are
largely dependent on the magnitude and distribution of these sources, on
210
Po
binding to suspended particulate matter and on particle scavenging in the water
column.
Polonium-210 in coastal waters is largely associated with suspended
particulate matter (around 70–80%), probably due to higher suspended loads
and intensive mixing in coastal waters than in open ocean waters. Lead-210 in
coastal sea water is also mainly associated with the suspended matter, although
to a lesser extent than
210
Po. The concentrations of these radionuclides in coastal
waters do not show a significant seasonal variation, and thus the atmospheric
depositions do not significantly modify those concentrations.
In open waters, the ocean is stratified and vertical mixing is reduced.
Within the water column,
210
Po is produced from radon decay. Given that radon
concentrations in the water column increase from a minimum in the surface
layer to a maximum in deep-sea water, and the radon distribution is mainly
dependent on diffusion in the water column and marine currents, surface water
concentrations of
210
Po in the open ocean are generally low.
Deposition from the atmosphere results in an excess of
210
Pb over
226
Ra in
the surface layer of the ocean. Absorption of
210
Pb and
210
Po onto particulates
and uptake by phytoplankton and zooplankton generates a downward flux of
particulate associated activity. Although these particles are, in part, recycled in
intermediate ocean layers, in their settling pathway through the deeper ocean
layers, they adsorb and remove soluble
210
Pb and
210
Po from the water column,
thus creating an imbalance between
226
Ra and its progeny. Some of the biogenic
particulate materials generated in the upper layer of the ocean may reach the
deep-sea (abyssal) floor and add
210
Pb and
210
Po to the top sediment layer. The
210
Pb flux arriving at the abyssal sea-floor is around double the
210
Pb atmospheric
flux entering the oceans at the surface. The mean residence times for
210
Po in
ocean water layers have been calculated to be around 6–12 months in the upper
layer and somewhat longer, around 2 years, in the deep-sea layers [4.52, 4.53].
44
CHAPTER 4
4.4.2.
210
Pb and
210
Po in marine organisms
The isotopes
210
Pb and
210
Po have been well studied in marine wildlife
owing to their relatively high concentrations in comparison with those in
terrestrial organisms. Currently known
210
Pb and
210
Po activity concentrations in
marine biota cover several orders of magnitude. With a few exceptions in the
case of abyssal fauna,
210
Po is in excess over
210
Pb. Reported
210
Po:
210
Pb ratios
are mostly in the range of 1–100
[4.54]. Carvalho [4.54] finds that organisms
occupying upper trophic levels (carnivores and top predators) generally display
lower
210
Po concentrations than planktivorous organisms (primary herbivores).
Furthermore, related species occupying equivalent ecological niches in different
ecosystems (e.g. filter feeding bivalves in the intertidal zone and in the abyssal
sea-floor) display similar
210
Po activity concentrations and
210
Po:
210
Pb ratios.
For higher trophic level organisms, such as shrimp, molluscs and fish, the
primary source of
210
Po uptake is from ingested food, with very little uptake
from water
[4.55–4.58]. Inside these organisms, the distribution of
210
Po is not
homogeneous, and the tissues and organs with higher concentrations are those
associated with the digestive system.
Concentrations of
210
Pb do not increase from phytoplankton to copepods
as much as
210
Po, and the
210
Pb transfer to planktivorous fish, such as sardines,
is less efficient than
210
Po transfer. Consequently, the
210
Po:
210
Pb ratio in marine
food webs appears to increase with the trophic level, at about 10 in phytoplankton
and zooplankton, 3–10 in herbivorous fish, 50–100 in piscivores (carnivorous
fish) and 200 in the muscle of marine mammals (top predators)
[4.59].
4.5. OCCURRENCE OF
210
Pb AND
210
Po IN FRESHWATER
ECOSYSTEMS
4.5.1.
210
Pb and
210
Po in groundwater
Rainwater infiltrating the ground interacts with soils, sediments and
rocks, and so it can accumulate polonium from a number of sources. This
includes polonium produced from naturally occurring sources within soils in the
unsaturated zone, polonium that has been naturally deposited onto the surface
from atmospheric sources, and anthropogenic sources. These sources add to the
polonium carried in the rainwater as wet deposition. Polonium is also available
from progenitor nuclides in the soils and is derived from similar sources. The
infiltrating waters then recharge the underlying groundwater systems.
Weathering of uranium bearing mineral phases will not normally be a major
source term for
210
Pb and
210
Po in groundwater, as it typically occurs at rates
45
OCCURRENCE AND CYCLING IN THE ENVIRONMENT
that are very long compared to the decay rate of the nuclides. Rather, they are
primarily available to the groundwater phase due to recoil following alpha decay
of progenitor atoms in the uranium decay series (see Sections
3.1.2 and 7.2). This
can take place from within the mineral grains, from absorption sites on grain
surfaces, and from within the groundwater phase.
Lead and polonium are both strongly particle reactive under most conditions
pertaining to groundwater. Consequently, the largest fraction of activity released
from mineral grains is adsorbed onto the aquifer rocks. The distribution of lead
and polonium in groundwater is therefore strictly controlled by the adsorption
characteristics of the surrounding minerals. Groundwater
210
Po concentrations
are typically in the range of 1 –30 mBq/L, although values of up to 19 Bq/L have
been recorded for brines.
The strong association of
210
Po with mineral surfaces and particles, and its
short half-life, means that significant transport of activity to a significant distance
from the site of production is unusual and measured concentrations generally
reflect the local supply rates and bulk adsorption coefficients. The study of
210
Po
in groundwater is therefore primarily of interest from a human health perspective
when the water is to be used for drinking or irrigation, and as a means of studying
geochemical processes.
Groundwater discharge into surface waters, such as lakes, rivers and
wetlands, as well as into coastal waters. These discharges can carry polonium,
although the concentrations may be modified at the sediment–water interface.
The relationship between polonium in groundwater and surface waters has not
been studied in detail.
4.5.2.
210
Pb and
210
Po in surface water and sediments
The freshwater environment encompasses lotic (moving) water, such as
rivers and streams, and standing water bodies (lentic), such as lakes, ponds,
wetlands and bogs. The concentrations of
210
Po within moving water (e.g. a river)
would be expected to reflect the environmental conditions in the catchment area
as well as both natural and anthropogenic sources. The concentrations throughout
the year will vary depending on variable precipitation carrying particulates from
the surrounding areas to the water body.
The levels of
210
Po in surface water in freshwater systems are influenced
by in
situ decay, fluvial inputs and atmospheric deposition. Within a standing
water body (e.g. a lake), there is uptake of
210
Pb and
210
Po by particulates
(particularly biomass) in the water column. As these particulates settle to the
bottom sediments, polonium is scavenged from the water column. Polonium has
also been shown to become volatile in both fresh and marine waters by the action
of microorganisms
[4.60].
46
CHAPTER 4
The levels of
210
Pb and
210
Po in sediment are influenced by the cycling of
iron and manganese as the redox conditions change in the sediment. Overall,
a portion of the lead and polonium in sediment may be reintroduced into the
water column by diffusive processes; the remainder becomes buried by continual
deposition of fresh settling matter. In addition to cycling in sediment, depending
on the characteristics of the lake, the bottom layer within the water column may
become anoxic during portions of the year, which will influence the levels of
polonium in the water column. During the anoxic period, levels of
210
Pb and
210
Po can be significantly increased in the hypolimnion; due to differences in the
cycling, the
210
Po:
210
Pb ratio can be greater than one.
The activity concentrations of
210
Po in oxic waters are mostly in the range
of 1–5
mBq/L and are higher, up to 17 mBq/L, in seasonally anoxic ponds [4.61].
On a local scale, there can be a large range of typical values.
4.5.3.
210
Pb and
210
Po in freshwater organisms
The levels of
210
Po have been measured in freshwater biota, but there are not
as many data available as for the marine environment. When compared to marine
biota, a similar pattern in freshwater biota is observed: high concentrations in
plankton and lower concentrations in higher trophic biota. The distribution of
210
Po within an organism shows higher concentrations in the kidneys, liver and
organs of the digestive system. A higher accumulation of
210
Po is found in the
soft tissue of molluscs than in shells, whereas
210
Pb has been detected in higher
levels in the shells. In the soft tissue of an organism,
210
Po is typically found at
levels greater than
210
Pb [4.51].
Whole organism CRs for lead (FW) range from approximately
5 (amphibians) [4.62] to as high as 6000 (bivalve molluscs) [4.63]; CRs for
polonium are generally higher, from 2000 (fish and vascular plants) up to
1.3 × 10
5
(bivalves) [4.64, 4.65]. Lead and polonium CRs for wildlife groups in
freshwater ecosystems can be found in Table 7.3, Section 7.3.5.
4.6. OCCURRENCE OF
210
Pb AND
210
Po IN FOOD
(INCLUDING SEAFOOD) AND DRINKING WATER
The consumption of food and drinking water is the most important exposure
pathway for
210
Pb and
210
Po for humans. Data on activity concentrations of
210
Pb and
210
Po in drinking water for different countries are given in Table 4.5.
Mean activity concentrations of
210
Po in drinking water are in the range of
0.04–7600
mBq/L, and for most regions the upper mean value is less than
4
mBq/L. Furthermore,
210
Po concentrations can vary by more than four orders of

47
OCCURRENCE AND CYCLING IN THE ENVIRONMENT
magnitude because of the diversity of water sources. High activity concentrations
of
210
Po are found in areas of high radiation background in Finland (up to
7600
mBq/L), while the lowest values are typical for public water supply systems
in countries with efficient water quality control systems, such as Italy, where the
concentrations of
210
Po in tap water are in the range of 0.1–5.9 mBq/L. Activity
concentrations of
210
Pb in drinking water are generally two to fivefold higher
than those measured for
210
Pb. The mean value for most countries is in the range
of 1.5–26.9
mBq/L.
TABLE 4.5. ACTIVITY CONCENTRATION OF
210
Pb AND
210
Po IN
DRINKING WATER
Country Origin
210
Pb (mBq/L)
210
Po (mBq/L)
Mean Range Mean Range
Brazil Mineral water 66 20–102 —
a
—
a
Czech Republic Public supply —
a
<40–350 9 <2–71
Finland Potable water —
a
0.2–21 000 —
a
0.2–7 600
Germany Potable water 1.5 0.2–170 0.5 0.1–40
India (Goa) Potable water —
a
2.2–11.5 —
a
1.7–7.0
Italy Tap water
Mineral water
—
a
—
a
—
a
0.13–5.9
<0.04–24
Poland Potable water 1.6 —
a
0.5 —
a
Portugal Public supply 18.5 2–390 —
a
—
a
Romania Water supply —
a
7.0–44 —
a
7.0–44
Russian Federation Water supply 26.9 2.3–68 2.72 1.2–7.1
Spain Public supply 18.7 0.23–59 3.63 1.8–19.3
United Kingdom Public supply —
a
40–200 —
a
—
a
USA Potable water —
a
0.1–1.5 —
a
—
a
Source: See Ref. [4.66].
a
—: data not available.

48
CHAPTER 4
Concentrations of
210
Pb and
210
Po in drinking water vary over a wide range
because of variations in the concentrations of uranium and its long lived progeny,
such as
226
Ra and
230
Th, in adjacent rocks, soil, atmosphere and groundwater.
High levels of
210
Pb and
210
Po have been found to be associated with regions of
uranium or thorium ore bodies. Mining and processing of uranium and other
minerals, and geothermal springs also lead to elevated concentrations of
210
Pb
and hence
210
Po in the environment.
Concentrations of
210
Pb and
210
Po in foods also vary over a wide range,
reflecting differences in uranium and its progeny in soil, the atmosphere
and water, differing farming practices and local features in food processing
(see Tables 4.6 and 4.7).
TABLE 4.6. CONCENTRATIONS OF
210
Pb AND
210
Po IN FOODS
Product Region Country
210
Pb (mBq/kg)
210
Po (mBq/kg)
Milk products North America USA 11 —
a
Asia China
India
16
—
a
13
15
Europe Germany
Poland
Romania
United Kingdom
5–280
18
10–15
35–88
2–80
16
13–140
20–220
Reference value 15 15
Meat products North America USA 18 —
a
Asia China
India
140
—
a
120
440
Europe Germany
Poland
Romania
United Kingdom
100–1 000
98–105
15–19
40–3 700
37–4 000
99–102
38–110
62–67 000
Reference value 80 60

49
OCCURRENCE AND CYCLING IN THE ENVIRONMENT
TABLE 4.6. CONCENTRATIONS OF
210
Pb AND
210
Po IN FOODS (cont.)
Product Region Country
210
Pb (mBq/kg)
210
Po (mBq/kg)
Grain products North America USA 33–81 —
a
Asia China
India
34
—
a
42
15–120
Europe Germany
Poland
Romania
United Kingdom
40–4 000
110–160
49–59
56–120
37–1 900
90–140
20–360
27–260
Reference value 50 60
Leafy vegetables North America USA 41 —
a
Asia China
India
360
—
a
430
320
Europe Germany
Poland
United Kingdom
4–4 100
43–51
16–3 300
4–7 400
40–67
37–3 300
Reference value 80 100
Root vegetables
and fruits
North America USA 8–150 —
a
Asia China
India
27
—
a
29
16–140
Europe Germany
Poland
Romania
United Kingdom
20–4 900
24–93
19–44
18–76
22–5 200
28–210
12–140
—
a
Reference value 30 40
Fish products North America USA 14–1 800 150–55 000
Europe Germany
Poland
Portugal
United Kingdom
20–4 400
81–93
—
a
180–4 800
50–5 200
3 100–3 800
80–120 000
60–53 000
Reference value
200 2 000
Source: See Ref. [4.67].
a
—: data not available.

50
CHAPTER 4
The lowest ranges in
210
Pb and
210
Po concentrations are in milk products.
Two to sixfold higher concentrations were observed in meat and plant products,
where root vegetables had slightly lower mean
210
Pb and
210
Po concentrations
compared with other plant products. The highest concentrations were found
to be in fish products, and leafy vegetables demonstrated
210
Pb concentrations
higher than in grain products. High
210
Po concentrations are consistently found
TABLE 4.7. CONCENTRATIONS OF
210
Pb AND
210
Po IN MUSCLES OF
COMMON SEAFOOD
Species Body weight
(g)
No. of
samples
210
Po
(Bq/kg, FW)
210
Pb
(Bq/kg, FW)
Sardine 51 4 66 ± 2 1.0 ± 0.02
Anchovy 5.5 6 9.4 ± 0.3 0.42 ± 0.01
Mackerel 120 4 19 ± 1 0.63 ± 0.4
Horse mackerel 200 2 5.2 ± 0.2 0.1 ± 0.02
Bigeye tuna 31 × 10
3
1 3.1 ± 0.09 0.46 ± 0.02
Hake 256 2 6.7 ± 0.3 0.15 ± 0.01
Red sea bream 275 1 2.4 ± 0.09 0.84 ± 0.02
Common sole 150 1 1.4 ± 0.04 0.14 ± 0.01
Skate 894 1 0.73 ± 0.03
0.12 ± 0.000 4
Squid 2 103 1 1.6 ± 0.04 0.41 ± 0.01
Common shrimp —
a
12 49 ± 1.5 1.1 ± 0.03
Clam —
a
6 152 ± 19 2.9 ± 0.1
Cockle —
a
5 9.4 ± 3 1.32 ± 0.06
Mussel —
a
12 132 ± 5 2.6 ± 0.1
Source: See Ref. [4.68].
Note: All samples were taken from the north-eastern Atlantic Ocean, off Portugal.
a
—: data not available.
51
OCCURRENCE AND CYCLING IN THE ENVIRONMENT
in seafood products, and with
210
Po:
210
Pb ratios much higher than unity, usually
2–100
[4.67–4.69].
The great importance of
210
Po to dietary intake is found in many coastal
countries, such as Japan, the Marshall Islands, Portugal and South Africa [4.67]. A
global review of
210
Po in marine food suggests that representative concentrations
are 2.4 Bq/kg in fish, 6.0 Bq/kg in crustaceans and 15.0 Bq/kg in molluscs [4.70].
Consumption of seafood varies widely, both between countries and within a
single country. In addition,
210
Pb and
210
Po concentrations in seafood species vary
within three orders of magnitude (see Table 4.7). If representative consumption
rates are 13 kg/a of fish and 1 kg/a each of molluscs and crustaceans, the intake
of
210
Po with these foods would be 52 Bq/a [4.67].
If there are processing or distribution delays for fish products between catch
and consumption, the activity intake will be reduced owing to the radioactive
decay of
210
Po. Statistics quoted by Aarkrog et al. [4.70] indicate that 30% of
seafood is eaten fresh, 30% frozen, 20% smoked and 20% canned. Application
of a correction factor of 0.6 [4.67] suggests an intake of 31 Bq/a in seafood and a
weighted concentration of
210
Po in fish products of 2.1 Bq/kg.
4.7. ANTHROPOGENIC SOURCES OF
210
Po IN THE ENVIRONMENT
4.7.1. Industrial uses of human-made
210
Po
There are a number of industrial uses of
210
Po, in particular as an alpha
emitter to eliminate static in manufacturing environments and to remove dust
under clean conditions. Owing to its short half-life, the
210
Po source needs to be
replaced approximately every twelve months. It has also been investigated as a
heat source for thermoelectric power devices for space applications, since the
energy released by decay is so great (140 W/g).
In nature,
210
Po occurs in extremely low concentrations; even uranium
ores contain less than 0.1 mg/t. However,
210
Po is generated in nuclear reactors
when stable
209
Bi is bombarded with neutrons. Around 100 g of
210
Po is produced
annually, largely at a single facility in the Russian Federation. There have not
been any widely reported releases of
210
Po from such facilities.
Earlier designs of nuclear weapons included a neutron source of beryllium
and polonium, and so weapons programmes required additional production of
210
Po. In the United Kingdom, this took place at Windscale Works, Sellafield. A
nuclear reactor fire there in 1957 led to the environmental release of radionuclides.
In addition to a study published in 1996 on the magnitude of the release of
210
Po
(see Ref. [4.71]), a 2007 study of
137
Cs,
131
I and
210
Po estimated an approximate
release of 40 TBq of
210
Po, with a range of 14–110 TBq [4.72].
52
CHAPTER 4
4.7.2. Environmental release of naturally occurring radioactive material
Naturally occurring
210
Po is continuously released from geological materials
processed for industrial use. In a 1976 study, Moore et
al. [4.73] estimate that
anthropogenic sources could constitute up to 7% of the total
210
Po fluxes in the
atmosphere, while elevated concentrations in soils and waters are found near
related industrial activities.
4.7.2.1. Phosphate fertilizer and phosphogypsum waste
Phosphate rich rocks are extensively mined, largely to produce phosphate
fertilizer. These rocks, especially those of sedimentary origin, can have high
concentrations of uranium and daughter
210
Po. Both the products and waste of these
operations can therefore release radionuclides to the environment
[4.74, 4.75].
Phosphate rocks contain up to 3700
Bq/kg of uranium [4.75], with high
uranium content generally corresponding to high phosphorus content. Physical
processing of ore can increase radionuclide concentrations by up to 300%
[4.76].
During chemical processing, phosphate rock is reacted with sulphuric acid
and converted into phosphoric acid to make fertilizer, while phosphogypsum
is generated as a by-product. While uranium and
210
Pb primarily partition into
the phosphoric acid, much of the
226
Ra and up to 99% of the
210
Po enter the
phosphogypsum
[4.74, 4.77].
Most phosphogypsum is stored in piles (referred to as stacks), although
some is discharged into surface waters. A study in the Netherlands examined the
impact of phosphogypsum discharged by the fertilizer industry into the Rhine
(~1.6
TBq/a) and transported by suspension to the North Sea [4.78]. The
210
Pb
and
210
Po settle with particulates, with some of the
210
Po becoming involved
in biological activity in the upper marine water column (see Chapter
8). The
sediments thus remain a potential source of
210
Po, although much of the
210
Pb and
226
Ra — and hence probably the daughter
210
Po — is trapped within insoluble
fractions
[4.79].
Radionuclides can also escape from processing plants or phosphogypsum
storage stacks. For example, a study around a fertilizer plant in the Syrian Arab
Republic found elevated levels of
210
Po in surrounding soil, water and vegetation,
attributable to deposition of daughter isotopes of
222
Rn escaping from the
facility
[4.80]. A fertilizer production facility along the Tagus estuary, Portugal,
has been found to contribute radionuclides to near-shore sediments through
discharge of process waters and leaching by rainwater of phosphogypsum
piles
[4.81]. Overall, the radiological impact of naturally occurring radioactive
material industries on the European population is dominated by discharges from
fertilizer plants (see Ref. [4.82]).
53
OCCURRENCE AND CYCLING IN THE ENVIRONMENT
The spread of phosphate fertilizer, containing
210
Pb, which decays to
210
Po,
can contribute to the naturally occurring radionuclides in soils. Phosphogypsum,
containing
210
Po, has also been used as a soil amendment for depleted soils in
some countries (see Ref.
[4.83]).
4.7.2.2. Fossil fuel production and combustion
Fossil fuels are often rich in uranium series nuclides, since the precursor
organic material was accumulated under reducing conditions favourable for
incorporation of uranium. Petroleum in the United States of America can contain
0.1–40
Bq/kg of
226
Ra [4.82], presumably with similar
210
Pb and
210
Po activities.
Production waste contains high levels of radium, with on average approximately
18
000 Bq/kg of radium in scale and 2800 Bq/kg of radium in sludge. These
wastes can be significant sources of
210
Pb and
210
Po when discharged, landfilled
or disposed of by land spreading
[4.84]. Waters discharged from production wells
can have high concentrations of
226
Ra, although low levels of
210
Po. For example,
waters from Norwegian wells have up to 16
Bq/L of
226
Ra but only 0.2–6 mBq/L
of
210
Po [4.85].
The behaviour of
210
Po in coal combustion power plants is considered
by Mora et
al. [4.86]. Coal typically has concentrations of 16–52 Bq/kg of
210
Po [4.75], which is volatilized during combustion. More than half of this is
condensed onto fly ash, although some also condenses within the installations,
and a small fraction remains in bottom ash. It is expected that
210
Pb behaves
similarly. Most of the process residues are deposited onto land, although some fly
ash is used in building materials
[4.75].
4.7.2.3. Metal production
One clear potential source of uranium series radionuclides is tailings
from uranium and radium mining sites, where leaching and runoff can disperse
contaminants. As discussed in Chapter 6, lead and polonium are relatively
immobile, except under highly acidic conditions. Radium is also relatively
immobile [4.87], and while uranium is more readily transported, in the absence
of other intermediate radionuclides, there will not be substantial
210
Po ingrowth
over short timescales (see Chapter 3). Therefore, the spread of
210
Po, and of
210
Pb followed by ingrowth of
210
Po, is often through discharge of acidic waters
and waste pile leachates, and the transport of airborne particles and suspended
sediments. The escape of
222
Rn to the atmosphere and subsequent deposition of
daughter nuclides is also an important route for spreading
210
Po (see Chapter 5).
Some of the geochemistry controlling radionuclide behaviour is summarized in
Ref. [4.88]. There have been various documented releases from mine tailings,
54
CHAPTER 4
in particular acute failures of containment structures: major events are listed in
Ref. [4.89] and an example of the release of
210
Po to the environment is presented
in chapter 7 of Ref. [4.90].
Ore processing for other metals can also release
210
Po and other
radionuclides. For example, Baxter et al. [4.91] report that raw materials for a
large tin smelter in the United Kingdom generally contain up to 2 × 10
3
Bq/kg
of
210
Po, and there was considerable loss of
210
Po in stack emissions, although
high concentrations were not found in surrounding soils. The technological
enhancement of radionuclides in primary products or waste materials can occur
during the processing of other metals as well [4.92]. Separation of zirconium
also releases radionuclides from zircon, which contains high concentrations of
uranium [4.93].
4.8. SUMMARY
The isotopes
210
Pb and
210
Po are widely distributed in the Earth’s crust
and soil. As polonium is only ever present at ultratrace levels and lead is usually
present at trace levels, their behaviour is heavily influenced by major ion
chemistry and processes such as adsorption, co-precipitation, and attachment to
colloids in aqueous systems and to aerosols in the atmosphere.
The activity concentrations of
210
Pb and
210
Po in the topsoil vary over a
wide range, with a range in most soils of 10–200 Bq/kg. The
210
Po:
210
Pb ratio in
soil is quite close to unity, implying that they are in secular equilibrium in soils.
Major sources to the atmosphere are decay of
222
Rn following its exhalation
from soils, and volcanic emissions (particularly for
210
Po), although there are
several other minor sources. Removal is primarily by deposition to the Earth’s
surface with precipitation.
For plants,
210
Po:
210
Pb activity ratios are in the range of 0.2–0.7 in above
ground biomass and 0.96–1.3 in roots, indicating different mobilities of
210
Pb and
210
Po in the soil–plant system as well as within plants. A greater variability in
concentrations is observed for animals, both terrestrial and aquatic. Lead-210 is
largely retained in bones, while
210
Po is distributed mainly in soft tissue. Target
tissues for
210
Po are the spleen, liver and kidneys. Relatively high concentrations
of
210
Po have been measured in marine animals, and ingestion of seafood is an
important pathway for radiological dose to humans.
Both lead and polonium are strongly particle reactive under most conditions
pertaining to groundwater, and most of the available activity is adsorbed onto
aquifer rocks. Their distribution is therefore strictly controlled by the adsorption
characteristics of the surrounding minerals. Groundwater
210
Po concentrations
55
OCCURRENCE AND CYCLING IN THE ENVIRONMENT
are typically in the range of 1–30 mBq/L, although values up to 19 Bq/L have
been recorded for brines.
The realistic behaviour of
210
Po in the environment can be described if
based on both predictions of environmental behaviour of
210
Pb as a source and
transfer of
210
Po itself, and so the environmental behaviour of these radionuclides
needs to be considered together.
REFERENCES TO CHAPTER 4
[4.1] GASCOYNE, M., “Geochemistry of the actinides and their daughters”, Uranium-
Series Disequilibrium: Applications to Earth, Marine, and Environmental Sciences
(IVANOVICH, M., HARMON, R.S., Eds), Clarendon Press, Oxford (1992)
34–61.
[4.2] RUDNICK, R.L., GAO, S., “Composition of the continental crust”, Treatise on
Geochemistry, Vol.
3: The Crust (HOLLAND, H.D., TUREKIAN, K.K., Eds),
Elsevier, Oxford (2003)
1–64.
[4.3] BALKANSKI, Y.J., JACOB, D.J., GARDNER, G.M., GRAUSTEIN, W.C.,
TUREKIAN, K.K., Transport and residence times of tropospheric aerosols inferred
from a global three-dimensional simulation of
210
Pb, J. Geophys. Res. 98
(1993)
20 573–20 586.
[4.4] BASKARAN, M., Po-210 and Pb-210 as atmospheric tracers and global atmospheric
Pb-210 fallout: A review, J. Environ. Radioact.
102 (2011) 500–513.
[4.5] VAARAMAA, K., ARO, L., SOLATIE, D., LEHTO, J., Distribution of
210
Pb and
210
Po
in boreal forest soil, Sci. Total Environ.
408 (2010) 6165–6171.
[4.6] BROWN, J.E., et al., Levels and transfer of
210
Po and
210
Pb in Nordic terrestrial
ecosystems, J. Environ. Radioact.
102 (2011) 430–437.
[4.7] SHEPPARD, S.C., SHEPPARD, M.I., ILIN, M., TAIT, J., SANIPELLI, B., Primordial
radionuclides in Canadian background sites: Secular equilibrium and isotopic
differences, J. Environ. Radioact.
99 (2008) 933–946.
[4.8] COPPIN, F., ROUSSEL-DEBET, S., Comportement du
210
Po en milieu terrestre: revue
bibliographique, Radioprot. 39 (2004) 39–58.
[4.9] SANTOS, P.L., GOUVEA, R.C., DUITA, I.R., GOUVEA, V.A., Accumulation of
210
Po in foodstuff cultivated in farms around the Brazilian mining and milling facilities
on Poços de Caldas plateau, J. Environ. Radioact.
11 (1990) 141–149.
[4.10] THOMAS, P.A., Radionuclides in the terrestrial ecosystem near a Canadian uranium
mill — Part
I: Distribution and doses, Health Phys. 78 (2000) 614–624.
[4.11] BUNZL, K., KRACKE, W., Distribution of
210
Pb and
210
Po, stable lead and fallout
137
Cs in soil, plants and moorland sheep of a heath, Sci. Total Environ. 39
(1984)
143–159.
[4.12] SCHÜTTELKOPF, H., KIEFER, H., “The Ra-226, Pb-210 and Po-210 contamination
of the Black Forest in natural radiation environment”, Natural Radiation Environment
(Proc. 2nd Special Symp. Bombay, 1981) (VOHRA, K.G., MISHRA, V., PILLAI, K.,
SADAÏWAN, S., Eds), John Wiley and Sons, New
York (1982).
56
CHAPTER 4
[4.13] ROSNER, G., BUNZL, K., HÖTZL, H., WINKLER, R., Low level measurements of
natural radionuclides in soil samples around a coal-fired power plant, Nucl. Instrum.
Methods Phys. Res.
223 (1984) 585–589.
[4.14] SINGH, D.R., NILEKANI, S.R., Measurement of polonium activity in Indian tobacco,
Health Phys.
31 (1976) 393–394.
[4.15] KARUNAKARA, D., et al., Distribution and enrichment of
210
Po in the environment
of Kaiga in South India, J. Environ. Radioact.
51 (2000) 349–362.
[4.16] SIDDAPPA, K., et al., Distribution of natural and artificial radioactivity components in
the environs of costal Karnataka, Kaiga and Goa (1991–94), Mangalore Univ. (1994).
[4.17] HOUMANI, Z.M.M., BRADLEY, D.A., MAAH, M.J., AHINED, Z., Concentration of
210
Po,
226
Ra and
228
Ac in non-siliceous environmental materials, Radiat. Phys.
Chem.
61 (2001) 665–668.
[4.18] MARTINEZ-AGUIRRE, A., GARCIA-LEON, M., Transfer of natural radionuclides
from soils to plants in a wet marshland, Appl. Radiat. Isot.
47 (1996) 1103–1108.
[4.19] SMITH-BRIGGS, J.L., BRADLEY, E.J., Measurement of natural radionuclides in UK
diet, Sci. Total Environ.
35 (1984) 431–440.
[4.20] UNITED STATES ATOMIC ENERGY COMMISSION, Polonium-210 in Soils and
Plants, USAEC Special Report, Vol.
11, USAEC (1980).
[4.21] LAPHAM, S.C., MILLARD, J.B., SAMET, J.M., Health implications of radionuclide
levels in cattle raised near U mining and milling facilities in Ambrosia
Lake,
New
Mexico, Health Phys. 56 (1989) 327–340.
[4.22] PARFENOV, Yu.D., Polonium-210 in the environment and in the human organism, At.
Energy Rev.
12 (1974) 75–143.
[4.23] PERSSON, B.R.R., HOLM, E., Polonium-210 and lead-210 in the terrestrial
environment: A historical review, J. Environ. Radioact.
102 (2011) 420–429.
[4.24] HARMSEN, K., DE HAAN, F.A.M., Occurrence and behaviour of uranium and
thorium in soil and water, Neth. J. Agric. Sci.
28 (1980) 40–62.
[4.25] SYED, H.S., Comparison studies adsorption of thorium and uranium on pure clay
minerals and local Malaysian soil sediments, J. Radioanal. Nucl. Chem.
241
(1999)
11–14.
[4.26] LAMBERT, G., BUISSON, A., SANAK, J., ARDOUIN, B., Modification of the
atmospheric polonium 210 to lead 210 ratio by volcanic emissions, J. Geophys. Res.
84
(1979)
6980–6986.
[4.27] PREISS, N., MÉLIÈRES, M.-A., POURCHET, M., A compilation of data on lead 210
concentration in surface air and fluxes at the air–surface and water–sediment interfaces,
J. Geophys. Res.
101 (1996) 28 847–28 862.
[4.28] ANAND, S.J.S., RANGARAJAN, C., Studies on the activity ratios of polonium-210
to lead-210 and their dry deposition velocities at Bombay in India, J. Environ.
Radioact.
11 (1990) 235–250.
[4.29] MARENCO, A., FONTAN, J., Sources of polonium-210 within the troposphere,
Tellus
24 (1972) 38–46.
[4.30] NHO, E.-Y., ARDOUIN, B., LE CLOAREC, M.F., RAMONET, M., Origins of
210
Po
in the atmosphere at Lamto, Ivory
Coast: Biomass burning and saharan dusts, Atmos.
Environ.
30 (1996) 3705–3714.
57
OCCURRENCE AND CYCLING IN THE ENVIRONMENT
[4.31] WINKLER, R., HÖTZL, H., CHATTERJEE, B., Analysis of
210
Pb and
210
Po
concentrations in surface air by an alpha spectrometric method, Health Phys.
41
(1981)
495–503.
[4.32] MOORE, H.E., POET, S.E., MARTELL, E.A.,
222
Rn,
210
Pb,
210
Bi and
210
Po profiles
and aerosol residence times versus altitude, J. Geophys. Res.
78 (1973) 7065–7075.
[4.33] LIU, H., JACOB, D.J., BEY, I., YANTOSCA, R.M., Constraints from
210
Pb and
7
Be on
wet deposition and transport in a global three-dimensional chemical tracer model
driven by assimilated meteorological fields, J. Geophys. Res.
106
(2001)
12 109–12 128.
[4.34] MARTIN, P., Uranium and thorium series radionuclides in rainwater over several
tropical storms, J. Environ. Radioact.
65 (2003) 1–18.
[4.35] MAHON, D.C., MATHEWES, R.W., Uptake of naturally occurring radioisotopes by
vegetation in a region of high radioactivity, Can. J. Soil Sci. 63 (1983) 281–290.
[4.36] LADINSKAYA, L.A., PARFENOV, Yu.D., ARUTYNOV, Ya.C., POPOVA, D.K.,
Natural concentrations of Pb-210 and Po-210 in plants of steppe ecosystems,
Ecologia
1 (1976) 32–35 (in Russian).
[4.37] HILL, C.R., Polonium-210 in man, Nature 208 (1965) 423–428.
[4.38] BEASLEY, T.M., PALMER, H.E., Lead-210 and polonium-210 in biological samples
from Alaska, Health Phys.
152 (1966) 1062–1064.
[4.39] HOLTZMAN, R.B., Natural levels of lead-210, polonium-210 and radium-226 in
humans and biota of the Arctic, Nature
210 (1966) 1094–1097.
[4.40] TASKAYEV, A.I., TESTOV, B.V., “Major components of doses to biological
organisms from incorporated primordial radionuclides”, Heavy Natural Radionuclides
in Biosphere (ALEXAKHIN, R.M., Ed.), Nauka, Moscow (1990) 201–217
(in Russian).
[4.41] CARVALHO, F.P., Contribution à l’étude du cycle du
210
Po et du
210
Pb dans
l’environnement, PhD Thesis, UFR Sciences, Univ. Nice Sophia Antipolis
(1990).
[4.42] JAWOROWSKI, Z., Radioactive lead in the environment and in the human body, At.
Energy Rev.
1 (1969) 3–45.
[4.43] SMITH-BRIGGS, J.L., Natural radionuclides near a coal-fired power station, Nucl.
Instrum. Meth. Phys. Res.
223 (1984) 590–592.
[4.44] KHANDEKAR, R.N., Polonium-210 in Bombay diet, Health Phys. 33 (1977) 148–150.
[4.45] LADINSKAYA, L.A., PARFENOV, Yu.D., POPOV, D.K., FEDOROVA, A.V.,
210
Pb
and
210
Po content in air, water, foodstuffs, and the human body, Arch. Environ.
Health
27 (1973) 254–258.
[4.46] GLOBEL, B., MUTH, H., “Natürliche Radioaktivität in Trinkwasser, Nahrungsmitteln
und im Menschen in Deutschland”, The Radiological Burden of Man from Natural
Radioactivity in the Countries of the European Communities (Proc. Sem. Paris, 1979),
Commission of the European Communities, Luxembourg (1979)
385–418.
[4.47] BUNZL, K., KRACKE, W., KREUZER, W.,
210
Pb and
210
Po in liver and kidneys of
cattle, I.
Animals from an area with little traffic or industry, Health Phys. 37
(1979)
323–330.
[4.48] IZAK-BIRAN, T., et al., Concentrations of U and Po in animal feed supplements, in
poultry meat and in eggs, Health Phys.
56 (1989) 315–319.
58
CHAPTER 4
[4.49] HILL, C.R., Identification of α emitters in normal biological materials, Health Phys. 8
(1962)
17–25.
[4.50] SMITH-BRIGGS, J.L., BRADLEY, E.J., POTTER, M.D., The ratio of lead-210 to
polonium-210 in UK diet, Sci. Total Environ.
54 (1986) 127–133.
[4.51] MARTIN, P., HANCOCK, G.J., JOHNSTON, A., MURRAY, A.S., Natural-series
radionuclides in traditional North Australian Aboriginal foods, J. Environ. Radioact.
40
(1998)
37–58.
[4.52] BACON, M.P., “Reactive radionuclides as tracers of oceanic particle flux”, Marine
Radioactivity (LIVINGSTON, H.D., Ed.), Elsevier, Amsterdam (2004)
139–164.
[4.53] CARVALHO, F.P., Distribution, cycling and mean residence time of
226
Ra,
210
Pb and
210
Po in the Tagus estuary, Sci. Total Environ. 196 (1997) 151–161.
[4.54] CARVALHO, F.P., Polonium (
210
Po) and lead (
210
Pb) in marine organisms and their
transfer in marine food chains, J. Environ. Radioact. 102 (2011) 462–472.
[4.55] CARVALHO, F.P., FOWLER, S.W., An experimental study on the bioaccumulation
and turnover of polonium-210 and lead-210 in marine shrimp, Mar. Ecol. Prog.
Ser.
102 (1993) 125–133.
[4.56] CARVALHO, F.P., FOWLER, S.W., A double-tracer technique to determine the
relative importance of water and food as sources of polonium-210 to marine prawns
and fish, Mar. Ecol. Prog. Ser.
103 (1994) 251–264.
[4.57] STEWART, G.M., et al., Contrasting transfer of polonium-210 and lead-210 across
three trophic levels in marine plankton, Mar. Ecol. Prog. Ser.
290 (2005) 27–33.
[4.58] WILDGUST, M.A., McDONALD, P., WHITE, K.N., Assimilation of
210
Po by the
mussel Mytilus edulis from the alga Isochrysis galbana, Mar. Biol.
136 (2000) 49–53.
[4.59] GODOY, J.M., SICILIANO, S., DE CARVALHO, Z.L., DE MOURA, J.F., GODOY,
M.L.D.P.,
210
Polonium content of small cetaceans from Southeastern Brazil, J. Environ.
Radioact. 106 (2012) 35–39.
[4.60] KIM, G., HUSSAIN, N., CHURCH, T.M., Excess
210
Po in the coastal atmosphere,
Tellus
52B (2000) 74–80.
[4.61] KIM, G., KIM, S.J., HARADA, K., SCHULTZ, M.K., BURNETT, W.C., Enrichment
of excess
210
Po in anoxic ponds, Environ. Sci. Technol. 39 (2005) 4894–4899.
[4.62] YANKOVICH, T.L., et al., Whole-body to tissue concentration ratios for use in biota
dose assessments for animals, Radiat. Environ. Biophys.
49 (2010) 549–565.
[4.63] RYAN, B., BOLLHÖFER, A., MARTIN, P., Radionuclides and metals in freshwater
mussels of the upper South Alligator River, Australia, J. Environ. Radioact.
99
(2008)
509–526.
[4.64] HOWARD, B.J., et al., The IAEA handbook on radionuclide transfer to wildlife,
J. Environ. Radioact. 121 (2013) 55–74.
[4.65] INTERNATIONAL ATOMIC ENERGY AGENCY, Handbook of Parameter Values
for the Prediction of Radionuclide Transfer to Wildlife, Technical Reports Series
No.
479, IAEA, Vienna (2014).
[4.66] UNITED NATIONS SCIENTIFIC COMMITTEE ON THE EFFECTS OF ATOMIC
RADIATION, Sources and Effects of Ionizing Radiation, UNSCEAR 2008 Report to
the General Assembly, with Scientific Annexes, Vol. II: Effects, United Nations,
New York (2008) Annex E.
59
OCCURRENCE AND CYCLING IN THE ENVIRONMENT
[4.67] UNITED NATIONS SCIENTIFIC COMMITTEE ON THE EFFECTS OF ATOMIC
RADIATION, Sources and Effects of Ionizing Radiation, UNSCEAR 2000 Report to
the General Assembly, with Scientific Annexes, Vol.
I: Sources, United Nations,
New
York (2000) Annex B.
[4.68] CARVALHO, F.P.,
210
Po and
210
Pb intake by the Portuguese population: The
contribution of seafood in the dietary intake of
210
Po and
210
Pb, Health Phys. 69
(1995)
469–480.
[4.69] LEE, C.W., et al., Assessment of
210
Po in foodstuffs consumed in Korea, J. Radioanal.
Nucl. Chem.
279 (2009) 519–522.
[4.70] AARKROG, A., et al., A comparison of doses from
137
Cs and
210
Po in marine food: A
major international study, J. Environ. Radioact.
34 (1997) 69–90.
[4.71] CHAMBERLAIN, A.C., Emissions from Sellafield and activities in soil, Sci. Total
Environ.
177 (1996) 259–280.
[4.72] GARLAND, J.A., WAKEFORD, R., Atmospheric emissions from the Windscale
accident of October
1957, Atmos. Environ. 41 (2007) 3904–3920.
[4.73] MOORE, H.E., MARTELL, E.A., POET, S.E., Sources of polonium-210 in
atmosphere, Environ. Sci. Technol.
10 (1976) 586–591.
[4.74] RUTHERFORD, P.M., DUDAS, M.J., SAMEK, R.A., Environmental impacts of
phosphogypsum, Sci. Total Environ.
149 (1994) 1–38.
[4.75] INTERNATIONAL ATOMIC ENERGY AGENCY, Extent of Environmental
Contamination by Naturally Occurring Radioactive Material (NORM) and
Technological Options for Mitigation, Technical Reports Series No.
419, IAEA,
Vienna
(2003).
[4.76] ROESSLER, C.D., SMITH, Z.A., BOLCH, W.E., PRINCE, R.J., Uranium and
radium-226 in Florida phosphate materials, Health Phys.
37 (1979) 269–277.
[4.77] HURST, F.J., ARNOLD, W.D., A discussion of the uranium control in phosphogypsum,
Hydrometall.
9 (1982) 69–82.
[4.78] VAN DER HEIJDE, H.B., KLIJN, P.-J., PASSCHIER, W.F., Radiological impacts of
the disposal of phosphogypsum, Radiat. Prot. Dosim.
24 (1988) 419–423.
[4.79] SANTOS, A.J.G., MAZZILLI, B.P., FÁVARO, D.I.T., SILVA, P.S.C., Partitioning of
radionuclides and trace elements in phosphogypsum and its source materials based on
sequential extraction methods, J. Environ. Radioact.
87 (2006) 52–61.
[4.80] AOUN, M., EL SAMRANI, A.G., LARTIGES, B.S., KAZPARD, V., SAAD, Z.,
Releases of phosphate fertilizer industry in the surrounding environment: Investigation
on heavy metals and polonium-210 in soil, J. Environ. Sci.
22 (2010) 1387–1397.
[4.81] CARVALHO, F.P.,
210
Pb and
210
Po in sediments and suspended matter in the Tagus
estuary, Portugal: Local enhancement of natural levels by wastes from phosphate ore
processing industry, Sci. Total Environ.
159 (1995) 201–214.
[4.82] BETTI, M., et al., Results of the European Commission Marina II Study — Part II:
Effects of discharges of naturally occurring radioactive material, J. Environ.
Radioact.
74 (2004) 255–277.
[4.83] BERETKA, J., “The current state of utilisation of phosphogypsum in Australia”,
Phosphogypsum (Proc. Third Int. Symp. Orlando, 1990), Publication No. 01-060-083,
Vol. I, Florida Institute of Phosphate Research, Bartow, FL (1990) 394–401.
60
CHAPTER 4
[4.84] BOU-RABEE, F., AL-ZAMEL, A.Z., AL-FARES, R.A., BEM, H., Technologically
enhanced naturally occurring radioactive materials in the oil industry (TENORM): A
review, Nukleonika
54 (2009) 3–9.
[4.85] NORWEGIAN RADIATION PROTECTION AGENCY, Natural Radioactivity in
Produced Water from the Norwegian Oil and Gas Industry in 2003, StrålevernRapport
2005:2, Østerås, NRPA, Oslo
(2004).
[4.86] MORA, J.C., ROBLES, B., CORBACHO, J.A., GASCO, C., GASQUEZ, M.J.,
Modelling the behaviour of
210
Po in high temperature processes, J. Environ.
Radioact.
102 (2011) 520–526.
[4.87] PORCELLI, D., “Investigating groundwater processes using U- and Th- series
nuclides”, U-Th Series Radionuclides in Aquatic Systems, Radioactivity in the
Environment, Vol.
13 (KRISHNASWAMI, S., COCHRAN, J.K., Eds), Elsevier,
Amsterdam (2008)
105–153.
[4.88] ABDELOUAS, A., Uranium mill tailings: Geochemistry, mineralogy, and
environmental impact, Elements
2 (2006) 335–341.
[4.89] INTERNATIONAL ATOMIC ENERGY AGENCY, The Long Term Stabilization of
Uranium Mill Tailings, IAEA-TECDOC-1403, IAEA, Vienna
(2004).
[4.90] INTERNATIONAL ATOMIC ENERGY AGENCY, The Environmental Behaviour of
Radium: Revised Edition, Technical Reports Series No. 476, IAEA, Vienna (2014).
[4.91] BAXTER, M.S., MacKENZIE, A.B., EAST, B.W., SCOTT, E.M., Natural decay series
radionuclides in and around a large metal refinery, J. Environ. Radioact.
32
(1996)
115–133.
[4.92] BAXTER, M.S., Technologically enhanced radioactivity: An overview, J. Environ.
Radioact.
32 (1996) 3–17.
[4.93] SELBY, J.H., “The industrial uses of zircon and zirconia and the radiological
consequences of these uses”, Naturally Occurring Radioactive Material (NORM
V),
(Proc. Int. Symp. Seville, 2007), IAEA, Vienna (2007)
95–116.
61
Chapter 5
210
Pb AND
210
Po IN ATMOSPHERIC SYSTEMS
5.1. INTRODUCTION TO THE ATMOSPHERIC ENVIRONMENT
The depth of the gaseous envelope surrounding Earth is not firmly fixed but
is estimated to extend, at a maximum, approximately 1000 km from the surface
of the crust. Despite comprising less than 15% of the total planetary radius, the
nature of the atmospheric system as a whole has an overwhelming influence on
almost all the biological and geochemical conditions on Earth. Not only does it
affect human exposure to external influences and how its processes occur, it also
influences many of the Earth’s physical characteristics.
The temperature and pressure of the atmosphere vary considerably
with height above the surface. Pressure decreases exponentially with height,
and altitude is often given in units of pressure (hPa) rather than distance. The
temperature variation is more complex and is a crucial determinant of atmospheric
mixing processes. The rate of decrease in temperature with height is termed the
temperature lapse rate (°C/km), whose sign changes several times with height,
giving rise to identifiable layers in the atmosphere (see Fig. 5.1). In the lowest
of these, the troposphere, the lapse rate is normally positive (i.e. temperature
decreases with height), resulting in rapid vertical mixing. In the stratosphere, the
lapse rate is zero or negative due to absorption of solar ultraviolet radiation by
ozone, and vertical mixing is slow. Above the stratosphere lie the mesosphere,
thermosphere and exosphere. Together, the troposphere and stratosphere
comprise the lower atmosphere, which is the region of interest here.
The actual temperature variations with height are crucial determinants
of atmospheric mixing processes and are more complex than those shown in
Fig. 5.1. They depend not only on the time of the year but also on variations
in the structure of the atmosphere: for example, the tropopause (the boundary
between the troposphere and stratosphere) is around 8 km over the poles to but
16 km over the tropics.
The troposphere can be further divided into the planetary boundary layer
(PBL) and the free atmosphere. The behaviour of the PBL is strongly influenced
by the Earth’s surface and the wind within it is affected by surface drag.
Furthermore, turbulence is common and vertical mixing is generally quite strong.
However, a negative temperature lapse rate can exist within the PBL, leading to
a layer of stable air above the surface called an inversion layer. This occurs when
the Earth’s surface is cooler than the air above, such as on nights with clear skies.

62
CHAPTER 5
The height of the PBL is about 1 km, although this depends on surface features
and atmospheric conditions.
In the atmosphere,
210
Bi,
210
Pb and
210
Po are attached to aerosol particles,
with a diameter distribution typically covering several orders of magnitude, from
around 10
−3
μm to 10
2
μm. The behaviour of these radionuclides reflect those
of the particles to which they are attached. Airborne particles may be broadly
classified into coarse particles (>1 μm) and fine particles (<1 μm). Fine particles
may be further categorized into Aitken nuclei (primary aerosols: 10
−3
–10
−1
μm)
and particles in the accumulation range (10
−1
–10
0
μm). These size range
classifications are approximate and vary depending on the situation [5.2, 5.3].
Coarse particles are primarily generated by mechanical means (e.g. dust
resuspension by wind), whereas fine particles originate from processes such as
condensation of gases. Aitken nuclei form particles in the accumulation range
through condensation and coagulation. Sedimentation (gravitational settling) is
a significant removal mechanism for coarse particles, but this is not the case for
fine particles.
The size distribution of particles to which radionuclides are attached
depends on several factors, including the source of the radionuclide and the
length of time it has been suspended. For example, airborne
210
Pb originating
from resuspension of dust will be predominantly on coarse particles, whereas that
Source: See Ref. [5.1].
FIG. 5.1. Vertical structure of the lower atmosphere.
63
ATMOSPHERIC SYSTEMS
produced by the decay of free
222
Rn present in the atmosphere is predominantly
on fine particles. In the latter case, on initial production, a large proportion of
the
210
Pb is attached to Aitken particles, but with time the proportion in the
accumulation mode increases.
5.2. SOURCES OF
210
Pb AND
210
Po IN THE ATMOSPHERE
The primary source of
210
Pb in the atmosphere is
222
Rn exhalation from the
Earth’s surface (mainly from soil) and its subsequent decay via short lived radon
progeny to
210
Pb. There are several other sources, including volcanic activity,
resuspension of soils, forest and savanna fires, and combustion of fossil fuel.
However, these other sources have been estimated to contribute less than 5% to
the total
210
Pb budget.
Exhalation of
222
Rn from the Earth’s surface with subsequent decay through
210
Pb to its progeny is one of the primary sources of
210
Po in the atmosphere.
However, due to the relative volatility of polonium, some other sources are
important. In particular, volcanic activity has been estimated to be as important
a contributor to the total budget as the exhalation of
222
Rn. Each of these sources
has different characteristics in terms of spatial and temporal variability, and
each has a different influence on the
210
Po:
210
Pb ratio and on the aerosol size
distribution to which
210
Pb and
210
Po are attached [5.4]. Overall global fluxes for
210
Pb and
210
Po have been estimated to be approximately 3.64 × 10
16
Bq/a and
3.82
× 10
15
Bq/a, respectively [5.5].
5.2.1. Radon exhalation
Radon exhalation flux densities from soil are dependent on a number of
factors, in particular the
226
Ra activity concentration in the soil, soil moisture,
bulk density and porosity
[5.6]. Although flux densities vary over several
orders of magnitude, sites where they are highly elevated are small on a
regional or global scale, and for the majority of soils, they are in the range of
10–50
mBq·m
−2
·s
−1
. Radon exhalation flux densities from the ocean surfaces
have been estimated to be about two orders of magnitude lower than those
from ice-free land surfaces
[5.5, 5.7]. As a result of these factors, flux densities
are highly variable over the Earth’s surface, and the major influences are the
proportion of ice-free land followed by soil properties, including the radium
activity concentration
[5.8].
Figure 5.2 shows the latitudinal dependence of radon flux densities, based
on the flux model of Schery and Wasiolek
[5.7]. In this plot, the latitude axis
is scaled such that the area enclosed by the curve between any two latitudes is

64
CHAPTER 5
proportional to the radon flux (Bq/s) for that area [5.10]. The region between the
Tropics of Cancer and Capricorn contributes around 38% to the total flux. Mean
latitudinal exhalation
222
Rn flux densities drop dramatically south of 30°S and
north of 70°N owing to the presence of large oceans and ice-covered land areas.
The southern hemisphere contributes only about 26% of the Earth’s
total radon flux. The radioactive half-life of radon (3.82
days) and the mean
lifetime of
210
Pb in the PBL, about 5–7 days [5.11], are considerably smaller
than the timescale required for mixing of air between the northern and southern
hemispheres. As a consequence, the lower radon flux results in lower average
210
Pb and
210
Po concentrations in the air, and lower deposition fluxes to the
Earth’s surface in the southern hemisphere
[5.12].
The radon exhalation flux density for ice-free soil is affected by a number
of factors. Of the temporally variable factors, soil moisture has the greatest
influence
[5.13]. The relationship is complex, but rainfall generally results in
a reduction in exhalation. Most studies of seasonal variability show reductions
in the wetter months: winter in temperate zones
[5.14] and wet monsoon in the
Mean latitudinal
222
Rn exhalation flux density
(mBq m
-2
s
-1
)
0 5 10 15 20 25
Latitude (degrees)
90
50
40
30
20
10
0
-10
-20
-30
-40
-50
-90
Source: Figure 1 of Ref. [5.9].
FIG. 5.2. Estimated mean annual latitudinal radon exhalation flux density.
65
ATMOSPHERIC SYSTEMS
tropics [5.15]. However, the variability is relatively small over an interannual
time frame.
Following exhalation, radon decays in the atmosphere to a series of short
lived progeny (
218
Po,
214
Pb,
214
Bi and
214
Po), which attach to fine (submicron)
aerosol particles. The activity concentrations of these short lived progeny have
been observed to be high, resulting in increased gamma dose rates close to the
ground during, and immediately following, rainstorms
[5.16, 5.17].
5.2.2. Volcanic activity
Volcanic activity releases thorium and uranium series radionuclides to the
atmosphere, including
210
Pb,
210
Po and
222
Rn, with
210
Po in excess over
210
Pb
in volcanic plumes due to its volatility
[5.18, 5.19]. The excess is sufficiently
high for
210
Po to be used as a tracer for long range transport of volcanic
plumes
[5.20]. Measurements close to Mount Sakurajima, Japan, show that
210
Po
is predominantly attached to fine particles smaller than 2
μm [5.21].
The fluxes of
210
Pb and
210
Po to the atmosphere from this source have
been estimated to be 6.0
× 10
13
Bq/a and 2.4 × 10
15
Bq/a, respectively [5.22].
In the case of
210
Po, this is of a similar order of magnitude to that from ingrowth
following
222
Rn exhalation from soil. However, this flux is difficult to quantify
accurately. Moreover, it greatly varies depending on the timing and location
of volcanic activity. One approach is to use of sulphate measurements as an
indicator for the influence of volcanic activity, in conjunction with
210
Pb and
210
Po measurements [5.23]. Volcanic eruptions can also lead to an injection
of
210
Pb and
210
Po into the stratosphere; in a 1979 study, Lambert et al. [5.24]
estimate this flux to be about 2% of that into the troposphere.
5.2.3. Resuspended soil dust
The suspension of dust can in some cases be a significant contributor to
concentrations of
210
Pb and its progeny in the atmosphere. This is especially
true in the PBL over continents. The sources often contribute only on a local or
regional scale, owing to the significant role sedimentation plays in the removal of
resuspended particles to the surface. Nevertheless dispersion can be significant,
even over relatively large distances
[5.20].
Globally, the flux has been estimated at 3.3
× 10
14
Bq/a for
210
Pb and
210
Po [5.5, 5.25], which means it contributes around 1% and 10%, respectively,
of the total budgets from all sources. However, because it primarily affects the
PBL above significant land masses, it can be a very significant contributor to the
observed concentrations at ground level.
66
CHAPTER 5
The isotopes
210
Bi,
210
Pb and
210
Po are normally in secular equilibrium in
topsoils, and
210
Pb is also normally in excess over its progeny in the troposphere.
Consequently, the resuspension of dust has the effect of increasing the
210
Bi:
210
Pb
and
210
Bi:
210
Po ratios. This can contribute to difficulties with using
210
Pb–
210
Bi
and
210
Pb–
210
Po couples to calculate atmospheric
210
Pb residence times
(see Section 5.4). One approach to counter this is to use a determination of a
member of the uranium decay chain above
210
Pb to apply a correction [5.26, 5.27].
5.2.4. Biomass burning
Polonium-210 has been shown to be enriched in fire plumes, due to the
volatility of elemental polonium and polonium compounds at temperatures in the
low hundreds of degrees Celsius
[5.28]. Estimates of total worldwide biomass
burning are around (2–5) × 10
13
kg CO
2
released per year [5.29], and the carbon
content of dried plant materials is around 45–50%
[5.30]. As noted in Chapter 4,
concentrations of
210
Po in vegetable matter vary considerably, but an indicative
average value is 10
Bq/kg. This would give a total annual flux of
210
Po from this
source of around 2
× 10
14
Bq/a if all of the available
210
Po were released. This
release would be at ground level, and the
210
Po would be available for removal by
wet and dry deposition processes.
On a global and time averaged scale, biomass burning should not be a
significant source of
210
Po (nor of
210
Pb). Although in the case of large burning
events, it can have a local or regional effect
[5.20, 5.31]. The convective draught
from fires can also transport
222
Rn to the free troposphere, with subsequent decay
to
210
Pb [5.32]. Biomass burning is discussed in more detail in Appendix I V.
5.2.5. Wind blown sea spray and emission of volatile polonium compounds
from water surfaces
The isotopes
210
Pb and
210
Po are enriched in the ocean surface microlayer,
with the
210
Po:
210
Pb activity ratio at around 2 [5.33]. Wind blown sea spray has
been shown to be a minor contributor to the atmospheric budget, estimated at
3.7
× 10
11
Bq/a and 7.4 × 10
11
Bq/a for
210
Pb and
210
Po, respectively [5.5, 5.25].
In addition, there is evidence that volatile compounds, such as dimethyl polonide,
can be formed and released from ocean and fresh water surfaces
[5.34–5.36].
5.2.6. Anthropogenic sources
There are many anthropogenic sources of
210
Pb and
210
Po. The most
significant are fossil fuel (particularly coal) burning and dispersion of
phosphate fertilizers and gypsum by-products. Global fluxes are estimated to be
67
ATMOSPHERIC SYSTEMS
8.2 × 10
12
Bq/a from fossil fuels for both
210
Pb and
210
Po, and 2.0 × 10
13
Bq/a and
6
× 10
11
Bq/a from phosphate for
210
Pb and
210
Po, respectively [5.5]. In addition,
much of the world’s biomass burning is anthropogenic (see Section 5.2.4).
Fossil fuel burning and most other anthropogenic sources impact most
heavily on concentrations in urban areas. A study in Seoul, Republic of
Korea
[5.37], concludes that the dominant fraction of
210
Po in precipitation
samples is linked to anthropogenic sources, primarily from the burning of coal,
with minor contributions from biomass burning.
5.3. PROCESSES FOR THE REMOVAL OF
210
Pb AND
210
Po FROM THE
ATMOSPHERE
Radioactive decay is only a minor contributor to the removal of
210
Pb and
210
Po on account of their short residence times in the troposphere. The primary
removal pathway is wet deposition to the Earth’s surface, with a secondary
pathway being dry deposition. Using the estimates of global source terms for
210
Pb
and
210
Po given in Section 5.2, the average global deposition flux density over
the Earth’s surface should be approximately 71
Bq·m
−2
·a
−1
and 7.5 Bq·m
−2
·a
−1
for
210
Pb and
210
Po, respectively. However, annual total deposition flux densities
for
210
Pb range from several Bq·m
−2
·a
−1
(as observed in Antarctica) to several
hundred Bq·m
−2
·a
−1
.
5.3.1. Wet deposition
Wet deposition refers to removal of atmospheric constituents to the ground
surface by precipitation (e.g. rain, snow and hail). Globally, wet deposition is
the primary process for removal of
210
Pb and
210
Po from the troposphere, and
occurs mainly by precipitation from either stratiform or convective clouds. The
removal of
210
Pb and
210
Po essentially reflects the removal of the aerosol particles
to which they are attached. The term rainout refers to the removal of aerosol
which has been scavenged within the cloud, while washout refers to scavenging
which occurs below the cloud during the precipitation event.
The concentrations of
210
Pb and
210
Po in precipitation are a complex result
of several factors and include the following:
— Their concentrations in the air column;
— The size distribution of the aerosol to which they are attached;
— The effective height of the precipitating column;
— The relative contributions from rainout and washout;

68
CHAPTER 5
— The growth in raindrop size within the cloud due to ascent in convective
updrafts;
— Evaporation of the raindrop during its descent;
— The type and duration of rainfall.
Reported values for
210
Pb concentration in rainwater vary
considerably at different locations, being most commonly in the range of
10–1000
mBq/L
3
with
210
Po:
210
Pb activity ratios of 0.005–1 or even greater.
Some measured concentrations in rainwater are provided in Table 5.1, showing
the variability in concentrations between locations and over extended periods at
one location.
In many cases,
210
Pb and
210
Po activity concentrations are greater at the start
of the precipitation event than later in the event, probably primarily due to the
reduction in washout as the air below the cloud becomes depleted
[5.27, 5.43].
A related effect is that activity concentrations in the precipitation are
generally inversely proportional to the amount of precipitation (e.g. see
Refs
[5.39, 5.40, 5.44]).
TABLE 5.1.
210
Pb AND
210
Po CONCENTRATIONS AND
210
Po:
210
Pb
ACTIVITY RATIOS IN RAINWATER
Location
210
Pb (mBq/L)
210
Po (mBq/L)
210
Po:
210
Pb
Ref.
Range Avg. Range Avg. Range Avg.
Izmir, Turkey 9–198 51 2–35 8 0.03–1.09 —
a
[5.38]
Geneva,
Switzerland
60–3300 —
a
—
a
—
a
—
a
—
a
[5.39]
Jabiru, Australia 46–1700 147 0.9–790 20 0.02–0.46 0.14 [5.27]
Galveston, USA 34–3600 132 —
a
—
a
—
a
—
a
[5.40]
Detroit, USA 105–1233 472 2.2–126 23 0.005–
0.283
0.049 [5.41]
Monaco —
a
—
a
—
a
—
a
—
a
0.25 [5.42]
a
—: data not available.
69
ATMOSPHERIC SYSTEMS
5.3.2. Dry deposition
Globally, dry deposition accounts for about 10–15% of total deposition for
210
Pb [5.5, 5.45]. However, this proportion varies spatially and temporally. The
contribution from dry deposition is greater for arid regions and can exceed the
wet fallout in these areas
[5.46].
Dry deposition of
210
Pb and
210
Po consists of two components: activity
present on resuspended dust; and activity from other sources and primarily
attached to fine particles. Deposition of activity on resuspended dust will be more
strongly influenced by sedimentation, although under some conditions such dust
can travel thousands of kilometres. Cases of such long distance transport have
been well documented for dusts originating from subtropical arid regions such as
North
Africa and the Middle East, Central Asia and Australia [5.20].
On its resuspension, the
210
Po:
210
Pb activity ratio of dust can be expected
to be approximately one. Since coarse particles may dominate the dry
deposition, this can result in a significantly higher
210
Po:
210
Pb ratio than in wet
deposition
[5.41].
5.3.3. Deposition fluxes
Figure 5.3 shows the results of an aerosol transport model simulation for
deposition flux density of
210
Pb on a latitudinal basis [5.47]. There is a peak in
deposition from convective rain in the tropics. The greater deposition flux in the
northern hemisphere compared with the southern hemisphere is due to the higher
222
Rn source term (see Fig. 5.1 and Section 5.4.1). The very low deposition flux
in Antarctica is due to a combination of the low southern hemisphere source
term, the low precipitation rate, the lack of significant ice-free land masses close
to Antarctica, and the band of strong westerly winds around 40–50°S, which act
as a barrier to low level movement of air from the land masses to the north.
As discussed, wet deposition dominates the total deposition at most
locations, and Fig.
5.3 shows that this is the case for all latitudinal bands. The
annual wet deposition flux (Bq·m
−2
·a
−1
) of a radionuclide can be calculated from
the product of the total precipitation over the year and the (precipitation weighted)
average concentration in the rainwater. Hence, both the amount of precipitation
and the concentration are important in evaluating these fluxes.
An example of the importance of multiple factors in determining deposition
fluxes is the waters on the east of Japan, where
210
Pb deposition fluxes of up to
800
Bq·m
−2
·a
−1
have been recorded. These high fluxes are due to a combination
of air masses with high
210
Pb concentrations from the surface layer of the Asian
continent being moved by strong winter monsoons, mixing with ascending
convection clouds over the body of water between Japan and the Asian mainland,

70
CHAPTER 5
and subsequent precipitation (rainfall and snowfall) over the coast and mountain
ridge of Japan with high
210
Pb concentrations in the precipitation [5.44, 5.48].
High
210
Po deposition fluxes and elevated
210
Po:
210
Pb ratios have also been
recorded in this region
[5.49].
The annual amount of precipitation is usually a very strong determinant
of depositional fluxes. This is true both between locations, and for interseasonal
and interannual variability at the same location
[5.39, 5.50]. However, Beks et
al.
[5.51] report that the
210
Pb depositional fluxes at two sites in the Netherlands
were mainly correlated with the number of times that there was heavy rain
or thunderstorms, rather than annual rainfall. The deposition fluxes were
approximately 75
Bq·m
−2
·a
−1
, which is considerably lower than the studies in
Japan on account of the predominantly westerly winds.
5.4. DISTRIBUTION OF
210
Pb AND
210
Po IN THE ATMOSPHERE
5.4.1. Concentrations at the land surface
In general, the most important influences on concentrations observed at the
surface are the presence of significant land masses within the prevailing wind
direction (as a source of
222
Rn), the amount of resuspension of dust, the degree of
wet deposition occurring in the region and the degree of vertical mixing of the air
Source: Figure 3 of Ref. [5.47].
FIG. 5.3. Zonally averaged annual deposition flux of
210
Pb (Bq·m
−2
·a
−1
) due to convective
rain, synoptic rain and dry deposition (reproduced with permission courtesy of American
Geophysical Union).

71
ATMOSPHERIC SYSTEMS
mass (see Section 4.2). The presence of other sources, such as volcanic activity
or forest fires, can also be important, especially for
210
Po.
Figure 5.4 shows average annual surface air concentrations of
210
Pb
for a number of locations against latitude
[5.12]. As a first approximation, the
latitudinal variations in concentrations reflect the variations in the
222
Rn source
term, which in turn, reflect the distribution of ice-free land masses. However,
for any specific location, factors such as predominant wind direction, degree of
vertical mixing and removal by precipitation also play important roles.
The highest
210
Pb concentrations in surface air are observed in the
subtropical and temperate latitudes of the northern hemisphere owing to the
relatively large land masses there. Values for average annual concentrations in
this region mainly lie in the range of 0.2–1
mBq/m
3
[5.12]. Between the tropics,
values are generally in the range of 0.1–0.5
mBq/m
3
, while south of around 30°S,
they quickly trend down to less than 0.1
mBq/m
3
, owing to the lack of large
ice-free land masses and consistent with the radon exhalation flux density trends
already discussed.
210
Pb concentration in surface air (mBq SCM
-1
)
0.0 0.2 0.4 0.6 0.8 1.0 1.2
Latitude (degrees)
90
50
40
30
20
10
0
-10
-20
-30
-40
-50
-90
Polar regions
Americas
Atlantic Ocean
Europe, Africa
Asia
Indian Ocean
Australia
Pacific Ocean
Source: Figure 2.13 of Ref. [5.10].
Note: Each point represents a separate measurement location.
FIG. 5.4. Mean annual
210
Pb concentration in surface air as a function of latitude (reproduced
with permission courtesy of Elsevier).
72
CHAPTER 5
The concentrations in the high latitude regions of the northern hemisphere
are about an order of magnitude higher than over Antarctica, primarily due to the
proximity of significant land masses to the south, and low level transport from
mid-latitudes to the Arctic in winter and spring, coincident with a pollution event
known as Arctic haze
[5.12, 5.52].
The concentrations are generally lower for the island or coastal sites (open
symbols in Fig. 5.4) than for continental sites. Since Fig. 5.4 shows data for land
based monitoring stations, the latitudinal average concentrations can be expected
to be lower than indicated, with the island sites being more representative of the
concentration over ocean areas.
There is clear evidence of a seasonality effect in
210
Pb and
210
Po
concentrations in surface air
[5.20, 5.53–5.55]. Concentrations are normally
higher during dry seasons due to higher exhalation of
222
Rn from the soil,
greater resuspension of soil dust and lower wet deposition rates, which can vary
locally. However, the effect of air mass origin can also play a significant role in
seasonality effects, depending on the local conditions
[5.56].
5.4.2. Vertical profiles
Mean vertical profiles of
222
Rn generally show a strong reduction in
concentration from the surface to the upper troposphere of a factor of 100 over
continental areas
[5.57–5.59]. This reflects the ground level source, and the fact
that radon is only removed by radioactive decay with a half-life of 3.8 days.
Nevertheless, vertical profiles are highly variable, reflecting geographical
variability in the source term, as well as vertical and horizontal mixing processes
in the PBL and the free atmosphere. Average profiles have been modelled using a
one dimensional turbulent diffusion equation
[5.60]. However, mixing processes
are much more dynamic than is implied by such models, including rapid
transport from the lower to the upper troposphere in convective clouds
[5.61].
Discontinuities have also been observed at the PBL–free atmosphere interface,
and between cloud layers and the above cloud layer
[5.59].
The vertical distributions of
210
Pb and
210
Po in the troposphere are
more complex than that of
222
Rn due to progressive ingrowth and removal by
entrainment in precipitation. For
210
Pb, the reduction between ground level
and upper tropospheric concentrations are generally less than a factor of
five, and the concentration in the upper troposphere can be higher than in the
cloud layer
[5.57, 5.62]. The reason for this is scavenging of
210
Pb by wet and
dry deposition in the lower troposphere, and by convective and stratiform
precipitation from the mid-layers
[5.53]. There is also a stratospheric contribution
resulting from stratosphere–troposphere exchange, which predominantly affects
upper tropospheric concentrations.
73
ATMOSPHERIC SYSTEMS
In oceanic and polar regions, the vertical distributions of
210
Pb and
210
Po can differ from those discussed above owing to the much reduced radon
exhalation flux densities at the surface. Their concentrations can increase with
altitude owing to a combination of the transport of radon to the free troposphere
due to convective events, with subsequent formation of
210
Pb [5.32, 5.63] and
contributions from stratospheric air
[5.52]. Elevated levels of
210
Pb in the free
troposphere over the South
Pacific are attributed to radon convection due to
fires, most likely in Africa
[5.32]. In a study of the western hemisphere Arctic,
210
Pb concentrations were elevated at 3–6 km compared to those below 1 km at
latitudes greater than 65°N
[5.64].
Average
210
Pb and
210
Po concentrations in the stratosphere are about
0.3
mBq/SCM (standard cubic metre) at 0°C and 1000 hPa, with relatively little
variation with altitude and latitude
[5.22, 5.65]. As discussed in Section 5.2,
some transfer of
210
Pb and
210
Po to the stratosphere can occur owing to
volcanic eruptions
[5.24]. Apart from this, direct transfers of
210
Pb and
210
Po
to the stratosphere are negligible due to their removal by ice crystals formed in
tropospheric air entering the lower stratosphere [5.66]. Rather,
222
Rn present in
these air masses decays to
210
Pb, with substantial subsequent ingrowth of
210
Po
due to the relatively long stratospheric residence times (~1 year). This transfer
occurs predominantly under conditions of strong convective activity, bringing
air from the lower troposphere with higher concentrations of radon and resulting
in sufficient radon being transferred to support the observed stratospheric
210
Pb
activity concentrations
[5.64]. Strong convective activity, as well as tropopause
folds, linked to the subtropical and polar jet streams, also result in the transfer of
stratospheric air to the troposphere
[5.67].
REFERENCES TO CHAPTER 5
[5.1] NATIONAL OCEANIC AND ATMOSPHERIC ADMINISTRATION, NATIONAL
AERONAUTICS AND SPACE ADMINISTRATION, UNITED STATES AIR
FORCE, US Standard Atmosphere, 1976, US Government Printing Office, Washington,
DC
(1976).
[5.2] SEINFELD, J.H., PANDIS, S.N., “Properties of the atmospheric aerosol”, Atmospheric
Chemistry and Physics: From Air Pollution to Climate Change, 2nd edn, Wiley and
Sons, Hoboken, NJ (2006)
350–395.
[5.3] PAPASTEFANOU, C., “Radioactive aerosol analysis”, Handbook of Radioactivity
Analysis, 3rd edn (L’ANNUNCIATA, M., Ed.), Elsevier, Amsterdam (2012)
727–767.
[5.4] GAFFNEY, J.S., MARLEY, N.A., CUNNINGHAM, M.M., Natural radionuclides in
fine aerosols in the Pittsburgh area, Atmos. Environ.
38 (2004) 3191–3200.
[5.5] BASKARAN, M., Po-210 and Pb-210 as atmospheric tracers and global atmospheric
Pb-210 fallout: A review, J. Environ. Radioact.
102 (2011) 500–513.
74
CHAPTER 5
[5.6] INTERNATIONAL ATOMIC ENERGY AGENCY, Measurement and Calculation of
Radon Releases from NORM Residues, Technical Reports Series No.
474, IAEA,
Vienna
(2013).
[5.7] SCHERY, S.D., WASIOLEK, M.A., “Modelling
222
Rn flux from the earth’s surface”,
Radon and Thoron in the Human Environment (Proc. 7th Tohwa Univ. Int. Symp.
Fukuoka, 1997) (KATASE, A., SHIMO, M., Eds), World Scientific, Tokyo
(1998)
207–217.
[5.8] Sources and Measurements of Radon and Radon Progeny Applied to Climate and Air
Quality Studies (Proc. Technical Mtg Vienna, 2009), IAEA, Vienna
(2012).
[5.9] MARTIN, P., “A note on the contribution of tropical regions to the Earth’s radon flux”,
ibid., pp.
75–76.
[5.10] MARTIN, P., McBRIDE, J.L., “Radionuclide behaviour and transport in the tropical
atmospheric environment”, Tropical Radioecology, Radioactivity in the Environment,
Vol. 18 (TWINING, J., Ed.), Elsevier, Amsterdam (2012) 59–91.
[5.11] RANGARAJAN, C., EAPEN, C.D., The use of natural radioactive tracers in a study of
atmospheric residence times, Tellus
B 42 (1990) 142–147.
[5.12] PREISS, N., MÉLIÈRES, M.-A., POURCHET, M., A compilation of data on lead 210
concentration in surface air and fluxes at the air–surface and water–sediment interfaces,
J. Geophys. Res.
101 (1996) 28 847–28 862.
[5.13] JHA, S., KHAN, A.H., MISHRA, U.C., A study of the
222
Rn flux from soil in the U
mineralised belt at Jaduguda, J. Environ. Radioact.
49 (2000) 157–169.
[5.14] WHITTLESTONE, S., ZAHOROWSKI, W., SCHERY, S.D., Radon flux variability
with season and location in Tasmania, Australia, J. Radioanal. Nucl. Chem.
236
(1998)
213–217.
[5.15] LAWRENCE, C.E., AKBER, R.A., BOLLHÖFER, A., MARTIN, P., Radon-222
exhalation from open ground on and around a uranium mine in the wet-dry tropics, J.
Environ. Radioact.
100 (2009) 1–8.
[5.16] FUJINAMI, N., Observational study of the scavenging of radon daughters by
precipitation from the atmosphere, Environ. Int.
22 (1996) 181–185.
[5.17] STÖHLKER, U., BLEHER, M., CONEN, F., BÄNNINGER, D., “Harmonization of
ambient dose rate monitoring provides for large scale estimates of radon flux density
and soil moisture changes”, Sources and Measurements of Radon and Radon Progeny
Applied to Climate and Air Quality Studies (Proc. Technical Mtg Vienna, 2009),
IAEA, Vienna (2012)
53–63.
[5.18] SATO, J., Natural radionuclides in volcanic activity, Appl. Radiat. Isot. 58
(2003)
393–399.
[5.19] GAUTHIER, P.-J., LE CLOAREC, M.-F., CONDOMINES, M., Degassing processes
at Stromboli volcano inferred from short-lived disequilibria (
210
Pb–
210
Bi–
210
Po) in
volcanic gases, J. Volcanol. Geotherm. Res.
102 (2000) 1–19.
[5.20] NHO, E.-Y., ARDOUIN, B., LE CLOAREC, M.-F., RAMONET, M., Origins of
210
Po
in the atmosphere at Lamto, Ivory
Coast: Biomass burning and Saharan dusts, Atmos.
Environ.
30 (1996) 3705–3714.
75
ATMOSPHERIC SYSTEMS
[5.21] ASHIKAWA, N., et al., The size distribution of
210
Po in the atmosphere around
Mt
Sakurajima in Kagoshima prefecture, Japan, J. Radioanal. Nucl. Chem. 230
(1998)
97–104.
[5.22] LAMBERT, G., ARDOUIN, B., POLIAN, G., Volcanic output of long-lived radon
daughters, J. Geophys. Res.
87 (1982) 11 103–11 108.
[5.23] SHENG, Z., KURODA, P.K., Atmospheric injections of polonium-210 from the recent
volcanic eruptions, Geochem. J.
19 (1985) 1–10.
[5.24] LAMBERT, G., BUISSON, A., SANAK, J., ARDOUIN, B., Modification of the
atmospheric polonium 210 to lead 210 ratio by volcanic emissions, J. Geophys. Res.
84
(1979)
6980–6986.
[5.25] ROBBINS, J.A., “Geochemical and geophysical applications of radioactive lead”, The
Biogeochemistry of Lead in the Environment (TRIAGE, J.O., Ed.), Elsevier,
Amsterdam (1978)
285–393.
[5.26] CARVALHO, F.P., Origins and concentrations of
222
Rn,
210
Pb,
210
Bi and
210
Po in the
surface air at Lisbon, Portugal, at the Atlantic edge of the European continental
landmass, Atmos. Environ.
29 (1995) 1809–1819.
[5.27] MARTIN, P., Uranium and thorium series radionuclides in rainwater over several
tropical storms, J. Environ. Radioact.
65 (2003) 1–18.
[5.28] LAMBERT, G., LE CLOAREC, M.-F., ARDOUIN, B., BONSANG, B., “Long-lived
radon daughters signature of Savanna Fires”, Global Biomass Burning (LEVIN, J.S.,
Ed.), MIT
Press, London (1991) 181–184.
[5.29] CRUTZEN, P.J., ANDREAE, M.O., Biomass burning in the tropics: Impact on
atmospheric chemistry and biogeochemical cycles, Science
250 (1990) 1669–1678.
[5.30] FRIEDL, A., PADOUVAS, E., ROTTER, H., VARMUZA, K., Prediction of heating
values of biomass fuel from elemental composition, Anal. Chim. Acta
544
(2005)
191–198.
[5.31] PAATERO, J., et al., Resuspension of radionuclides into the atmosphere due to forest
fires, J. Radioanal. Nucl. Chem.
282 (2009) 473–476.
[5.32] DIBB, J.E., et al., Constraints on the age and dilution of Pacific Exploratory Mission:
Tropics biomass burning plumes from the natural radionuclide tracer
210
Pb, J. Geophys.
Res.
104 (1999) 16 233–16 241.
[5.33] BACON, M.P., ELZERMAN, A.W., Enrichment of
210
Pb and
210
Po in the sea-surface
microlayer, Nature
284 (1980) 332–334.
[5.34] KIM, G., HUSSAIN, N., CHURCH, T.M., Excess
210
Po in the coastal atmosphere,
Tellus
52B (2000) 74–80.
[5.35] MOMOSHIMA, N., SONG, L.-X., OSAKI, S., MAEDA, Y., Biologically induced Po
emission from fresh water, J. Environ. Radioact.
63 (2002) 187–197.
[5.36] CHURCH, T.M., SARIN, M.M., “U- and Th-series nuclides in the atmosphere: Supply,
exchange, scavenging and applications to aquatic processes”, Radioactivity in the
Environment, Vol.
13 (KRISHNASWAMI, S., COCHRAN, J.K., Eds), Elsevier,
Amsterdam (2008)
11–45.
[5.37] YAN, G., CHO, H.-M., LEE, I., KIM, G., Significant emissions of
210
Po by coal
burning into the urban atmosphere of Seoul, Korea, Atmos. Environ.
54 (2012) 80–85.
76
CHAPTER 5
[5.38] UĞUR, A., ÖZDEN, B., FILIZOK, I., Determination of
210
Po and
210
Pb concentrations
in atmospheric deposition in İzmir (Aegean Sea-Turkey), Atmos. Environ.
45
(2011)
4809–4813.
[5.39] CAILLET, S., ARPAGAUS, P., MONNA, F., DOMINIK, J., Factors controlling
7
Be
and
210
Pb atmospheric deposition as revealed by sampling individual rain events in the
region of Geneva, Switzerland, J. Environ. Radioact.
53 (2001) 241–256.
[5.40] BASKARAN, M., COLEMAN, C.H., SANTSCHI, P.H., Atmospheric depositional
fluxes of
7
Be and
210
Pb at Galveston and College Station, Texas, J. Geophys. Res. 98
(1993)
20 555–20 571.
[5.41] McNEARY, D., BASKARAN, M., Residence times and temporal variations of
210
Po in
aerosols and precipitation from southeastern Michigan, United States, J. Geophys.
Res.
112 (2007).
[5.42] TATEDA, Y., CARVALHO, F.P., FOWLER, S.W., MIQUEL, J.-C., Fractionation of
210
Po and
210
Pb in coastal waters of the NW Mediterranean continental margin, Cont.
Shelf Res.
23 (2003) 295–316.
[5.43] GAVINI, M.B., BECK, J.N., KURODA, P.K., Mean residence times of the long-lived
radon daughters in the atmosphere, J. Geophys. Res.
79 (1974) 4447–4452.
[5.44] HIROSE, K., KIKAWADA, Y., DOI, T., SU, C.-C., YAMAMOTO, M,
210
Pb deposition
in the far East Asia: Controlling factors of its spatial and temporal variations, J.
Environ. Radioact.
102 (2011) 514–519.
[5.45] BALKANSKI, Y.J., JACOB, D.J., GARDNER, G.M., GRAUSTEIN, W.C.,
TUREKIAN, K.K., Transport and residence times of tropospheric aerosols inferred
from a global three-dimensional simulation of
210
Pb, J. Geophys. Res. 98 (1993)
20
573–20 586.
[5.46] RASTOGI, N., SARIN, M.M., Atmospheric
210
Pb and
7
Be in ambient aerosols over
low- and high-altitude sites in semiarid region: Temporal variability and transport
processes, J. Geophys. Res.
113 (2008).
[5.47] GUELLE, W., BALKANSKI, Y.J., SCHULZ, M., DULAC, F., MONFRAY, P., Wet
deposition in a global size-dependent aerosol transport model: 1.
Comparison of a
1
year
210
Pb simulation with ground measurements, J. Geophys. Res. 103 (1998)
11
429–11 445.
[5.48] YAMAMOTO, M., et al., Seasonal and spatial variation of atmospheric
210
Pb and
7
Be
deposition: Features of the Japan Sea side of Japan, J. Environ. Radioact.
86
(2006)
110–131.
[5.49] TATEDA, Y., IWAO, K., High
210
Po atmospheric deposition flux in the subtropical
coastal area of Japan, J. Environ. Radioact.
99 (2008) 98–108.
[5.50] BASKARAN, M., A search for the seasonal variability on the depositional fluxes of
7
Be and
210
Pb, J. Geophys. Res. 100 (1995) 2833–2840.
[5.51] BEKS, J.P., EISMA, D., VAN DER PLICHT, J., A record of atmospheric
210
Pb
deposition in the Netherlands, Sci. Total Environ.
222 (1998) 35–44.
[5.52] DIBB, J.E., et al., Estimation of stratospheric input to the Arctic troposphere:
7
Be and
10
Be in aerosols at Alert, Canada, J. Geophys. Res. 99 (1994) 12 855–12 864.
77
ATMOSPHERIC SYSTEMS
[5.53] ANAND, S.J.S., RANGARAJAN, C., Studies on the activity ratios of polonium-210
to lead-210 and their dry-deposition velocities at Bombay in India, J. Environ.
Radioact.
11 (1990) 235–250.
[5.54] MARENCO, A., FONTAN, J., Sources of polonium-210 within the troposphere,
Tellus
24 (1972) 38–46.
[5.55] WINKLER, R., HÖTZL, H., CHATTERJEE, B., Analysis of
210
Pb and
210
Po
concentrations in surface air by an alpha spectrometric method, Health Phys.
41
(1981)
495–503.
[5.56] ALI, N., et al., The effect of air mass origin on the ambient concentrations of
7
Be and
210
Pb in Islamabad, Pakistan, J. Environ. Radioact. 102 (2011) 35–42.
[5.57] MOORE, H.E., POET, S.E., MARTELL, E.A.,
222
Rn,
210
Pb,
210
Bi and
210
Po profiles
and aerosol residence times versus altitude, J. Geophys. Res.
78 (1973) 7065–7075.
[5.58] LIU, S.C., McAFEE, J.R., CICERONE, R.J., Radon 222 and tropospheric vertical
transport, J. Geophys. Res.
89 (1984) 7291–7297.
[5.59] ZAHOROWSKI, W., et al., “
222
Rn observations for climate and air quality studies”,
Sources and Measurements of Radon and Radon Progeny Applied to Climate and Air
Quality Studies (Proc. Tech. Mtg Vienna, 2009), IAEA, Vienna (2012)
77–96.
[5.60] JACOBI, W., ANDRÉ, K., The vertical distribution of radon 222, radon 220 and their
decay products in the atmosphere, J. Geophys. Res.
68 (1963) 3799–3814.
[5.61] KRITZ, M.A., LE ROULLEY, J.-C., DANIELSEN, E.F., The China Clipper: Fast
advective transport of radon-rich air from the Asian boundary layer to the upper
troposphere near California, Tellus
42B (1990) 46–61.
[5.62] LIU, H., JACOB, D.J., BEY, I., YANTOSCA, R.M., Constraints from
210
Pb and
7
Be on
wet deposition and transport in a global three-dimensional chemical tracer model
driven by assimilated meteorological fields, J. Geophys. Res.
106 (2001)
12
109–12 128.
[5.63] GIANNAKOPOULOS, C., et al., A three-dimensional modelling study of the
correlations of
210
Pb with HNO
3
and peroxyacetylnitrate (PAN) at remote oceanic
sites, J. Geophys. Res.
105 (2000) 1947–1956.
[5.64] DIBB, J.E., TALBOT, R.W., GREGORY, G.L., Beryllium 7 and lead 210 in the
western hemisphere Arctic atmosphere: Observations from three recent aircraft-based
sampling programs, J. Geophys. Res.
97 (1992) 16 709–16 715.
[5.65] FEELY, H.W., SEITZ, H., Use of lead 210 as a tracer of transport processes in the
stratosphere, J. Geophys. Res.
75 (1970) 2885–2894.
[5.66] KRITZ, M.A., et al., Radon measurements in the lower tropical stratosphere: Evidence
for rapid vertical transport and dehydration of tropospheric air, J. Geophys. Res.
98
(1993)
8725–8736.
[5.67] BARAY, J.-L., ANCELLET, G., RANDRIAMBELO, T., BALDY, S., Tropical cyclone
Marlene and stratosphere–troposphere exchange, J. Geophys. Res.
104 (1999)
13
953–13 970.
79
Chapter 6
210
Pb AND
210
Po IN TERRESTRIAL SYSTEMS
6.1. DESCRIPTION OF THE TERRESTRIAL ENVIRONMENT
For the purposes of this chapter, the terrestrial environment is arbitrarily
defined as encompassing the following range of related components: the
atmosphere (particularly the troposphere); the lithosphere (particularly soils);
and the surface and groundwater with which they are associated. Within these
compartments, the terrestrial environment is also populated by a diverse range of
plants and animals. The exchanges within and between these compartments are
the environmental processes on which terrestrial ecosystems depend. However,
the interactions among the various abiotic and biotic environments vary from
location to location. The International Union of Radioecology (IUR) suggests
that interaction matrices could present information on relevant processes in the
terrestrial environments in a synthesized and structured way [6.1].
Each environment has its own unique chemical, physical and biological
characteristics. Generic interaction matrices developed by the IUR [6.1] provide
a convenient checklist for the initial evaluation of relevant environmental
processes. The IUR indicates that the major components of the terrestrial
environment closely relate to those discussed in the BIOMASS project [6.2]. In
the IUR matrices, however, plants and animals are treated as distinct components
rather than aggregated biota. Similarly, soils are subdivided into several distinct
components. The main components in any terrestrial system are thus the
following [6.1]:
(a) The atmosphere, particularly the troposphere, which comprises the region
above the soil surface, including the regions both above and below the plant
canopy.
(b) Surface aquatic bodies (i.e. rivers, streams and lakes) and groundwater,
including aquifers.
(c) Vegetation, including macroscopic vegetation.
(d) Animals (macrofauna) in the terrestrial environment, inhabiting both the
soil surface and areas within the soil (i.e. burrowing animals).
(e) Soil microbiota, comprising microflora and protozoa but excluding larger
organisms such as earthworms and burrowing mammals.
80
CHAPTER 6
(f) The soil, comprising minerals and organic material, aqueous solutions and
the content of air in a soil sample. The characteristics of these various parts
are influenced by the biotic and abiotic conditions in operation.
(g) The interface with the geosphere. This is an artificial frontier. In terrestrial
environments, it will normally be represented by weathered, saturated or
unsaturated materials.
6.2. MAJOR TRANSFER PROCESSES AND PATHWAYS
The decay of
238
U is the ultimate source of
210
Pb and
210
Po (see also
Chapter 4). As a primordial radioisotope,
238
U is present in every component of
the environment to varying degrees. The decay of
238
U leads to the formation
of a series of different elements, including
210
Pb and
210
Po, each with its own
characteristic environmental behaviour. As environmental processes are always
dynamic, there may be disequilibrium between members of the decay series,
depending on how open the system is to external influences (e.g. see Ref.
[6.3]).
The major environmental processes which cause disequilibria and the transfer
of
210
Pb and
210
Po, or their relatively long lived progenitors (including
226
Ra
and
230
Th), from primary sources in the crust to other compartments include the
following:
— Weathering of exposed rocks;
— Leaching or dissolution of rocks by groundwater;
— Volcanic eruptions;
— Fires and burning fossil fuels;
— Exhalation of
222
Rn.
Given that
210
Pb and
210
Po are intrinsically linked by their radiogenic
association, a combined consideration of both of these radionuclides is necessary
to explain the observed patterns of
210
Po transfer in the environment (see Fig. 6.1).
The two main categories of biotic transfer are flora and fauna. Terrestrial
plants can be exposed to
210
Pb and
210
Po via their surface (bark and cuticle of
foliage), interaction with the atmosphere or via their roots, which can take up
the radionuclides from the soil solution. Atmosphere–plant interactions are
explored in Section
6.3. The mobility of radioisotopes in soil and their uptake by
plant roots is discussed in Sections
6.4 and 6.5, and
210
Po transfer in animals in
Section 6.6.

81
TERRESTRIAL SYSTEMS
6.3. ATMOSPHERE–PLANT INTERACTIONS
Polonium and lead activity concentrations on the surfaces of plants can be
affected by many processes, including:
(a) Interception of gases, aerosols or dusts directly from the atmosphere, or by
processes such as rain splash from the soil;
(b) Weathering, which results in the removal of radionuclides from plant
surfaces by various physical processes and comprises the loss of
radionuclides from the plant surface due to various processes, including
wash-off due to rain or irrigation and surface abrasion;
(c) Biological processes (e.g. tissue ageing and leaf fall);
(d) Translocation of radionuclides from the plant surface to other, internal
tissues of the plant.
References [6.4, 6.5] provide detailed discussions about the concepts and
processes involved in air to plant interactions and how these processes can be
quantified. This section provides a broad overview of these ideas and identifies
information specific to polonium.
Plants can come into contact with
210
Pb and
210
Po by deposition of
aerosols from
222
Rn decay or from resuspended dust. The contribution of
atmospheric deposition to the total amount of radionuclides in and on plants
FIG. 6.1. Pathways controlling the movement and disposition of
210
Po in terrestrial
environments.
82
CHAPTER 6
is significant [6.6, 6.7] and can account for up to 95% [6.8]. The two basic
mechanisms governing the rate of radionuclide deposition from the atmosphere
to plants are wet and dry deposition processes. Wet deposition is the transport of
radionuclides from the atmosphere with precipitation (rain, snow or hail); and
dry deposition is a direct flux of radionuclides from the atmosphere in the form
of gases or particulates. Pietrzak-Flis and Skowrońska-Smolak [6.8] find that the
incorporation of the radionuclides occurs mainly as a result of wet deposition.
In addition to deposition, losses of radioactivity through weathering
influence the overall amount of radioactivity retained on the plant surface.
Pietrzak-Flis and Skowrońska-Smolak [6.8] measure these processes and
determine experimentally that the deposition of
210
Po onto a variety of grasses
and crops is in the range of 2–23 Bq/m
2
(6–62 Bq/m
2
in the case of
210
Pb), with
retention proportional to their surface area and the presence of surface features
(in this example possibly due to pockets on barley chaff, but may include factors
such as glabrous or hirsute leaves).
With regard to translocation of the deposited radioactivity, there is
unfortunately limited information available specifically on polonium, thus
restricting the capacity to quantitatively model the relevant foliar uptake
processes. If polonium specific data are absent, appropriate values might be
identified from suitable analogues
[6.9]. Although similar chemical properties of
different elements do not necessarily mean similar behaviour inside the plant, this
approach is still often used in practice, in the absence of other data. Analogues
used for polonium include sulphur, selenium and tellurium (see Section
3.2), all
of which are in Group
16 in the periodic table (chalcogens).
6.3.1. Interception
The extent to which plants can retain radionuclides depends on many
physical, chemical and biological factors. The simplest parameter used for
quantification is the interception fraction f, which is defined as the ratio of
the activity initially retained by the standing vegetation (immediately after
deposition) to the total activity deposited
[6.10]. Since the interception fraction
depends on the stage of development of the plant, the interception fraction is
normalized to the standing biomass B (dry mass) to yield the mass interception
fraction f
B
(m
2
/kg) [6.10]. As the leaf is the main interface between the atmosphere
and vegetation, it is also possible to normalize the interception fraction to the leaf
area index f
LAI
[6.10].
There are no specific data on interception fractions for
210
Po. Using
33
S
as a chemically similar analogue, an f
B
of 0.35 was derived across five species
based on wet deposition
[6.11]. This value was slightly higher than that derived

83
TERRESTRIAL SYSTEMS
for
131
I (0.27) and was around three times lower compared with the other
radionuclides used in the experiment (
109
Cd,
144
Ce,
51
Cr and
85
Sr).
Particle size is another key parameter in determining the extent of dry
deposition along with the vegetation type (often characterized by surface
roughness). Interception is more effective for small particles and reactive
gases
[6.10]. No specific information on interception of polonium by dry
deposition has been identified in the literature. However, data on interception for
different particle sizes, across a range of radionuclides and vegetation types, can
be found in Ref. [6.10].
6.3.2. Physical and biological weathering
Weathering is the loss of contamination from plants. The magnitude of the
weathering loss of a radionuclide depends on many factors, including its solubility,
strength of adsorption to the plant surface, degree of penetration into the inner
flesh and leachability from the interior [6.12]. Biological factors (e.g. regular or
seasonal exfoliation) also play a part in the weathering process. Thus, a complex
interaction of those factors leads to the observed difference in weathering loss
among radionuclides, plant species and their growth stages [6.12]. Despite this
complexity, in models of environmental radionuclide behaviour, weathering is
normally described by a single exponential function characterized by a first order
rate constant λ
w
or a weathering half-life T
w
[6.12].
W
ln2
T
l =
(6.1)
Data from numerous studies have shown limited differences in weathering
between cationic species (such as caesium, cobalt, manganese, ruthenium and
strontium) for most plant species, and that T
w
is dependent on plant characteristics
as well as on plant growth stage at the time of deposition
[6.12]. In general,
therefore, for different elements and plant groups, an assumption can be made
that the weathering half-life for polonium will be in the range of 7.9–49
days (see
table 1 of Ref. [6.12]).
6.3.3. Foliar translocation
Translocation is the process by which elements, water and nutrients
are moved within vascular plants in the phloem and xylem, and can refer to
contaminants either taken up by plants roots or absorbed from the surface of
plants and transferred to other parts that have not been contaminated directly. The
rate of this process reflects the mobility of an element within the plant and can
84
CHAPTER 6
vary greatly. Translocation from roots is discussed in Section 6.5. To quantify this
process for material deposited directly onto the foliage, the translocation factor f
tr
normally used represents the proportion of foliage activity per 1
m
2
of crop at the
time of deposition (Bq/m
2
) associated with the activity in the edible part per 1 m
2
of crop at harvest time (Bq/m
2
) expressed as a percentage [6.13].
There is a lack of information on polonium translocation values; hence, an
analogue could be considered here. However, there is no appropriate analogue
for translocation based on foliar deposition (f
tr
). The only analogue data available
are the proportional translocation of tellurium in root vegetables, reported as
0.8
[6.14, 6.15]. However, Ham et al. [6.7] and Pietrzak-Flis and Skowrońska-
Smolak
[6.8] report that the movement of polonium from roots is negligible.
This highlights the uncertainty associated with the analogue approach, as the
experimental data for polonium have not indicated a translocation factor as high
as that reported for tellurium.
6.4. MOBILITY OF POLONIUM IN SOILS
Surface soils are the interface between the atmosphere, the parent rocks
and the subsurface hydrological system. There are radionuclide fluxes between
these environmental compartments which govern
210
Pb and
210
Po activity
concentrations and how they change in the surface soil. Generally,
210
Po is at, or
very close to, secular equilibrium with
210
Pb in almost all soils [6.16]. However,
the distribution of
210
Po in soil is rather inhomogeneous (see Chapter 4) [6.17].
The concentrations of radionuclides in surface soils vary depending on a number
of processes and are discussed in Sections 6.4.1–6.4.4.
6.4.1. Resuspension
In general, three types of process are used to describe the lateral spread of
contaminants which have been deposited onto surface soil
[6.18, 6.19]: surface
creep, saltation and resuspension. Resuspension occurs when wind exerts a
force exceeding the degree of adherence of particles to the surface material. The
forces in action are the weight of the particle and the adherence, as well as the
aerodynamic loads relating to the flow of wind. There are two basic approaches
used to model resuspension. The resuspension factor is the ratio of volumetric
air concentration to soil contamination. The resuspension rate approach is based
on the ratio of particle flow density to soil contamination, which depends on
the resuspended material (particle size, shape and adherence), the surface type
(roughness and humidity), the time lapsed after deposition, and mechanical
actions (soil processing), if any.
85
TERRESTRIAL SYSTEMS
No specific information is available for polonium. However, because the
variability of measured resuspension factors and rates are very high and the
accuracies of these models’ predictions are rather low, there is no substantial
evidence that the elemental characteristics of radionuclides influence the
processes governing resuspension. This means that the data and models validated
for other radionuclides can also be used for polonium. Overall, the resuspension
factors measured directly after the acute depositions are around 10
−5
/m in
residential areas, on a site undergoing cleanup operations and on an arid site, and
from 10
−8
/m to 10
−6
/m in humid areas. The resuspension factor decreases by up
to 10
−9
/m over 3–4 years after the deposition. Resuspended dust can be a very
localized phenomenon or can reach global scales, such as with the resuspension
of dusts from nuclear test sites in China to Japan and even further
[6.20].
6.4.2. Radon exhalation
One of the major mechanisms which determines changes in
210
Pb
concentrations (and hence
210
Po) in topsoil is exhalation of a fraction of the noble
gas
222
Rn from surface soils to the atmosphere, with subsequent decay to
210
Pb
in the atmosphere and removal to the Earth’s surface by wet and dry deposition
(see Section 5.3). Depending on the local soil and meteorological conditions,
these processes can result in either an excess or deficiency of
210
Pb relative to
226
Ra in the topsoil (primarily in the top 1 m of soil).
Since exhalation flux densities of
222
Rn from soils are about a hundred
times greater than that from oceans, deposition of soil-origin
210
Pb to the oceans
can be expected to cause a deficiency of
210
Pb and
210
Po relative to
226
Ra in
soils. Globally, approximately 90% of
210
Pb deposition is by wet deposition, and
local deposition greatly depends on rainfall
[6.21, 6.22]. A confounding factor
is
210
Pb in leaf litter, which can lead to excess
210
Pb and
210
Po in the surface
layer
[6.23, 6.24]. Owing to the permanent flux from the atmosphere and other
processes, activity concentrations in the topsoil (0–10
cm) tend to be higher
compared with the deeper soil, where
210
Po is in approximate equilibrium with
the
210
Pb and
222
Rn values, within a few tens of Bq/kg [6.17].
6.4.3. Partitioning of the soil Po:Pb ratio into solution
A portion of the radioactivity present in the soil will be adsorbed to soil
particles, while some will dissolve into soil water and hence become available for
root uptake by plants. Significant progress has been made to describe radionuclide
sorption in heterogeneous solids. Nevertheless, models describing sorption are
still mostly based on empirical, solid–liquid distribution coefficient (K
d
) values,

86
CHAPTER 6
which are estimated from sorption studies using reasonably well characterized
homogeneous surfaces (see Section 2.1).
A summary of available K
d
values for lead and polonium for soils, grouped
on the basis of texture and organic matter content criteria, as well as data on
the effect of pH (excluding organic soils), are given in Table 6.1. Soils are
grouped according to the sand and clay mineral percentages, and the organic
matter content of the soil. Some variation is due to pH (8%) and clay content
(24%) but not cation exchange capacity [6.25]. These numbers are estimates,
since adsorption is controlled by a larger range of conditions, including the
concentration of competing ions, the mineralogical and organic composition of
the soils, and the grain size of the constituents (and so the available surface area
for adsorption per gram).
TABLE 6.1. K
d
VALUES FOR SOILS GROUPED ACCORDING TO THE
TEXTURE/ORGANIC MATTER CRITERION (L/kg)
Element Soil group
N
GM GSD AM SD Min. Max.
Pb All soils 23 2.0 × 10
3
1.0 × 10
1
1.5 × 10
4
3.3 × 10
4
2.5 × 10
1
1.3 × 10
5
Sand 9 2.2 × 10
2
4 4.0 × 10
2
4.3 × 10
2
2.5 × 10
1
1.3 × 10
3
Loam 5 1.0 × 10
4
3 1.5 × 10
4
1.6 × 10
4
3.6 × 10
3
4.3 × 10
4
Clay 2 n.a. n.a. 6.6 × 10
4
n.a. 5.4 × 10
3
1.3 × 10
5
Organic 5 2.5 × 10
3
3 3.7 × 10
3
3.8 × 10
3
8.8 × 10
2
1.0 × 10
4
Unspecified 2 n.a. n.a. 5.9 × 10
4
n.a. 1.6 × 10
4
1.0 × 10
5
Po All soils 44 2.1 × 10
2
5 5.6 × 10
2
1.1 × 10
3
1.2 × 10
1
7.0 × 10
3
Sand 14 1.0 × 10
2
6 7.4 × 10
2
1.9 × 10
3
1.7 × 10
1
7.0 × 10
3
Loam 27 2.3 × 10
2
4 4.6 × 10
2
4.6 × 10
2
1.2 × 10
1
1.8 × 10
3
Clay 2 n.a. n.a. 1.7 × 10
3
n.a. 7.2 × 10
2
2.7 × 10
3
Organic 1 n.a. n.a. 6.6 × 10
3
n.a. n.a. n.a.
Source: See Ref. [6.5].
Note: The sand group is ≥65% sand and <18% clay; the clay group is ≥35% clay; the
organic group is ≥20% organic matter; and the rest is assigned to the loam group and
unspecified. AM — arithmetic mean; GM — geometric mean; GSD — geometric
standard deviation; n.a. — not applicable; SD — standard deviation.
87
TERRESTRIAL SYSTEMS
Table 6.1 shows that K
d
values for polonium tend to be lower than those for
lead. This difference can lead to some disequilibrium between these radionuclides
in the fraction assumed to be available for root transfer.
6.5.
210
Pb AND
210
Po TRANSFER FROM SOIL TO PLANTS
6.5.1. Processes governing
210
Pb and
210
Po transfer to plants
Polonium can enter plants via several routes. Most large scale contamination
is generally through air deposition or from contaminated soil. Contamination
can also occur via contaminated surface water (inundation and irrigation) or
contaminated groundwater (upwelling and irrigation)
[6.8, 6.26]. The relative
importance of these pathways depends on the concentration of the radionuclides
in the soil, the soil–plant concentration ratio (CR) and the rate of deposition onto
plant parts above ground. An important implication is that radionuclide activity
concentrations in crops such as in roots, tubers, cereals and legumes, where the
edible portion is protected by inedible plant parts, should not be significantly
affected by direct deposition; whereas the converse is true for leafy vegetables.
Plant type and the ecosystem influence the values thus derived. Transfer factors
(TFs) range across orders of magnitude, depending on the plant and soil type
variations assessed
[6.5].
6.5.2.
210
Pb and
210
Po transfer to mosses, plants and lichens
The factors that determine variability in radionuclide transfer to plants
generally including the following [6.27]:
(a) The form in which the activity enters or is present in the soil (e.g. as
particles, as aerosol or in solution);
(b) The physicochemical properties of the radionuclide;
(c) The time after entry into the soil;
(d) The type of soil and its physicochemical characteristics;
(e) The type of crop;
(f) Crop management practices (the application of fertilizers, irrigation,
ploughing and liming);
(g) Climatic conditions;
(h) The experimental conditions under which the TFs were obtained.
88
CHAPTER 6
The significance of these factors varies depending on the radionuclide of
interest and the contamination scenario. Table 6.2 presents data from the 1970s
on the influence of different steppe ecosystems on
210
Po transfer to plants.
From Table 6.2, the activity concentrations in the above ground biomass
are in the range of 3.3–16.3
Bq/kg (DW) for
210
Po and 9.3–49.6 Bq/kg (DW) for
210
Pb. For both
210
Pb and
210
Po, there are higher concentrations in the underground
biomass (roots). Maximum activity concentrations of
210
Pb and
210
Po occur in
perennial sawn grasses, probably because of the application of mineral fertilizers
containing a mixture of naturally occurring radionuclides
[6.29]. The
210
Po:
210
Pb
ratios in plants are lower than those for topsoils, indicating higher transfer rates
for
210
Pb compared with
210
Po [6.29].
Taskayev and Testov
[6.29] present data on
210
Po transfer to 24 species of
plants and mosses representative of northern taiga and collected from areas with
naturally elevated radiation backgrounds (see Table 6.3).
One type of vegetation of particular interest in the assessment of
210
Po is
lichen. Lichens are slow growing symbiotic associations (fungi plus algae or
cyanobacteria) that grow on trees, rocks and soils. They have no true roots and,
despite accessing some mineral nutrition from rocks or organic nutrients from
bark, are highly dependent on the atmosphere for nutrition. They have a long
lifespan; thus, activity concentrations of
210
Po are supported by
210
Pb ingrowth
in addition to direct deposition. Lichens are important for human populations in
northern climates because reindeer or caribou (which are key components of the
human diet) ingest large quantities in winter. The importance of lichens has not
been considered to the same extent for food chains involving herbivorous species
in other climatic zones.
In a 1978 study, Ermolayeva-Makovskaya and Litver
[6.30] report a high
mean value of 260
Bq/kg (DW) for both
210
Pb and
210
Po in lichens of the polar
and subpolar zones of the Russian Federation, from the Cola Peninsula to Chukot.
Slightly lower values (70–212
Bq/kg, DW) of
210
Po activity concentrations in
lichens in Nordic terrestrial ecosystems are presented in Refs [6.24, 6.31].
Data on lichens in northern Canada show mean activity concentrations of
275
Bq/kg (DW) for Cladina mitis and 622 Bq/kg (DW) for Cetraria
nivalis
[6.32]. The data indicate that the highest activity concentrations of
210
Pb
and
210
Po in above ground biomass occur in mosses and lichens, followed by
club-mosses and ferns, and then grasses.
6.5.3.
210
Pb and
210
Po transfer to berries, mushrooms and understory
species
Several comprehensive studies in Finland and France have greatly extended
the information on
210
Pb and
210
Po transfers to forest products, mainly mushrooms

89
TERRESTRIAL SYSTEMS
TABLE 6.2. ACTIVITY CONCENTRATIONS OF
210
Po AND
210
Pb IN MEADOW PLANTS OF DIFFERENT STEPPE
ECOSYSTEMS
Ecosystem
210
Po (Bq/kg, DW)
210
Pb (Bq/kg, DW)
210
Po:
210
Pb ratio
Above ground
biomass
Roots Ratio Above ground
biomass
Roots Ratio Above ground
biomass
Roots
Natural steppe 7.0 ± 4.4 42 ± 14 0.17 ± 0.1 17.5 ± 8.1 44 ± 15 0.41 ± 0.1 0.39 ± 0.1 0.96 ± 0.1
Saline meadow 3.7 ± 0.7 25 ± 24 0.16 ± 0.1 9.3 ± 2.6 31 ± 18 0.42 ± 0.3 0.4 ± 0.05 1.25 ± 0.2
Bog meadow 3.3 ± 2.0 23 ± 7 0.14 ± 0.1 11.8 ± 2.0 18 ± 4 0.62 ± 0.3 0.28 ± 0.05 1.25 ± 0.2
Sawn grasses 16.3 ± 6.7 —
a
—
a
49.6 ± 23.7 —
a
—
a
0.34 ± 0.08 —
a
Trees/bushes 13.7 ± 4.4 —
a
—
a
32.6 ± 13.3 —
a
—
a
—
a
—
a
Mean
8.1
± 5.9 30 ± 19 0.19 ± 0.1 21.5 ± 17 32 ± 17 0.47 ± 0.3 0.38 ± 0.12 0.97 ± 0.3
Source: See Ref. [6.28].
a
—: data not available.

90
CHAPTER 6
and berries, at different forest sites (Scots pine, Norway spruce, mixed forest and
Downy birch).
Vaaramaa et al.
[6.23] present data on
210
Pb and
210
Po in wild berries
(blueberry and lingonberry) and mushrooms in boreal forest ecosystems in
Finland. The lingonberry is an evergreen dwarf shrub and the blueberry is a
deciduous plant (see Table 6.4).
TABLE 6.3. CONCENTRATIONS OF
210
Po IN PLANTS OF
NORTHERN TAIGA
Group Species
210
Po
(Bq/kg, DW)
Mosses
Conocephalum conicum
1480
Polytrichum commune
353
Mnium cinclidioides
293
Pleurozium schreberi
292
Sphagnum
274
Club-mosses
Lycopodium annotinum L.
100
Gymnocarpium dryopteris
80
Ferns
Dryopteris filix-mas L.
70
Grasses
Solidago virgaurea L.
60
Trientalis L.
55
Caltha palustris
47
Polygonum L.
46
Geum rivale L.
39
Filipendula ulmaria
37
Veratrum lobelianum
34
Anemone
34
Chamerion angustifolium
33
Equisetum arvense
27
Juncus L.
20
Sanguisorba officinalis
13
Source: See Ref. [6.29].
Note: Range is 13–1480 Bq/kg (DW); overall mean is 145 Bq/kg (DW);
standard deviation is 300 Bq/kg (DW); geometric mean is
61 Bq/kg (DW); median is 42 Bq/kg (DW); geometric standard
deviation is 1.1.

91
TERRESTRIAL SYSTEMS
TABLE 6.4. ACTIVITY CONCENTRATIONS OF
210
Po AND
210
Pb IN WILD BERRIES IN FINLAND
Wild berry Site
210
Pb
(Bq/kg, DW)
210
Po
(Bq/kg, DW)
Blueberry N. Finland
S. Finland
0.7 ± 0.1
1.7 ± 0.3
3.2 ± 0.6
2.5 ± 0.4
Lingonberry N. Finland
S. Finland
1.4 ± 0.2
3.2 ± 0.3
2.2 ± 0.3
7.5 ± 1.0
Source: See Ref. [6.23].
The activity concentrations of
210
Pb and
210
Po in wild berries do not show
clear dependence on the type of forest and are quite similar to those reported by
Solatie et al.
[6.33] for Finnish Lapland and McDonald et al. [6.34] for samples
taken in England and Wales.
The highest
210
Pb (
210
Po) concentrations (dry weight) in blueberry shrubs
were found to be the following
[6.23]:
— Lignified brown stem of the blueberry shrub: 35 (80) Bq/kg;
— Roots: 33 (48) Bq/kg;
— Green stem 30 (29) Bq/kg;
— Leaves 16 (27) Bq/kg.
For lingonberry shrubs, the highest
210
Po concentrations (dry weight) were
found in the roots and rhizome (100 Bq/kg), followed by the current year stem
(90 Bq/kg), the lignified brown stem (30 Bq/kg), and the current year and old
leaves (22–26 Bq/kg) [6.23]. In general, the activity concentrations of
210
Pb and
210
Po in leaves and small tree branches were higher than those in above ground
biomass of grassy plants because of higher interception of the radionuclides from
the atmosphere, due to a higher leaf area index and a longer period of vegetation
during the year.
Although the
210
Po:
210
Pb activity ratios in the organic horizon of the
studied forests was close to 0.9, in most cases the concentrations of
210
Po in plant
tissues exceeded those of
210
Pb (
210
Po:
210
Pb varied from 1.5 to 2.5), except in
the lignified stem of lingonberry where the concentration of
210
Pb was 1.5 times
higher than
210
Po [6.23]. This might indicate that
210
Po is characterized by both
a higher transfer rate than
210
Pb from forest soil to plant compartments and by a
higher mobility within the plant. However, more precise research is needed to
confirm or reject these statements.
92
CHAPTER 6
According to the literature, fungi have been shown to accumulate stable
lead to much higher concentrations than berries
[6.35]. However, because of
their short lifespan, direct atmospheric deposition on the surfaces of the fruiting
bodies of mushrooms cannot be the major uptake route for elements
[6.36]. Thus,
environmental properties of the site where fungi grow can be more important for
radionuclide transfer
[6.23, 6.35, 6.36] provide information on
210
Pb and
210
Po
transfer to many species of mushrooms.
Vaaramaa et
al. [6.23] find that the activity concentrations for
210
Pb and
210
Po in mushrooms in Finland are generally higher than wild berries, especially in
the case of
210
Po. The dry weight range for
210
Pb is 0.97–16.2 Bq/kg, and is much
wider for
210
Po, 13.9–1200 Bq/kg. They find the highest mean concentration of
210
Po in the fruiting bodies of the following families [6.23]:
— Boletaceae: 380 Bq/kg (DW);
— Russulaceae: 32.2 Bq/kg (DW);
— Cortinariaceae: 10 Bq/kg (DW).
They find the minimum mean values for
210
Po for mushrooms in the
following families [6.23]:
— Hygrophorus: 2.5/3.1 Bq/kg (DW) in the cap/stipe;
— Albatrellus: 3.8 Bq/kg (DW) in the whole fruit body;
— Ratio of the activity concentration between the caps and stipes: 1.6–7.1.
As for
210
Pb, considering the same species in different forest types,
Vaaramaa et al. [6.23] find the lowest activity concentrations in mushrooms
of the Boletaceae family (3.0 Bq/kg, DW) and the highest in Russulaceae
(11.1 Bq/kg, DW) and Cortinariaceae (11.1 Bq/kg, DW) [6.23]. Overall, these
data are similar to the
210
Pb concentrations (1.76–36.5 Bq/kg, DW) measured in
various mushrooms collected from the forests of France
[6.37].
In the northern Finland site, Vaaramaa et al. [6.23] report:
“No differences between forest types [Norway spruce, Scots pine, Downy
birch and mixed forest] were found in the concentrations of
210
Pb in the
whole fruiting bodies (cap and stipe combined), except in the activity
concentration of
210
Pb in Cortinarius armillatus L. collected from mixed
forest….
.......
93
TERRESTRIAL SYSTEMS
“The activity concentrations of
210
Pb were higher in the caps of the
mushrooms than in the stipes for Lactarius rufus
L. and Russula paludosa L.
The average cap-to-stipe ratio of
210
Pb in the Boletaceae family was 1.0,
which is in agreement with the mean ratio for
210
Pb (1.1) in Xerocomus
badius
L. (Boletaceae family) analyzed by Malinowska et al. [Ref. [6.38]].”
These values were statistically significantly higher than those for
210
Pb,
reflecting higher mobility of
210
Po among tissues of mushrooms. Vaaramaa et
al. [6.23] find that:
“The
210
Po/
210
Pb ratio in the mushrooms varied widely from 0.87 to 322 in
whole fruiting bodies, mainly being higher than one. The lowest value was
recorded in R.
paludosa L. and the highest in L. vulpinum L. In S. luteus L.
the ratio of
210
Po/
210
Pb has been reported to be 22 and 47 in Finnish Lapland
(Solatie et
al. [Ref. [6.33]]). In the present study, the ratio was 17 for the
same species in Scots pine forest of the northern Finland site.... The results
may indicate that
210
Po is more effectively taken up from soil to the fruiting
bodies than
210
Pb.”
6.5.4.
210
Pb and
210
Po transfer to agricultural plants
Several comprehensive reviews of radionuclide transfer from soil to plants,
including recommended TF values for both natural and artificial radionuclides
can be found in Refs
[6.4, 6.5] and in Table 6.5 for lead and polonium. The
TF values are for 14 plant groups according to the classifications presented in
Chapter
4 (see Ref. [6.5] for assignments of individual plants to these groups,
and the plant compartments considered). The TF values depend on the plants and
soil types assessed. Among the lowest values for both elements is maize grain
(5.2 × 10
−4
for lead and 1.8 × 10
−5
for polonium); while the highest are 1.0 for
polonium in pasture vegetation and 25 for lead in leafy vegetables.
However, when analysing the data in Table 6.5, it should be noted that
the radioactivity in the plant (or plant compartments) used for assessing the TF
(or CR) values is not only acquired through root transfer. The above ground
biomass might also be contaminated because of resuspension or direct deposition
of radionuclides from the atmosphere [6.8] (see also Section 6.3). These
atmospheric fluxes are of secondary importance for many radionuclides (such as
137
Cs and
90
Sr), but that might not be the case for
210
Pb and
210
Po, for which the
foliar pathway dominates in some contamination scenarios. In this context, plants
with edible compartments located above ground do not experience the same
contamination conditions as root crops and tubers, which can be contaminated

94
CHAPTER 6
TABLE 6.5. SOIL TO PLANT TRANSFER FACTORS FOR LEAD AND POLONIUM
Element Plant group Plant compartment Soil group
N
Mean/value GSD/SD
a
Min. Max.
Temperate environments
Pb Cereals Grain All 9 1.1 × 10
−2
3.6 1.9 × 10
−3
4.8 × 10
−2
Stems and shoots All 4 2.3 × 10
−2
3.5 5.1 × 10
−3
9.6 × 10
−2
Maize Grain All 9 1.2 × 10
−3
2.3 5.2 × 10
−4
3.8 × 10
−3
Stems and shoots All 3 2.8 × 10
−3
6.6 6.0 × 10
−4
2.3 × 10
−2
Leafy vegetables Leaves All 31 8.0 × 10
−2
1.3 × 10
1
3.2 × 10
−3
2.5 × 10
1
Sand 4 7.3 × 10
−2
1.5 4.9 × 10
−2
1.1 × 10
−1
Loam 3 8.2 × 10
−1
1.0 7.9 × 10
−1
8.6 × 10
−1
Clay 7 2.8 × 10
−2
4.1 4.1 × 10
−3
1.2 × 10
−1
Non-leafy vegetables Fruits, heads, berries,
buds
All 5 1.5 × 10
−2
2.6 × 10
1
1.5 × 10
−3
3.9
Stems and shoots All 2 8.8 × 10
−3
—
b
5.8 × 10
−3
1.2 × 10
−2

95
TERRESTRIAL SYSTEMS
TABLE 6.5. SOIL TO PLANT TRANSFER FACTORS FOR LEAD AND POLONIUM (cont.)
Element Plant group Plant compartment Soil group
N
Mean/value GSD/SD
a
Min. Max.
Pb Leguminous vegetables Seeds and pods All 17 5.3 × 10
−3
1.2 × 10
1
4.6 × 10
−4
4.9
Sand 3 2.7 × 10
−3
3.2 6.5 × 10
−4
8.9 × 10
−3
Loam 5 1.4 × 10
−3
4.4 6.5 × 10
−4
8.9 × 10
−3
Clay 4 8.0 × 10
−4
1.0 4.6 × 10
−4
1.0 × 10
−2
Stems and shoots All 1 8.0 × 10
−4
—
b
—
b
—
b
Root crops Roots All 27 1.5 × 10
−2
1.6 × 10
1
2.4 × 10
−4
3.3
Sand 5 6.4 × 10
−2
1.6 4.2 × 10
−2
1.2 × 10
−1
Loam 5 2.3 × 10
−3
4.7 2.4 × 10
−4
1.7 × 10
−2
Stems and shoots All 12 6.3 × 10
−2
150 3.0 × 10
−3
1.6 × 10
1
Tubers Tubers All 30 1.5 × 10
−3
7.4 1.5 × 10
−4
2.6
Sand 5 6.4 × 10
−3
3.5 1.6 × 10
−3
3.9 × 10
−2
Loam 17 5.2 × 10
−4
2.4 1.5 × 10
−4
2.3 × 10
−3
Grasses Stems and shoots All 17 3.1 × 10
−1
1.8 1.1 × 10
−1
1.0

96
CHAPTER 6
TABLE 6.5. SOIL TO PLANT TRANSFER FACTORS FOR LEAD AND POLONIUM (cont.)
Element Plant group Plant compartment Soil group
N
Mean/value GSD/SD
a
Min. Max.
Pb Pasture Stems and shoots All 34 9.2 × 10
−2
4.8 2.2 × 10
−3
1.0
Leguminous fodder Stems and shoots All 1 1.6 × 10
−2
n.a.
c
n.a.
c
n.a.
c
Po Cereals Grain All 2 2.4 × 10
−4
—
b
2.2 × 10
−4
2.6 × 10
−4
Maize Grain All 2 2.4 × 10
−4
—
b
1.8 × 10
−5
4.7 × 10
−4
Leafy vegetables Leaves All 12 7.4 × 10
−3
6.9 2.5 × 10
−4
5.0 × 10
−2
Non-leafy vegetables Stems and shoots All 2 1.9 × 10
−4
—
b
1.6 × 10
−5
3.7 × 10
−4
Leguminous vegetables Seeds and pods All 4 2.7 × 10
−4
3.9 6.0 × 10
−5
1.0 × 10
−3
Root crops Roots All 10 5.8 × 10
−3
4.3 2.4 × 10
−4
4.9 × 10
−2
Stems and shoots All 2 7.7 × 10
−2
—
b
5.8 × 10
−2
9.7 × 10
−2
Tubers Tubers All 9 2.7 × 10
−3
5.8 1.4 × 10
−4
3.4 × 10
−2
Pasture Stems and shoots All 10 1.2 × 10
−1
4.2 2.2 × 10
−2
1.0
Leguminous fodder Stems and shoots All 2 1.1 × 10
−2
—
b
2.6 × 10
−5
2.2 × 10
−4

97
TERRESTRIAL SYSTEMS
TABLE 6.5. SOIL TO PLANT TRANSFER FACTORS FOR LEAD AND POLONIUM (cont.)
Element Plant group Plant compartment Soil group
N
Mean/value GSD/SD
a
Min. Max.
Tropical environments
Pb
d
Cereals Grain Sand 1 2.5 × 10
−3
n.a.
c
n.a.
c
n.a.
c
Grasses Leaves Sand 9 2.1 × 10
−1
2.2 5.9 × 10
−2
1.00
Herbs Leaves Sand 2 3.7 × 10
−1
—
b
2.0 × 10
−2
7.1 × 10
−1
Leguminous
vegetables
Grain All 9 3.3 × 10
−3
2.4 6.5 × 10
−4
8.9 × 10
−3
Sand 3 3.4 × 10
−3
1.3 2.8 × 10
−3
4.4 × 10
−3
Loam 6 3.2 × 10
−3
3.0 6.5 × 10
−4
8.9 × 10
−3
Other crops Sand 18 2.3 × 10
−1
2.7 1.4 × 10
−2
1.0
Non-leafy vegetables All 2 7.0 × 10
−3
—
b
7.0 × 10
−3
7.0 × 10
−3
Pasture Stems and shoots Unspecified 1 3.0 × 10
−1
n.a.
c
n.a.
c
n.a.
c
Root crops Roots Loam 3 2.4 × 10
−3
1.5 1.8 × 10
−3
4.0 × 10
−3

98
CHAPTER 6
TABLE 6.5. SOIL TO PLANT TRANSFER FACTORS FOR LEAD AND POLONIUM (cont.)
Element Plant group Plant compartment Soil group
N
Mean/value GSD/SD
a
Min. Max.
Pb
d
Tubers Tubers All 16 5.7 × 10
−4
2.4 1.5 × 10
−4
2.3 × 10
−3
Sand 1 1.6 × 10
−3
n.a.
b
n.a.
b
n.a.
b
Loam 15 5.3 × 10
−4
2.4 1.5 × 10
−4
2.3 × 10
−3
Maize Grain All 6 8.5 × 10
−4
2.1 5.2 × 10
−4
3.8 × 10
−3
Sand 1 5.2 × 10
−4
n.a.
b
n.a.
b
n.a.
b
Loam 5 9.3 × 10
−4
2.2 5.9 × 10
−4
3.8 × 10
−3
Source: See Ref. [6.5].
a
Geometric standard deviation/standard deviation.
b
—: data not available.
c
n.a.: not applicable.
d
Data for polonium not available.
99
TERRESTRIAL SYSTEMS
through root uptake. Therefore, observed concentrations for leafy vegetables can
be up to 3–5 times higher due to deposition effects. It is also known that slowly
maturing plants have much lower transfer coefficients for
210
Po than rapidly
maturing plants under greenhouse conditions [6.39].
On the other hand, the concept of soil to plant TFs is adopted as a
reasonable empirical measure of plant contamination under steady state
conditions, when radionuclide fluxes corresponding to different pathways are
already well balanced. A general point is that the data in Table
6.5 also reflect
existing differences in mobility between radionuclides in soil and tend to be in
compliance with the data on K
d
values given in Table 6.1. In particular, TF values
for clay soils (where available) are normally about three times lower than those
for light sandy soils, and this ratio is close to the ratio for the K
d
values for the
same radionuclides and soil types.
6.6.
210
Pb AND
210
Po TRANSFER TO ANIMALS
In this section, transfer to animals of both lead and polonium are considered
because
210
Pb transferred to animal tissues decays to
210
Po. Therefore, its transfer
characteristics and rates to various animals are also relevant for polonium.
6.6.1. Processes governing distribution of
210
Pb and
210
Po in animals
Lead-210 is largely retained in bone, while
210
Po is distributed mainly
in soft tissue (see Table
6.6). Target tissues for
210
Po are the spleen, liver and
kidneys. However, the observed
210
Po:
210
Pb ratios in tissues are time dependent
and can be substantially modified by continuing radioactive decay of
210
Pb
resulting in support for
210
Po, as well as by the difference in biological half-lives
of these radionuclides in the different tissues. The rates of these processes greatly
depend on the animals and tissues of interest, and can vary across a wide range.
Following
210
Po administration to rats under laboratory conditions, the
highest concentrations are observed in the spleen, kidney, liver and other soft
tissue [6.40]. In wild animals (e.g. roe deer), the highest concentrations are
observed in kidney, liver and bone [6.30].
Some of the most detailed evidence of animal dependent distribution of
210
Po to
210
Pb is given by Maslov and Testov [6.41] in the 1970s, who report data
on
210
Pb and
210
Po activity in small mouse-like rodents (bank vole, six months
old) and reindeer (six years old) (see Table 6.7).
Differences in the accumulation of
210
Po, and the ratio with
210
Pb, in small
rodents and reindeer can be explained by the origin of the contamination. In the
bank vole, the
210
Po accumulated in some target organs (e.g. spleen, kidney and

100
CHAPTER 6
other soft tissue) originates from the diet and not, to any great extent, from
210
Pb
decay in the body. Therefore, the
210
Po:
210
Pb ratios in different tissues of these
small animals (with relatively short lifespans) typically exceed unity. In contrast,
the ratios in reindeer are normally less than one (0.02–0.3), since bone contains a
substantial amount of older
210
Pb accumulated in the animals as a consequence of
their longer lifespan. Data for other species are given in Table
6.8.
The highest accumulation of
210
Po was observed in small rodents and
earthworms, followed by reptiles and amphibians. For
210
Pb, the highest activity
concentrations (around 50 Bq/kg, DW) were found in earthworms. The mobility
of
210
Po varies in different species:
210
Po:
210
Pb ratios were highest for rodents,
TABLE 6.6. CONCENTRATIONS OF
210
Pb AND
210
Po:
210
Pb RATIOS IN
BONE AND MUSCLE OF ANIMALS IN FINLAND
Animal
Bone Muscle
210
Pb
(Bq/kg, DW)
210
Po:
210
Pb
210
Pb
(Bq/kg, DW)
210
Po:
210
Pb
Deer 1338 0.56 0.17 24
Moose 18.5 0.54 0.06 12.5
Wolf 9.6 0.69 0.02 100
Wolverine 22.9 0.67 0.08 90
TABLE 6.7. ACTIVITY OF
210
Po IN ANIMALS WITH DIFFERENT
LIFESPANS
Tissue
Bank vole Reindeer
210
Po
(mBq)
210
Po ratio
to bone
210
Po
(mBq)
210
Po ratio
to bone
Bone 1–7.8 1 765.9 1
Muscle 27 1.5 23.7 0.02
Liver 23 1.3 261.2 0.3
Spleen 177.6 10 45.9 0.05
Source: See Ref. [6.41].

101
TERRESTRIAL SYSTEMS
while for earthworms they were close to unity. Ratios greater than 100 have also
been reported in the muscle of wild boar [6.42].
6.6.2. Quantification of
210
Pb and
210
Po transfers to agricultural animals
6.6.2.1. Absorption
The International Commission on Radiological Protection (ICRP)
recommends a relatively high fractional gastrointestinal absorption value of 0.5
for polonium [6.43]. Considering ruminants in particular, data specifically for
polonium gastrointestinal absorption have not been identified; however, a lower
mean fractional gastrointestinal absorption value of 0.04 for lead is reported by
TABLE 6.8. WHOLE BODY (EXCLUDING GASTROINTESTINAL TRACT)
ACTIVITY CONCENTRATIONS AND ESTIMATED
210
Po:
210
Pb RATIOS IN
NORDIC WILD SPECIES
Species N
210
Po (Bq/kg, DW)
210
Pb (Bq/kg, DW)
210
Po:
210
Pb
a
Mean SD Mean SD
Common shrew 9 37.2 19 0.3 0.4 258
Bank vole 8 65.1 17.3 1.5 1.1 77
Willow grouse 5 3.3 1.2 2.1 1.6 2.2
Red earthworm 2 48.6 28.7 50 8 1.0
Grey worm 5 57.2 42.3 47 23 1.5
Viper 1 20.9
21.84
—
b
—
b
13.05
12.09
—
b
—
b
—
b
Frog 1 5.65
5.88
—
b
—
b
3.39
2.98
—
b
—
b
—
b
Source: Adapted from Ref. [6.24].
Note: SD — standard deviation. The intestinal tracts of the worms were not removed.
a
The
210
Po:
210
Pb ratios are averages based on individual sample values rather than species
means.
b
—: data not available.
102
CHAPTER 6
Howard et al. [6.44]. The value is within the range of 0.01–0.1 recommended for
polonium in humans by the ICRP [6.43].
6.6.2.2. Transfer to animal food products
The major pathway for polonium transfer to animals is through ingestion
of feed and water. Parameters used to estimate that transfer are the transfer
coefficient — F
m
and F
f
for milk and flesh, respectively — and the CR (for a
definition, see Chapter
2). Given its easy absorption in the gut, the transfer to
meat and milk is relatively high for some animal products. As lead accumulates
in bone, its transfer to milk and meat (although higher in some offal) might be
expected to be lower than polonium. However, data to make the comparison are
presently inadequate to test this assumption for most products (see Table 6.9).
The transfer coefficient for polonium to cow milk is based on four data
sources and is lower than that for goat milk [6.45–6.48]. The difference does not
mean that higher activity concentrations are expected in goat milk, as transfer
coefficients include the amount of feed ingested [6.4, 6.5, 6.49]. Since CRs for
milk for all ruminants are similar, it is likely that the polonium CR for cow milk
would be a reasonable value to use for polonium in goat and sheep milk [6.49].
The polonium CR for beef is based on only one data source
[6.50] and there is
some uncertainty concerning its derivation (lack of information on weight basis),
so it has not been included in Table
6.9.
Using data from the United Nations Scientific Committee on the Effects
of Atomic Radiation (UNSCEAR), the reference concentration (FW) for
210
Po
in milk and meat is 15
mBq/kg and 60 mBq/kg, respectively [6.51]. When this
is compared with a mean value (33.5 Bq/kg, DW) of natural steppe and sawn
grass (see Table
6.2) and all grass species (see Table 6.3), the respective CRs are
4.5
× 10
−4
and 1.8 × 19
−3
. These CRs are based on specific types of ecosystem for
vegetation and unassociated milk and are therefore uncertain. However, the value
for cow milk is similar to Ref.
[6.5]. A direct comparison of transfer of lead and
polonium is only possible for cow milk. In this case, both F
m
and CRs are similar
for the two elements. The CR for beef would be expected to be lower than for
reindeer meat, so the value given based on UNSCEAR data
[6.51] might be more
appropriate than the value in Ref. [6.5].
The transfer coefficient for polonium is relatively high for poultry meat and
egg contents. However, this might be the result of the values being derived from
unrelated feed and food samples
[6.52].
The equivalent transfer values for lead are given in Table
6.10. In
addition, similar values for cow milk of F
m
and CR of 3.6 × 10
−4
and 7.3 × 10
−3
,
respectively, are reported in Ref.
[6.53], which also reports higher values of
F
f
and CR for beef (n = 3) for lead of 0.01 and 0.11, respectively. The values

103
TERRESTRIAL SYSTEMS
incorporate review information from animal nutrition studies as well as other
literature on metal transfer.
6.6.3. Transfer to terrestrial animals
The transfer of polonium to products such as game species is not quantified
in Refs
[6.4, 6.5], and therefore, data specifically on the human food chain are
not available. However, the transfer of radionuclides to wildlife has recently
been published in Refs
[6.54, 6.55], in which the transfer to terrestrial animals is
reported as CRs between the fresh weight whole body (excluding shell, gut and
contents, feathers and pelt) and dry weight soil for a few species (see Table
6.11).
The CR
wo-soil
for polonium transfer to terrestrial animals is high compared
to many other radionuclides. These values are not directly comparable to those
given above for beef, as the CRs are for the whole organism (including bone),
and the denominator is soil, not ingested feed. The especially high transfer of
polonium to reindeer is well known and has already been discussed
[6.56]. The
CR
wo-soil
for different mammal categories varies widely, with a much lower value
for herbivores than those carnivores or omnivores. The high CR
wo-soil
for reptiles
is influenced by relatively high values from Australia
[6.57] and may be due to
acidic spray from mine tailings
[6.58].
TABLE 6.9. TRANSFER COEFFICIENTS AND CONCENTRATION RATIOS
FOR POLONIUM TRANSFER TO ANIMALS AND FOODSTUFFS
Animal product
Transfer coefficient (d/L or d/kg) Concentration ratio
N
Mean GSD Min. Max.
N
CR
Cow milk 4 2.1 × 10
−4
1.8 8.9 × 10
−5
3. × 10
−4
1 2.4 × 10
−3
Goat milk 2 2.2 × 1
−3
n.a.
a
1.8 × 10
−3
2.7 × 10
−3
—
b
—
b
Poultry 1 2.4 n.a.
a
n.a.
a
n.a.
a
—
b
—
b
Reindeer meat
c
—
b
5 × 10
−2
—
b
—
b
—
b
—
b
5 × 10
−2
Egg 1 3.1 n.a.
a
n.a.
a
n.a.
a
—
b
—
b
Sources: See Refs [6.5, 6.31].
Note: CR — concentration ratio; GSD — geometric standard deviation.
a
n.a.: not applicable.
b
—: data not available.
c
Assuming lichen intake [6.31].

104
CHAPTER 6
TABLE 6.11. TRANSFER OF POLONIUM TO THE WHOLE BODY OF
TERRESTIAL ANIMALS
Wildlife group
CR
wo-soil
N
AM ASD GM GSD Min. Max.
Annelid 7 1.0 × 10
−1
3.9 × 10
−2
9.6 × 10
−2
1.4 —
a
—
a
Bird
Herbivorous
5
1.0 × 10
−2
2.9 × 10
−3
9.6 × 10
−3
1.3
—
a
—
a
Mammal
Carnivorous
Herbivorous
Omnivorous
Rangifer spp.
67
11
38
10
199
8.6 × 10
−2
1.2 × 10
−1
2.9 × 10
−3
2.1 × 10
−1
2.5
2.1 × 10
−1
8.7 × 10
−2
1.9 × 10
−3
1.2 × 10
−1
3.7
3.3 × 10
−2
9.7 × 10
−2
2.4 × 10
−3
1.8 × 10
−1
1.4
4.0
1.9
1.8
1.7
3.0
2.4 × 10
−4
1.9 × 10
−2
2.4 × 10
−4
7.5 × 10
−4
5.9 × 10
−1
1.1
1.4 × 10
−1
9.5 × 10
−3
2.6 × 10
−1
21
Reptile 15 9.5 23 3.6 4.0 1.9 × 10
–2
11
Source: See Refs [6.54, 6.55].
Note: CR
wo-soil
is the activity concentration in the whole organism (in Bq/kg, FW) divided by
the activity concentration in the soil (in Bq/kg, DW). Rangifer spp. include reindeer
and caribou. AM — arithmetic mean; ASD — arithmetic standard deviation; CR —
concentration ratio; GM — geometric mean; GSD — geometric standard deviation.
a
—: data not available.
TABLE 6.10. TRANSFER COEFFICIENTS FOR LEAD TRANSFER TO
ANIMALS AND FOODSTUFFS
Animal product
Transfer coefficient (d/L and d/kg) Concentration ratio
N
Mean GSD Min. Max.
N
CR
Cow milk 4 1.9 × 10
−4
1.0 7.3 × 10
−6
1.2 × 10
−3
1 2.4 × 10
−3
Goat milk 1 6.0 × 10
−3
n.a.
a
n.a.
a
n.a.
a
1 9.0 × 10
−3
Sheep milk 1 3.5 × 10
−2
n.a.
a
n.a.
a
n.a.
a
1 3.0 × 10
−2
Beef 5 7.0 × 10
−4
2.5 2.0 × 10
−4
1.6 × 10
−3
11 7.7 × 10
−2
Mutton 2 7.1 × 10
−3
—
b
4.0 × 10
−3
1.0 × 10
−2
3 1.2 × 10
−2
Pork —
b
—
b
—
b
—
b
—
b
2 6.6 × 10
−1
Source: See Ref. [6.5].
Note: CR — concentration ratio; GSD — geometric standard deviation.
a
n.a.: not applicable.
b
—: data not available.

105
TERRESTRIAL SYSTEMS
The CR
wo-soil
for lead transfer to terrestrial animals (see Table 6.12) are
generally lower than those for polonium. As for polonium, there is relatively
high transfer to Rangifer species. The mammal values are similar for all of
the subcategories. The mammal values in Tables
6.11 and 6.12 are not directly
comparable to those given for beef in Tables
6.9 and 6.10 as the transfer
coefficient includes dietary intake and the CR compares muscle (not whole body)
with ingested feed and not soil.
TABLE 6.12. TRANSFER OF LEAD TO THE WHOLE BODY OF DIFFERENT
WILDLIFE GROUPS
Wildlife group
CR
wo-soil
N
AM ASD GM GSD Min. Max.
Amphibian 24 1.2 × 10
−1
5.2 × 10
−1
2.7 × 10
−2
5.6 8.8 × 10
−4
2.8 × 10
−1
Annelid 647 5.2 × 10
−1
7.5 × 10
−1
2.9 × 10
−1
2.9 2.3 × 10
−3
2.8
Arachnid 2 5.3 × 10
−2
—
a
—
a
—
a
4.3 × 10
−2
6.2 × 10
−2
Arthropod 561 4.0 × 10
−1
4.7 × 10
−1
2.6 × 10
−1
2.5 4.6 × 10
−3
1.0
Bird
Carnivorous
424
6.2 × 10
−2
1.7 × 10
−1
2.1 × 10
−2
4.4
—
a
—
a
Mammal
Carnivorous
Herbivorous
Omnivorous
Rangifer spp.
515
368
92
51
270
3.8 × 10
−2
4.7 × 10
−2
2.0 × 10
−2
1.2 × 10
−2
3.6
3.6 × 10
−2
2.8 × 10
−2
2.7 × 10
−2
6.3 × 10
−2
3.3
2.8 × 10
−2
4.0 × 10
−2
1.2 × 10
−2
2.2 × 10
−3
2.7
2.2
1.7
2.8
6.3
2.2
2.7 × 10
−4
8.8 × 10
−3
1.9 × 10
−3
2.7 × 10
−4
4.0 × 10
−1
2.0 × 10
−1
7.7 × 10
−2
2.0 × 10
−1
3.9 × 10
−2
18
Gastropod 47 7.3 × 10
−3
1.3 × 10
−2
3.6 × 10
−3
3.3 6.1 × 10
−4
3.8 × 10
−2
Reptile
Carnivorous
45
32
3.7 × 10
−1
3.8 × 10
−2
1.0
1.6 × 10
−1
1.3 × 10
−1
8.7 × 10
−3
4.3
5.6
1.4 × 10
−3
1.4 × 10
−3
1.2
7.0 × 10
−2
Source: See Refs [6.54, 6.55].
Note: CR
wo-soil
is the activity concentration in the whole organism (in Bq/kg, FW) divided by
the activity concentration in the soil (in Bq/kg, DW). Rangifer spp. include reindeer
and caribou. AM — arithmetic mean; ASD — arithmetic standard deviation; CR —
concentration ratio; GM — geometric mean; GSD — geometric standard deviation.
a
—: data not available.
106
CHAPTER 6
Generally, the behaviour of
210
Po in terrestrial ecosystems depends on
many processes which have been studied little. The available data are largely
based on field observations that attempt to evaluate the role of individual
factors governing the behaviour of
210
Po. However, it is not always possible to
identify these processes in uncontrolled conditions. Furthermore, the quantity
of
210
Po in environmental compartments depends to some extent on the ambient
concentrations of
210
Pb and the behaviour of
210
Pb and
210
Po. Therefore, both
aspects should be considered when trying to explain any observed patterns
of
210
Po accumulation in specific environmental receptors. The generic data
presented in this chapter can be used for assessing the impacts of polonium on
the environment. However, these values are often based on only a few measured
values or single observations, which are sometimes contradictory. Therefore, the
data presented here should be considered with some caution, bearing in mind the
relatively high uncertainty associated with many of the values.
REFERENCES TO CHAPTER 6
[6.1] INTERNATIONAL UNION OF RADIOECOLOGY, Recommendations for Improving
Predictions of the Long-term Behaviour of
14
C,
36
Cl,
99
Tc,
237
Np and
238
U, IUR
Report
6, IUR, Warrington (2006).
[6.2] INTERNATIONAL ATOMIC ENERGY AGENCY, ‘Reference Biospheres’ for Solid
Radioactive Waste Disposal, Report of BIOMASS Theme 1 of the BIOsphere
Modelling and ASSessment (BIOMASS) Programme, Part of the IAEA Co-ordinated
Research Project on Biosphere Modelling and Assessment (BIOMASS),
IAEA-BIOMASS-6, IAEA, Vienna (2003).
[6.3] IVANOVICH, M., HARMON, R.S. (Eds), Uranium Series Disequilibrium:
Applications to Environmental Problems, Clarendon Press, Oxford
(1982).
[6.4] INTERNATIONAL ATOMIC ENERGY AGENCY, Quantification of Radionuclide
Transfer in Terrestrial and Freshwater Environments for Radiological Assessments,
IAEA-TECDOC-1616, IAEA, Vienna
(2009).
[6.5] INTERNATIONAL ATOMIC ENERGY AGENCY, Handbook of Parameter Values
for the Prediction of Radionuclide Transfer in Terrestrial and Freshwater Environments,
Technical Reports Series No.
472, IAEA, Vienna (2010).
[6.6] MAHON, D.C., MATHEWES, R.W., Uptake of naturally occurring radioisotopes by
vegetation in a region of high radioactivity, Can. J. Soil Sci.
63 (1983) 281–290.
[6.7] HAM, G.J., EWERS, L.W., WILKINS, B.T., Variations in Concentrations of Naturally
Occurring Radionuclides in Foodstuffs, NRPB-M892, National Radiological
Protection Board, Chilton
(1998).
[6.8] PIETRZAK-FLIS, Z., SKOWROŃSKA-SMOLAK, M., Transfer of
210
Pb and
210
Po to
plants via root system and above-ground interception, Sci. Total Environ.
162
(1995)
139–147.
107
TERRESTRIAL SYSTEMS
[6.9] LECLERC, E., TAGAMI, K., UCHIDA, S., VARGA, B., “Use of analogues”,
Quantification of Radionuclide Transfer in Terrestrial and Freshwater Environments
for Radiological Assessments, IAEA-TECDOC-1616, IAEA, Vienna (2009)
605–614.
[6.10] PRÖHL, G., “Interception”, ibid., pp 29–43.
[6.11] HOFFMAN, F.O., THIESSEN, K.M., RAEL, R.M., Comparison of interception and
initial retention of wet-deposited contaminants on leaves of different vegetation types,
Atmos. Environ.
29 (1995) 1771–1775.
[6.12] LECLERC, E., CHOI, Y.H., “Weathering”, Quantification of Radionuclide Transfer in
Terrestrial and Freshwater Environments for Radiological Assessments,
IAEA-TECDOC-1616, IAEA, Vienna (2009)
45–48.
[6.13] LECLERC, E., COLLE, C., MADOZ-ESCANDE, C., CHOI, Y.H., “Translocation”,
ibid., pp
49–62.
[6.14] BAEZA, A., PANIAGUA, J.M., RUFO, M., STERLING, A., BARANDICA, J.,
Radiocaesium and radiostrontium uptake by turnips and broad beans via leaf and root
absorption, Appl. Radiat. Isot.
50 (1999) 467–474.
[6.15] BRAMBILLA, M., FORTUNATI, P., CARINI, F., Foliar and root uptake of
134
Cs,
85
Sr
and
65
Zn in processing tomato plants (Lycopersicon esculentum Mill.), J. Environ.
Radioact.
60 (2002) 351–363.
[6.16] SHEPPARD, S.C., SHEPPARD, M.I., ILIN, M., TAIT, J., SANIPELLI, B., Primordial
radionuclides in Canadian background sites: Secular equilibrium and isotopic
differences, J. Environ. Radioact.
99 (2008) 933–946.
[6.17] COPPIN, F., ROUSSEL-DEBET, S., Comportement du
210
Po en milieu terrestre: revue
bibliographique, Radioprot.
39 (2004) 39–58.
[6.18] JOURDAIN, F., “Resuspension”, Quantification of Radionuclide Transfer in Terrestrial
and Freshwater Environments for Radiological Assessments, IAEA-TECDOC-1616,
IAEA, Vienna (2009) 63–68.
[6.19] ALFARO, S.C., GAUDICHET, A., GOMES, L., MAILLÉ, M., Modelling the size
distribution of a soil aerosol produced by sandblasting, J. Geophys. Res.
102 (1997)
11
239–11 249.
[6.20] KIDO, H., FUJIWARA, H., JAMSRAN, U., ENDO, A., The simulation of long-range
transport of
137
Cs from East Asia to Japan in 2002 and 2006, J. Environ. Radioact. 103
(2012)
7–14.
[6.21] BALKANSKI, Y.J., JACOB, D.J., GARDNER, G.M., GRAUSTEIN, W.C.,
TUREKIAN, K.K., Transport and residence times of tropospheric aerosols inferred
from a global three-dimensional simulation of
210
Pb, J. Geophys. Res. 98 (1993)
20
573–20 586.
[6.22] BASKARAN, M., Po-210 and Pb-210 as atmospheric tracers and global atmospheric
Pb-210 fallout: A review, J. Environ. Radioact.
102 (2011) 500–513.
[6.23] VAARAMAA, K., ARO, L., SOLATIE, D., LEHTO, J., Distribution of
210
Pb and
210
Po
concentrations in wild berries and mushrooms in boreal forest ecosystems, Sci. Total
Environ.
408 (2009) 84–91.
[6.24] BROWN, J.E., et al., Levels and transfer of
210
Po and
210
Pb in Nordic terrestrial
ecosystems, J. Environ. Radioact.
102 (2011) 430–437.
108
CHAPTER 6
[6.25] VANDENHOVE, H., GIL-GARCIA, C., RIGOL, A., VIDAL, M., New best estimates
for radionuclide solid–liquid distribution coefficients in soils — Part
2: Naturally
occurring radionuclides, J. Environ. Radioact.
100 (2009) 697–703.
[6.26] PETTERSSON, H.B.L., HALLSTADIUS, L., REDVALL, R., HOLM, E.,
Radioecology in the vicinity of prospected uranium mining sites in the subarctic
environment, J. Environ. Radioact.
6 (1988) 25–40.
[6.27] SANZHAROVA, N., FESENKO, S., REED, E., “Processses governing radionuclide
transfer to plants”, Quantification of Radionuclide Transfer in Terrestrial and
Freshwater Environments for Radiological Assessments, IAEA-TECDOC-1616,
IAEA, Vienna (2009)
123–138.
[6.28] LADINSKAYA, L.A., PARFENOV, Yu.D., ARUTYNOV, P.S., POPOVA, D.K.,
Natural concentrations of Pb-210 and Po-210 in plants of steppe ecosystems,
Ehkologiya 1 (1976) 32–35 (in Russian).
[6.29] TASKAYEV, A.I., TESTOV, B.V., “Major components of doses to biological
organisms from incorporated primordial radionuclides”, Heavy Natural Radionuclides
in Biosphere (ALEXAKHIN, R.M., Ed.), Nauka, Moscow (1990) 201–217
(in Russian).
[6.30] ERMOLAYEVA-MAKOVSKAYA, A.P., LITVER, B.Y., Lead-210 and polonium-210
in biosphere, Atomizdat, Moscow (1978) (in
Russian).
[6.31] SKUTERUD, L., GWYNN, J.P., GAARE, E., STEINNES, E., HOVE, K.,
90
Sr,
210
Po
and
210
Pb in lichen and reindeer in Norway, J. Environ. Radioact. 84 (2005) 441–456.
[6.32] THOMAS, P.A., SHEARD, J.W., SWANSON, S., Transfer of
210
Po and
210
Pb through
the lichen–caribou–wolf food chain of northern Canada, Health Phys.
66
(1994)
666–677.
[6.33] SOLATIE, D., JUNTTILA, M., VESTERBACKA, P.,
210
Po and
210
Pb in the food chain
lichen–reindeer–man, Radiochemistry (Radiokhimiya)
48 (2006) 632–633.
[6.34] McDONALD, P., JACKSON, D., LEONARD, D.R.P., McKAY, K., An assessment of
210
Pb and
210
Po in terrestrial foodstuffs from regions of England and Wales, J. Environ.
Radioact.
43 (1999) 15–29.
[6.35] PELKONEN, R., ALFTHAN, G., JÄRVINEN, O., Cadmium, Lead, Arsenic and
Nickel in Wild Edible Mushrooms, The Finnish Environment
17, Finnish Environment
Institute, Helsinki
(2006).
[6.36] SVOBODA, L., ZIMMERMANNOVÁ, K., KALAČ, P., Concentrations of mercury,
cadmium, lead and copper in fruiting bodies of edible mushrooms in an emission area
of a copper smelter and a mercury smelter, Sci. Total Environ.
246 (2000) 61–67.
[6.37] KIRCHNER, G., DAILLANT, O., Accumulation of
210
Pb,
226
Ra and radioactive
cesium by fungi, Sci. Total Environ.
222 (1998) 63–70.
[6.38] MALINOWSKA, E., SZEFER, P., BOJANOWSKI, R., Radionuclides content in
Xerocomus badius and other commercial mushrooms from several regions of Poland,
Food Chem.
97 (2006) 19–24.
[6.39] WATTERS, R.L., HANSEN, W.R., The hazards implication of the transfer of
unsupported
210
Po from alkaline soil to plants, Health Phys. 18 (1970) 409–413.
[6.40] PARFENOV, Yu.D., Polonium-210 in the environment and in the human organism, At.
Energy Rev.
12 (1974) 75–143.
109
TERRESTRIAL SYSTEMS
[6.41] MASLOV, V.B., TESTOV, B.V., Activity Concentrations and Distribution of
210
Po and
210
Pb in Animals, Inhibiting Areas with Elevated Radiation Background, Results of
Radioecological Research in Natural Environment, Syktuvkar, Komi Branch, Academy
of Science of the Former USSR (1971)
98–106.
[6.42] MARTIN, P., HANCOCK, G.J., JOHNSTON, A., MURRAY, A.S., Natural-series
radionuclides in traditional North Australian Aboriginal foods, J. Environ. Radioact.
40
(1998)
37–58.
[6.43] INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION, Human
Alimentary Tract Model for Radiological Protection, Publication 100, Elsevier,
Amsterdam (2007).
[6.44] HOWARD, B.J., BERESFORD, N.A., BARNETT, C.L., FESENKO, S.,
Gastrointestinal fractional absorption of radionuclides in adult domestic ruminants, J.
Environ. Radioact.
100 (2009) 1069–1078.
[6.45] WATTERS, R.L., McINROY, J.F., The transfer of
210
Po from cattle feed to milk,
Health Phys.
16 (1969) 221–225.
[6.46] JOHNSON, J.E., WATTERS, R.L.,
210
PoO
2
Metabolism in Ruminants, C00-2044-5,
Colorado State Univ., Colorado
(1972).
[6.47] MOROZ, B.B., PARFENOV, Yu.D., Metabolism and biological effects of
polonium-210, At. Energy Rev.
10 (1972) 175–232.
[6.48] SCHÜTTELKOPF, H., KIEFER, H., “The Ra-226, Pb-210 and Po-210 contamination
of the Black Forest in natural radiation environment”, Natural Radiation Environment
(Proc. 2nd Special Symp. Bombay, 1981) (VOHRA, K.G., MISHRA, V., PILLAI, K.,
SADAÏWAN, S., Eds), John Wiley and Sons, New
York (1982).
[6.49] HOWARD, B.J., BERESFORD, N.A., BARNETT, C.L., FESENKO, S., Quantifying
the transfer of radionuclides to food products from domestic farm animals, J. Environ.
Radioact.
100 (2009) 767–773.
[6.50] MILOSEVIC, Z., HORSIC, E., KLJAJIC, R., BAUMAN, A., “Distribution of
uranium,
226
Ra,
210
Pb and
210
Po in the ecological cycle in mountain regions of central
Yugoslavia”, IRPA
5, Jerusalem, International Radiation Protection Association,
Fontenay-aux-Roses (1980)
1123–1126.
[6.51] UNITED NATIONS SCIENTIFIC COMMITTEE ON THE EFFECTS OF ATOMIC
RADIATION, Sources and Effects of Ionizing Radiation UNSCEAR 2000 Report to
the General Assembly, with Scientific Annexes, Vol. I: Sources, United Nations,
New York (2000) Annex B.
[6.52] IZAK-BIRAN, T., et al., Concentrations of U and Po in animal feed supplements, in
poultry meat and in eggs, Health Phys.
56 (1989) 315–319.
[6.53] SHEPPARD, S.C., LONG, J.M., SANIPELLI, B., Verification of radionuclide transfer
factors to domestic-animal food products, using indigenous elements and with
emphasis on iodine, J. Environ. Radioact.
101 (2010) 895–901.
[6.54] INTERNATIONAL ATOMIC ENERGY AGENCY, Handbook of Parameter Values
for the Prediction of Radionuclide Transfer to Wildlife, Technical Reports Series
No.
479, IAEA, Vienna (2014).
[6.55] HOWARD, B.J., et al., The IAEA handbook on radionuclide transfer to wildlife, J.
Environ. Radioact.
121 (2013) 55–74.
110
CHAPTER 6
[6.56] PERSSON, B.R.R., HOLM, E., Polonium-210 and lead-210 in the terrestrial
environment: A historical review, J. Environ. Radioact.
102 (2011) 420–429.
[6.57] READ, J., PICKERING, R., Ecological and toxicological effects of exposure to an
acidic, radioactive tailings storage, Environ. Monit. Assess.
54 (1999) 69–85.
[6.58] WOOD, M.D., BERESFORD, N.A., SEMENOV, D.V., YANKOVICH, T.L.,
COPPLESTONE, D., Radionuclide transfer to reptiles, Radiat. Environ. Biophys.
49
(2010)
509–530.

111
Chapter 7
210
Pb AND
210
Po IN FRESHWATER AND
GROUNDWATER SYSTEMS
7.1. DESCRIPTION OF THE HYDROLOGICAL CYCLE
The continuous movement of water on, above and below the surface of the
Earth is called the hydrological cycle. Throughout this cycle, water changes from
one phase to another: from solid to liquid to vapour (see Fig.
7.1).
Precipitation runs off the land surface into streams and rivers to fill
standing bodies of water, such as natural lakes and artificial impoundments, on
its way to the sea and comes into contact with river and lake-bed sediments as it
moves. Some evaporates, but a large proportion of precipitation also infiltrates
the ground, where it interacts with soils and rocks. The infiltrating waters then
recharge the underlying groundwater systems (see Section
7.2). Groundwater
discharges into surface waters, such as lakes, rivers and wetlands, as well as into
coastal waters. All these waters and discharges can carry polonium.
The surface water environment includes streams, rivers, lakes, bogs,
fens and wetlands, along with the sediment in each of these water bodies (see
Section 7.3). Polonium in surface water can be directly transferred to other
surface water bodies, or can deposit and migrate in bottom sediments. It can also
FIG. 7.1. Illustration of the hydrological cycle.
112
CHAPTER 7
be taken up by freshwater organisms, thus entering the food chain. The transfer in
surface water occurs through a combination of processes, including
[7.1]:
(a) Diffusion and dispersion caused by gradients of concentration and the
turbulent motion of water;
(b) Transport caused by water currents;
(c) Exchanges of contaminants between the dissolved and solid phases
(suspended solids);
(d) Deposition and remobilization following interaction with suspended matter
and bottom sediments.
Hydraulic processes responsible for radionuclide transfer by water are
mainly independent of radionuclide properties. Exchanges of radionuclides
between the dissolved and solid phases, as well as interaction with abiotic
suspended matter and bottom sediments, are strongly radionuclide specific.
Quantification of radionuclide redistribution in freshwater ecosystems requires
the evaluation of the physicochemical properties of radionuclides in water
and sediments. A description of how these processes can be represented in a
quantitative manner is provided in Ref. [7.2].
Polonium in water can then transfer to biota, such as plants, phytoplankton,
zooplankton, other invertebrates (including molluscs and crustaceans), fish and
water based amphibians, as well as to reptiles, mammals and birds that obtain
dietary components from the aquatic environment.
7.2. GROU NDWATER
7.2.1. Groundwater environment
Groundwater is an important component of the hydrological cycle and
source of drinking water for humans. A simplified typical aquifer system is shown
in Fig. 7.2. While water recharging the groundwater contains some
210
Po from
rainwater, any surface deposition or releases and interactions in the vadose zone,
the
210
Po concentrations further in the aquifer are controlled by local processes,
as steady state conditions are established over very short distances and tend to
break down over longer distances. In recharge zones, as precipitation enters the
subsurface, there are a number of sources of polonium in the water:
— Polonium contained in the rainwater or deposited on soils from the
atmosphere (i.e. wet or dry deposition; see Chapter
5);
— Polonium already in soils (see Chapter 6);

113
FRESHWATER AND GROUNDWATER SYSTEMS
— Anthropogenic releases (see Chapter 4);
— Polonium produced by progenitor nuclides in the
238
U decay series
(see Fig. 3.1, in Chapter 3) that have similar origins.
However, owing to its short half-life,
210
Po from sources confined to the near
surface cannot migrate far before decaying away. As the water begins to interact
with the surrounding minerals, both within the vadose zone and the underlying
aquifers, there are a number of factors that control the distribution of polonium
in the groundwater, including: the decay of the parent
210
Bi (which is the very
short lived daughter of
210
Pb) that is dissolved or adsorbed; the decay of
210
Po,
supply by recoil from mineral grains; and adsorption. The short half-life means
that the concentration is determined by the conditions in the immediate area.
Groundwater discharges into surface waters (see Section 7.3) and coastal waters
and are thus important inputs to these environments. These inputs are likely to
be moderated by interactions within sediments at the sediment–water interface,
although there is insufficient data to document the general characteristics of the
relationships between discharging groundwater and surface waters.
The behaviour of polonium in groundwater has been studied much less than
on other radionuclides — presumably because it is relatively immobile and short
lived. However, it has sometimes been found to be a significant contributor to the
overall gross alpha activity of groundwater, which has in turn led to a number of
regional studies.
7.2.2. Sources of
210
Pb and
210
Po in groundwater
An important input of
210
Po is the decay of parent isotopes dissolved within
the groundwater or adsorbed onto aquifer surfaces. Therefore, the distribution of
FIG. 7.2. A simplified view of groundwater flow and the dominant processes controlling the
distribution of
210
Po within aquifers.

114
CHAPTER 7
progenitor atoms is important, with the distribution of uranium controlling the
distribution and release of
230
Th, which, in turn, controls that of
226
Ra, which
affects the release of
222
Rn by recoil, and so the concentrations of the short lived
nuclides further along the chain (see Section
7.2.5).
A dominant input to groundwater for many constituents is weathering of
aquifer rocks and thus chemical releases from mineral structures
[7.3]. This is
important for uranium, thorium and radium (all progenitors of
210
Po), and is
responsible for much of the redistribution of the long lived nuclides. For short
lived nuclides, however, this is generally not important compared to other
sources, and so can be neglected
[7.4].
The most important mechanism for the release of short lived nuclides from
within mineral structures is recoil (see Figs
7.2 and 7.3). Whenever an atom
is subject to energetic alpha decay, the daughter atom is recoiled in a random
direction (though directly opposite to that of the alpha particle) over a distance
of approximately 20 nm in most mineral lattices [7.3]. When this recoil path
crosses a grain boundary (mineral surface), the daughter atom can be released to
a solution in the groundwater
[7.5, 7.6]. Atoms implanted into adjacent phases
may escape as well, along the alpha track defect in the mineral lattice generated
during the implantation event
[7.3].
The stopping power of water is substantially lower than mineral lattices,
and so atoms can cross pores and become implanted in adjacent grains, from
which the ion may migrate back to the solution [7.3]. This distance is somewhat
dependent on the lattice characteristics as well as on the decay energy (see the
review in Ref. [7.4]), although the recoil characteristics of the alpha decay
Source: Figure 2 of Ref. [7.3].
FIG. 7.3. A major source of short lived nuclides in groundwater is recoil (reproduced with
permission courtesy of Elsevier).
115
FRESHWATER AND GROUNDWATER SYSTEMS
products along the decay series are similar. Quantifying this input is discussed
further in Section 7.2.5.
Owing to the short half-life of
210
Po, its levels in groundwater are generally
in a steady state, such that the removal rates are equal to the input rates. While
much of the polonium is adsorbed, this may be readily exchangeable with
dissolved species (see Section 7.2.5). The main removal process of adsorbed
and dissolved
210
Pb is simply decay. Irreversible incorporation of
210
Po into
precipitating phases is generally too slow to be a significant removal flux.
7.2.3. Groundwater concentrations
Porcelli [7.3] reports that reconnaissance studies of groundwater across the
United States of America [7.7] and across California [7.8] find groundwater
210
Po
concentrations to be typically less than 40 mBq/L. Values as high as 16 Bq/L
have been recorded in a well in Finland [7.9], and brines in Australia can have
values of up to 19 Bq/L [7.10]. A number of factors control the variations in
concentrations of
210
Po in groundwater, including variations in input and removal
rates. These factors are considered below, followed by an evaluation of polonium
adsorption coefficients.
7.2.4. Particles and colloids in groundwater
Polonium has been found to be associated with colloids and particles in
groundwater, as well as being dissolved. This is expected because polonium is
very particle reactive, and particles are often composed of clays and compounds
of manganese and iron that have strong adsorption coefficients for polonium
(see Chapter 3). Not separating these from truly dissolved constituents can lead
to misleading conclusions regarding chemical behaviour. These species are
usually operationally defined by filtration methods, with particles being material
excluded from 0.2 μm or 0.45 μm filters, and colloids constituting material
passing this filter but not molecular filters or ‘ultrafilters’ of 5–20 kilodaltons.
Some large molecules, such as humic acids, are in the colloidal pool, while iron
and manganese oxyhydroxides and clays may be present at sizes that can be
either colloidal or particulate.
There have been several polonium speciation studies of groundwater
from Finland. In low ionic strength Ca–HCO
3
waters, 35–68% of
210
Po was
bound to particles, while more than 90% was bound to particles in higher
salinity Na–Cl waters that have high concentrations of iron, manganese and
humic acids [7.11]. The colloid fraction (between 5 kilodaltons and <0.45 μm)
contained approximately less than 10% [7.12]. Vesterbacka et al. [7.13] report
that, on average, 86% of
210
Pb in groundwater is found in the large particle

116
CHAPTER 7
fraction (>0.45 μm). However, for waters that are rich in organics, iron and
manganese, and which have high Fe:Mn ratios, the majority of
210
Po is found in
either the intermediate particle (100 kilodaltons and 0.45 μm) or small particle
(<100 kilodaltons) fractions.
Polonium, with such low natural elemental concentrations (due to its
relatively short half-life), clearly does not form its own particles but rather is
adsorbed onto, or incorporated into, other phases. While early laboratory
experiments suggested that polonium in simple solutions can form colloids [7.14],
it is present in extremely low concentrations compared to other colloid forming
elements and is highly reactive with surfaces; so that in natural environments, it
is likely to be associated with colloids of mixed composition [7.12].
Studies of groundwater from aquifers in Florida [7.15] and Nevada [7.16],
United States of America, provide comparisons between filtered and unfiltered
samples (see Fig. 7.4). Data cluster around the line for equal concentrations,
especially for the Nevada samples, although significant fractions of
210
Po appear
to be present on particles (data above the line). However, there are significant
deviations in the Florida samples. Filtering of particles from Nevada groundwater
removed 5–47% of
210
Po [7.16]. One of the highest concentrations recorded
(95 Bq/L) is for an unfiltered Florida groundwater; the filtered sample contained
17%
210
Po [7.17]. Such a high concentration suggests that particles with high
226
Ra were present. The most perplexing are some samples that appear to have
higher concentrations when filtered. This can only be a sampling or analytical
artefact.
The strong association of polonium with particles has a number of
implications for interpreting polonium behaviour. Particles can be mobile,
Source: See Refs [7.15, 7.16].
FIG. 7.4. Data for groundwater (filtered and unfiltered) from aquifers in Florida and Nevada,
United States of America.
117
FRESHWATER AND GROUNDWATER SYSTEMS
although not necessarily at the same rate as dissolved species. There might be
sampling biases in quantifying the particle load, since the particle load in samples
collected from wells might not be representative of aquifer groundwater; removal
of particles can occur in the filter pack around the well; or increased mobilization
of particles around the well can occur from pumping, resulting in enhanced flow
rates. Furthermore, if particle bound polonium is not separated, the amount of
dissolved species will be overestimated. In some cases, a significant fraction
of polonium can also be colloid bound. Overall, such effects should be further
investigated, especially where unusual occurrences of polonium occur. Routine
practice should include reporting data for both unfiltered and filtered samples,
and filtration should be done during collection, or as soon as possible after, since
association with particles can change during storage.
7.2.5. In situ adsorption coefficients
7.2.5.1. Quantifying polonium adsorption
As with most trace elements, the extent of
210
Po adsorption can be
determined from the analysis of aquifer rocks or laboratory experiments.
However, it is difficult to obtain a bulk average aquifer value for a process that
is expected to vary substantially between samples due to the heterogeneous
distribution of secondary phases. Furthermore, as discussed in Chapter 4, this has
been done for polonium on only a small number of soils, sediments and aquifer
materials. Porcelli [7.3] finds that:
“Studies involving decay-series systematics of nuclides in groundwater have
quantified parameters of water–rock interaction from direct measurements
of waters alone, with source terms and adsorption coefficients inferred from
comparison between the different radionuclides. Sampling of large volumes
or from a number of wells can obtain parameter values for larger volumes
of aquifer. In particular, the rate of recoil supply to groundwater, which
supplies all of the daughter nuclides at similar rates, and the evolution and
steady state concentrations of the short-lived nuclides can be determined.
Then bulk adsorption partition coefficients can be obtained.”
7.2.5.2. Calculating average aquifer adsorption coefficients
Porcelli [7.3] reports that mathematical treatments of simple aquifer models
have been extensively developed [7.18–7.23] and summarized in Refs [7.4, 7.24].
These provide operational rates of transport and of water–rock interaction
118
CHAPTER 7
processes, regardless of the actual mechanisms involved, and so complement
physical chemistry studies that can identify the controlling chemical processes.
These studies have so far largely considered steady state groundwater profiles;
that is, they assume that the conditions at each location within the aquifer do
not change, and so groundwater radionuclide concentrations are constant at each
location but may change along flow lines. Furthermore [7.3]:
“In general, the decay series systematics for groundwater behavior can
become quite complex, as there are a number of parameters that affect each
isotope, and the resulting distribution then affects the production of the
next isotope in the chain. However, the element with the simplest and most
predictable behavior is
222
Rn, an unreactive noble gas, and the distribution
of isotopes further along the
238
U decay series, including
210
Po, can be
estimated from consideration of the distribution of
222
Rn.
“The section of the
238
U decay series that is most directly relevant to
understanding
210
Po is below
226
Ra, as shown in Fig. [7.5]. For each nuclide,
the sum of dissolved and adsorbed atoms, that is the atoms that have
been released from aquifer solids and are chemically exchanging, can be
considered together as the mobile pool. Further, in most cases, radioisotope
concentrations will have reached a steady state concentration, where the
supply of the isotope from within the minerals and from decay of mobile
atoms of the parent will together equal the decay rate (that is, the activity)
of the mobile pool of the isotope. For the short-lived nuclides below
222
Rn,
it is generally the case (see Porcelli and Swarzenski, 2003 [Ref. [7.24]])
that for very short-lived nuclides, recoil is much more important as an
input than weathering, which can then be ignored. Also, the decay rates
of short-lived adsorbed and dissolved nuclides are generally much greater
than precipitation rates in groundwaters, and so this also can generally be
ignored
(Porelli, 2008 [Ref. [7.4]]). Of course, there are circumstances
when these factors do need to be considered, e.g. in rapidly weathering
horizons, or preferential rapid weathering of minerals rich in U (and so also
rich in all daughter nuclides) minerals, or where abrupt changes in aquifer
chemistry cause rapid precipitation. However, in the absence of evidence
for these, weathering and precipitation can be ignored.”
The models have been applied to the available data to obtain recoil supply
rates for
210
Po, as well as K
d
values for both
210
Pb and
210
Pb [7.3]. Porcelli [7.3]
states that the distribution of
210
Po can be evaluated by starting with
222
Rn. The
radionuclide
222
Rn does not precipitate or adsorb, so that groundwater (
222
Rn)

119
FRESHWATER AND GROUNDWATER SYSTEMS
activity is equivalent to
222
Rn recoil plus the production from mobile
226
Ra [7.4],
which is otherwise expressed as [7.3]:
( ) ( ) ( )
( )
222 226 226
222 266
W RW
Rn Ra Ra Kbe=+
(7.1)
where
b
is the ratio of rock mass to water mass per unit volume;
ε
222
is the fraction of
226
Ra decays that recoil daughter atoms to the
groundwater;
(
226
Ra)
R
is the concentration of
226
Ra in the aquifer rock;
(
226
Ra)
W
is the concentration of dissolved
226
Ra;
and (K
226
) is the ratio of atoms of
226
Ra adsorbed to atoms in solution.
Porcelli [7.3] continues:
“The
222
Rn in solution will produce
218
Po, which will largely adsorb. In
addition, there will be recoil of
218
Po into solution from decay of
222
Rn
within the minerals. The decay rate of mobile
218
Po (adsorbed and dissolved)
will equal the input rates. Mobile
218
Po will then produce mobile
214
Po,
along with further recoil of
214
Pb, so that mobile
214
Pb activity will be equal
to that of dissolved
222
Rn plus the addition of two recoil fluxes. The same
will apply to
210
Pb (produced by decay of
214
Pb to the very short-lived
214
Bi,
then the very short-lived
214
Po), which will have an activity of adsorbed
and dissolved atoms of
222
Rn plus 3 recoil fluxes. Since
210
Bi and
210
Po are
Source: Figure 3 of Ref. [7.3].
FIG. 7.5. The
238
U decay series from
226
Ra to
206
Pb, showing the recoil fluxes from within the
aquifer solid and so supplying the mobile pool of dissolved and adsorbed radionuclides in the
aquifer (reproduced with permission courtesy of Elsevier).

120
CHAPTER 7
produced by low energy beta decay, there are no recoil contributions to
these nuclides (see Fig. [7.5]). Therefore, the activity of mobile
210
Pb,
210
Bi
and
210
Po will be equal.”
Combining these effects together gives
[7.3]:
( )
( ) ( )
( )
Pb
226 222
210
210
RW
W
210
3 Ra Rn
Pb
K
be +
=
(7.2)
The activity of mobile
210
Pb is equal to that of mobile
210
Po, so that [7.3]:
( )
( )
Po
Pb
210
210
W
210
210
W
Pb
K
K
Po
=
(7.3)
If the production of
222
Rn is largely from recoil, so that the term for
production from adsorbed
226
Ra can be neglected, then [7.3]:
( ) ( )
222 226
222
WR
Rn Rabe=
(7.4)
and
( ) ( )
Pb
210
210 222
WW
4
K
Pb Rn
=
(7.5)
Porcelli [7.3] concludes by stating that there may be some circumstances in
which there is sufficient
226
Ra adsorbed to dominate
222
Rn production, which will
reduce K
210Pb
by a factor of four. However, the range in K
210Pb
is substantially
greater and so this will generally not be a major consideration. Moreover [7.3]:
“As discussed above, the values for K
210Po
and K
210Pb
are equal to the
number of atoms adsorbed divided by the number of atoms in solution
within any volume of aquifer (i.e. in units of mol/mol). In order to translate
this into the more familiar values of atoms per kg of rock divided by atoms
per liter of groundwater (i.e. L/kg), the porosity of the aquifer must be
known. Since this is often not reported or not well constrained, the values
calculated below are for moles adsorbed on aquifer solids relative to moles
in solution, or mol/mol.”

121
FRESHWATER AND GROUNDWATER SYSTEMS
Further information can be found in Ref. [7.3], including site specific
adsorption coefficients for groundwater in Australia, Brazil, Scandanavia and the
United States of America.
7.3. SURFACE WATER
The freshwater environment encompasses lotic (moving) water, such as
rivers and streams, and lentic water bodies (standing), such as lakes and ponds.
The concentrations of
210
Po within moving water depend on the environmental
conditions in the catchment area and reflecte both natural and anthropogenic
sources. The concentrations throughout the year is expected to vary, since
precipitation carries particulates from the surrounding areas into the water body.
Polonium in water can transfer to biota such as plants, phytoplankton,
zooplankton, invertebrates, fish, water based amphibians, crustaceans, mammals
and birds with dietary components from the aquatic environment (see Fig. 7.6).
FIG. 7.6. Conceptual model of an aquatic ecosystem.
122
CHAPTER 7
The interactions among the various abiotic and biotic environments
depends on the location. The International Union of Radioecology (IUR) suggests
that interaction matrices provide a convenient way to consider information on
relevant processes in the aquatic environment in a synthesized and structured
way
[7.25]. Each environment has its own unique chemical, physical and
biological characteristics. Nonetheless, the generic interaction matrices for the
aquatic environment provided by the IUR
[7.25] and the associated generic
discussions provide a convenient checklist for initial discussions of the relevant
processes.
The aquatic environment is characterized by the flow regime, water and
sediment quality, and local species, including primary producers, and primary
and secondary consumers. The IUR identifies the following main components for
the aquatic environment:
(a) The atmosphere, which is the region above the surface;
(b) Water, including surface water bodies (e.g. rivers, streams and lakes);
(c) Abiotic suspended matter, which is inorganic particles (>0.45 µm)
suspended in water;
(d) Deposited matter (sediment), which is particulate matter (inorganic and
organic) deposited at the bottom of rivers, streams and lakes, including
interstitial water;
(e) Primary producers, which are autotrophic organisms, such as phytoplankton,
macrophytes and aquatic plants;
(f) Primary consumers, which are animals that feed on primary producers
(e.g. zooplankton and macrobenthos);
(g) Secondary consumers, which are animals that feed on primary consumers
(e.g. omnivorous fish);
(h) Decomposers, which comprise microflora and protozoa.
7.3.1. Sources in surface fresh water
The sources of lead and polonium in surface waters include:
— Atmospheric deposition of resuspended dusts or aerosols;
— Fluvial inputs;
— In situ decay from progenitors (particularly
226
Ra in sediment; depending on
mixing rates, the interceding
222
Rn is often likely to decay before diffusing
to the water surface);
— Wash-off from the terrestrial environment;
— Anthropogenic inputs (e.g. phosphate ore processing operation and uranium
mining).
123
FRESHWATER AND GROUNDWATER SYSTEMS
Radionuclides dispersed in the environment can be deposited into surface
water or onto surfaces within the watershed, which, in the latter case, can
represent a long term source of radionuclides for freshwater ecosystems within
the affected region.
7.3.2. Levels of
210
Po in the freshwater environment
The activity of
210
Po in normally oxic waters is in the range of 1–5 mBq/L
and is higher, up to 17 mBq/L, in seasonally anoxic ponds [7.26]. Table 7.1
provides a summary of
210
Po measurements in the freshwater environment of
rivers and lakes. The amount of data for freshwater biota is less than for the
marine environment (see Table 7.2).
A similar pattern in freshwater biota has been observed when compared to
marine biota (see Chapter 8), in that higher concentrations have been reported
in the soft tissue of molluscs than in shells, whereas
210
Pb has been detected
in higher levels in the shells. A mollusc shell is largely composed of inorganic
substances, such as calcium carbonate, while the exoskeleton of prawn consists
of organic substances, such as chitin. As
210
Po has an affinity for organic moieties,
this accounts for the relatively higher concentration of
210
Po in the exoskeleton of
prawn [7.28].
7.3.3. Behaviour of
210
Po in standing water bodies
The behaviour of
210
Po in a water body is influenced by a combination
of physical and chemical processes. Within a standing water body (e.g. lake),
there is uptake of
210
Pb and
210
Po by particulates (particularly biomass) in the
water column. As these particulates settle to the bottom sediment, polonium is
scavenged from the water column. Within a few months, equilibrium between
210
Pb and
210
Po can be established in the sediment. However, changing redox
conditions in the sediment and water column also influence the polonium levels.
The chemical form (speciation) of sulphur in sediment is redox dependent and
can, in turn, affect
210
Pb and
210
Po behaviour (lead forms sulphides, e.g. PbS).
The cycling of polonium in sediment can be affected by microbial activity in the
bottom sediment, as the release of polonium can be enhanced in sulphur enriched
bacteria
[7.42]. Polonium has also been shown to become volatile in both fresh
and marine waters by the action of microorganisms
[7.43, 7.44] (see Section 3.2).
In addition to cycling in sediment, depending on the characteristics
of the lake, there can be zones within the water column that become anoxic
during periods of the year, which influences the behaviour of polonium in the
water column. Polonium has several oxidation states (−2, +2, +4 and +6), of
which the insoluble tetravalent state Po(IV) is the most stable in oxic aqueous

124
CHAPTER 7
solutions [7.45]. However, it can hydrolyse and form hydroxides. Polonium is
influenced by the cycling of iron and manganese
[7.26, 7.36, 7.46]. As oxygen
levels decrease, iron and manganese are reduced and enter into solution, bringing
with them transition metals, including
210
Pb and
210
Po, which are adsorbed onto
TABLE 7.1. BASELINE CONCENTRATIONS OF
210
Po IN SURFACE WATER
AND SEDIMENTS
Location
210
Po in water
(mBq/L)
210
Po in sediments
(Bq/kg)
a
Ref.
Kaveri River, India
b
0.77–1.27 14.4–26.5 [7.28]
Mutharasanallur Pond, India
b
1.4 60 [7.29]
Rostov, Russian Federation
b
1.8 × 10
−3
—
c
[7.30]
Vistula River, Poland 1.94–3.21
d
—
c
[7.31]
Tributaries of the Vistula River, Poland 2.15–6.03
d
—
c
[7.31]
Oder River, Poland 1.46–2.39
d
—
c
[7.32]
Tributaries of the Oder River, Poland 1.02–3.64
d
—
c
[7.32]
Saskatchewan, Canada (control site) —
c
240
e
[7.33]
Saskatchewan, Canada (unimpacted lake
near the McArthur River site)
2 50
e
[7.34]
Crystal Lake, Wisconsin, USA 1.6 —
c
[7.35]
Bickford Reservoir, Massachusetts, USA 1.3 —
c
[7.36]
Four Norwegian lakes 1.6–2 —
c
[7.37]
South Alligator River, Australia
(control site)
1.94 filtered
2.51 particulate
—
c
[7.38]
a
Fresh weight unless otherwise stated.
b
Adapted from Ref. [7.27].
c
—: data not available.
d
May be influenced by anthropogenic sources.
e
Bq/kg (DW).

125
FRESHWATER AND GROUNDWATER SYSTEMS
TABLE 7.2. CONCENTRATIONS OF
210
Po IN FRESHWATER BIOTA
SPECIES
Sample Part
210
Po
(Bq/kg)
a
Concentration ratios
(L/kg)
a
Ref.
Plankton
b
19–29 (2.1–2.5) × 10
4
[7.28]
Water hyacinth
(Eichhornia crassipes)
Shoot
Root
2.3–6.5
6.7–31
(2.7–4.6) × 10
3
(0.73–2.2) × 10
4
[7.28]
[7.29]
Bivalve mollusc
(Lamellidens marginalis)
Shell
Soft tissue
1.2–4.5
53–106
(0.86–5.3) × 10
3
(3.8–8.4) × 10
4
[7.28]
[7.29]
Bivalve mollusc
(Anodonta cygnea)
Shell
Soft tissue
0.1
c
19.3
c
—
d
6.2 × 10
3e
[7.39]
Bivalve mollusc
(Anodonta sp.)
Soft tissue
Shell
Whole
5.7
2.5
4.7
2.1 × 10
3
9.4 × 10
2
1.7 × 10
3
[7.37]
Bivalve mollusc
(Velesunio angasi)
Soft tissue 302–416
c
4.7 × 10
3
[7.38]
Crustacean
(crab, prawn)
Exoskeleton
Muscle
8.6–16.6
12–20
(0.81–1.2) × 10
4
(0.93–1.6) × 10
4
[7.28]
[7.29]
Snail
(Pila virens)
Shell
Soft tissue
0.2–3.9
33–46
(0.14–5.3) × 10
3
(2.6–4.2) × 10
4
[7.28]
[7.29]
Fish
(Mystus vittatus,
Orechromis mossambicus
,
Puntius chola)
Bone
Muscle
1.3–17
1.9–27
(0.13–1.2) × 10
4
(0.22–1.9) × 10
4
[7.28]
[7.29]
Fish
(perch, bream)
b
—
d
0.36–0.43 —
d
[7.30]
Fish
b
Bone 0.37–0.43
c
—
d
[7.40]
Fish Whole
Edible
1.0–6.5
0.08–1.9
(0.63–9.3) × 10
3
—
d
[7.37]
Fish
(Cyprinus carpio,
Sander lucioperca)
Bone
Muscle
Liver
8–14
2–7
11–183
—
d
—
d
[7.41]
a
Fresh weight unless otherwise stated.
b
Adapted from Ref. [7.27].
c
Bq/kg (DW).
d
—: data not available.
e
L/kg (DW).

126
CHAPTER 7
oxides. The insoluble Po(IV) is also reduced to Po(II) near the same redox
potential that Mn(IV) is reduced to Mn(II)
[7.36. 7.46]. The dissolved ions diffuse
upwards and, as they become oxidized again, they form precipitates. Thus,
changing redox conditions can have a significant effect on the levels of polonium
in a water body. As a result, much higher levels of
210
Po have been measured in
the anoxic zone of a water body (hypolimnion) compared to the epilimnion, and
an enrichment of
210
Po compared to
210
Pb is observed [7.26, 7.36, 7.46].
Under certain conditions, polonium can diffuse out of the sediments and,
the sediments can act as a sink for polonium.
7.3.4. Quantification of water and sediment quality
The interaction of radionuclides with bottom sediments and suspended
particles is controlled by many environmental processes, such as sedimentation
and resuspension (see Fig.
7.7).
C
Radionuclide
in water (dissolved)
D
f
Radionuclide
particulate form (fast
exchange process)
D
s
Radionuclide
particulate form (slow
exchange process)
K
s
K
fw
K
wf
K
fs
K
sf
K
ws
K
sw
Source: Figure 2 of Ref. [7.47].
Note: The fast processes are marked in the subscript by ‘f’, while the slow processes are
marked by ‘s’.
FIG. 7.7. Schematic structure of contaminant fluxes and environmental compartments
involved in the processes of radionuclide migration to and from bottom sediment.

127
FRESHWATER AND GROUNDWATER SYSTEMS
Monte et al. [7.47] describe three active compartments:
— Dissolved radionuclide in water (C);
— Particulate radionuclide: rapid exchange component (D
f
);
— Particulate radionuclide: slow exchange component (D
s
).
Accordingly
[7.47]:
“The fluxes (Bq·s
−1
) from a compartment can be calculated as the product
of the total amount of radionuclide in the compartment (Bq) multiplied by
the ‘rates’ (s
−1
) of migration (K
wf
, K
fw
, K
sf
, K
fs
, K
ws
, K
sw
, K
s
).
“The seven radionuclide fluxes can be schematized as follows:
(a) Radionuclide fluxes from dissolved form to particulate form and vice
versa — rapid exchange processes (K
wf
·C and K
fw
·D
f
);
(b) Radionuclide fluxes from D
f
to D
s
and vice-versa (K
fs
·D
f
and K
sf
·D
s
);
(c) Radionuclide fluxes from water to D
s
and vice-versa (K
ws
·C and
K
sw
·D
s
);
(d) Radionuclide irreversible burial in inactive sediments (K
s
·D
s
).”
Under oxic conditions, an assessment of the interaction of dissolved
radionuclides with solid particles in suspension or deposited, is often based on
the K
d
concept, assuming a presence of an equilibration between the dissolved
(C
w
, Bq/m
3
) and the adsorbed phases (C
s
, Bq/kg) of a radionuclide [7.47]:
s
d
w
C
K
C
=
(7.6)
There are very limited data available to determine a K
d
for polonium.
Ciffroy et al. [7.48] did not find a sufficient database of information. One study of
polonium in a pond derived an overall K
d
of 4.3 × 10
4
L/kg (FW) [7.29], whereas
K
d
values in a river were estimated at 1.5 × 10
5
L/kg (FW) for suspended matter
to dissolved and 3
× 10
4
L/kg (FW) for bottom sediment to dissolved [7.49].
Application of these values should be treated with caution, as they are based on
limited information and K
d
values can vary widely.
7.3.5. Freshwater aquatic biota
The accumulation of
210
Po by aquatic biota is a dynamic process, with
many pathways of exposure being involved. However, it is often simplified by
128
CHAPTER 7
assuming that abiotic and biotic media have reached steady state conditions
and a concentration ratio (CR) can thus be established that relates the biota
concentration to the concentration in the abiotic reference medium (water or
sediment). These CRs can also be derived for those non-aquatic animals that
depend on the aquatic environment, including mammals and birds.
There are two basic sets of data on radionuclide accumulation in aquatic
organisms. The first approach is based on assessments of CRs for edible tissues
of freshwater biota. Within the second approach, intended mainly for assessments
of dose to biota, activity concentrations of radionuclides are calculated (or
measured) for the whole organism. Yankovich et
al. [7.50] describe methods for
converting tissue concentrations to whole body values for a variety of species
using CRs. The empirically derived CRs for lead and polonium are summarized
in Table
7.3.
Until fairly recently, the Handbook of Parameter Values for the Prediction
of Radionuclide Transfer in Temperate Environments
[7.65] provided the
basis for environmental assessments. Since that publication was released, new
datasets have become available, and an update of that publication was considered
appropriate. Two large scale reviews, in the framework of the international
IAEA projects, Environmental Modelling for Radiation Safety (EMRAS
I and
EMRAS
II), compiled the available data covering both transfers to human
foodstuffs and the available quantitative data on the transfer of radionuclides to
wildlife. The update was accomplished with the publication of the Handbook of
Parameter Values for the Prediction of Radionuclide Transfer in Terrestrial and
Freshwater Environments
[7.66]. Much of the earlier data on biological uptake
for many radionuclides was often on specific tissues and not the whole organism.
The recent reviews were much wider in context, based on many sources and,
hence, many more data for polonium were compiled. The CRs for
210
Pb and
210
Po
are presented in Table 7.3 for broad wild biota groups and comprise a subset from
Handbook of Parameter Values for the Prediction of Radionuclide Transfer to
Wildlife
[7.67] — an overview of which is provided in Ref. [7.51].
Although Ref. [7.66] represents a key reference for radioecologists,
modellers and authorities by providing data for environmental impact
assessments, it includes just a few freshwater CRs for lead and polonium. Hence,
it has limited applicability for the impact assessment of naturally occurring
radioactive material on freshwater species and their contamination of freshwater
food.
From the available tissue data (see Table 7.2), the distribution of
210
Po within an organism shows higher concentrations in the kidney, liver
and other organs in the digestive system. A higher accumulation of
210
Po is
found in the soft tissue of molluscs than in shells, whereas
210
Pb has been
detected at higher levels in shells. In the soft tissue of an organism,
210
Po is

129
FRESHWATER AND GROUNDWATER SYSTEMS
TABLE 7.3.
210
Pb AND
210
Po CONCENTRATION RATIO (CR
WO-WATER
) VALUES FOR WILDLIFE GROUPS IN
FRESHWATER ECOSYSTEMS
Wildlife group
CR
wo-water
Ref.
N
AM ASD GM GSD Min. Max.
210
Pb
Amphibian 2 5.3 —
a
—
a
—
a
1.7 8.9 [7.52]
Crustacean 5 3.9 × 10
1
4.7 × 10
1
2.5 × 10
1
2.6 —
a
—
a
[7.53]
Fish
Benthic feeding
Forage
Piscivorous
379
148
30
201
2.5 × 10
2
1.8 × 10
2
2.6 × 10
1
3.5 × 10
2
7.0 × 10
2
6.3 × 10
2
6.2 × 10
1
7.8 × 10
2
8.7 × 10
1
4.8 × 10
1
9.9
1.4 × 10
2
4.3
5.0
4.0
3.8
2.0
3.2
2.0
8.3
7.5 × 10
3
7.5 × 10
3
3.5 × 10
2
5.7 × 10
3
[7.52–7.58]
[7.52–7.55, 7.58]
[7.52, 7.53, 7.55–7.57]
[7.52–7.55]
Mollusc (bivalve) 32 6.0 × 10
3
—
a
2.3 × 10
3
4.0 1.1 × 10
2
2.9 × 10
4
[7.38, 7.54, 7.59, 7.60]
Reptile 12 4.4 × 10
2
6.2 × 10
2
2.5 × 10
2
2.9 1.3 × 10
1
1.9 × 10
3
[7.61]
Vascular plant 21 6.2 × 10
1
7.0 × 10
1
4.1 × 10
1
2.5 1.3 × 10
1
1.9 × 10
2
[7.52, 7.60]

130
CHAPTER 7
TABLE 7.3.
210
Pb AND
210
Po CONCENTRATION RATIO (CR
WO-WATER
) VALUES FOR WILDLIFE GROUPS IN
FRESHWATER ECOSYSTEMS (cont.)
Wildlife group
CR
wo-water
Ref.
N
AM ASD GM GSD Min. Max.
210
Po
Crustacean 12 8.3 × 10
3
7.0 × 10
3
6.3 × 10
3
2.1 1.2 × 10
3
1.6 × 10
4
[7.28, 7.29, 7.53]
Fish
Benthic feeding
Forage
Piscivorous
203
90
18
95
2.0 × 10
3
1.6 × 10
3
7.6 × 10
3
1.3 × 10
3
6.6 × 10
3
4.4 × 10
3
1.2 × 10
4
6.7 × 10
3
5.9 × 10
2
5.7 × 10
2
4.2 × 10
3
2.6 × 10
2
4.8
4.2
3.0
6.1
4.9 × 10
1
6.3 × 10
1
1.3 × 10
2
4.9 × 10
1
3.7 × 10
4
1.9 × 10
4
2.6 × 10
4
3.7 × 10
4
[7.28, 7.29, 7.49, 7.53, 7.54, 7.62]
[7.28, 7.29, 7.49, 7.53, 7.54]
[7.28, 7.29, 7.53, 7.62]
[7.53, 7.54, 7.63]
Mollusc
Bivalve
147
141
1.2 × 10
5
1.3 × 10
5
5.2 × 10
4
4.9 × 10
4
1.1 × 10
5
1.2 × 10
5
1.5
1.5
1.7 × 10
3
1.7 × 10
3
1.7 × 10
5
1.7 × 10
5
[7.28, 7.29, 7.38, 7.54, 7.62, 7.64]
Reptile 7 3.6 × 10
3
2.3 × 10
3
3.1 × 10
3
1.8 1.5 × 10
3
7.3 × 10
3
[7.61]
Vascular plant 31 2.0 × 10
3
1.5 × 10
3
1.6 × 10
3
2.0 5.5 × 10
2
4.6 × 10
3
[7.28, 7.29, 7.62]
Source:
See Ref. [7.51].
Note: CR
wo-water
is the activity concentration in the whole organism (in Bq/kg, FW) divided by the activity concentration in the water
(in Bq/kg, DW). AM — arithmetic mean; ASD — arithmetic standard deviation; CR — concentration ratio; GM — geometric mean;
GSD — geometric standard deviation.
a
—: data not available.
131
FRESHWATER AND GROUNDWATER SYSTEMS
typically found at higher levels than
210
Pb. Since
210
Po concentrations in soft
tissue are normally higher than those in bones and comprise most of the edible
tissues of freshwater species, the data in Table 7.3 can also be used to assess
210
Po concentrations in human foodstuffs. For polonium, the ratio of whole
body to tissue is expected to be approximately 1.1 for freshwater fish and 2 for
mammals [7.50]. However, there is a paucity of data for most species. The use
of CRs in Table 7.3 represents a cautious approach to estimating
210
Po in edible
tissues when sufficient data are unavailable. Additional reasons supporting the
use of
210
Po CRs for assessment of human foodstuff contamination include the
following [7.67]:
(a) For aquatic ecosystems, the whole organism CR
wo-media
values for bivalve
molluscs, large crustaceans and marine gastropods do not include the shell.
This is consistent with commonly used dosimetric approaches.
(b) For vertebrate wildlife groups, whole organism CR
wo-media
values typically
do not include the gastrointestinal tract contents, although there may be
some exceptions, such as when animals have been monitored live and in
the case of small fish.
Table 7.3 also provides CRs for
210
Pb, since it serves as a source of
210
Po
in wildlife. Such information is also often valuable for modelling purposes. The
data in Table 7.3 clearly show that
210
Po CRs tend to be one to two orders of
magnitude greater than those for
210
Pb, thus demonstrating higher mobility in
freshwater ecosystems. The highest
210
Po CRs are in bivalve molluscs, followed
by crustaceans and some foraging species, indicating the sensitivity of some
freshwater organisms to contamination by polonium.
In summary,
210
Po in groundwater occurs over a wide range of
concentrations. Once the differences due to release from aquifer minerals of
222
Rn by recoil are considered, there are very large differences due to adsorption
of
210
Po onto surfaces. Bulk adsorption coefficients are difficult to predict
because of wide variations in grain size and mineralogy over even very small
spatial scales. Therefore, while earlier studies can provide some guidance on
what might be expected, each aquifer needs to be characterized individually.
In order to do so, accompanying data on
222
Rn are valuable for characterizing
adsorption. Limited data also suggest that the concentrations of
210
Po on particles
and colloids can be important under some circumstances. Future studies on
the chemistry of polonium in dynamic groundwater regions, such as oxic and
anoxic transitions and areas of salt water intrusion, are necessary. The behaviour
of polonium within the vadose zone, which is most vulnerable to anthropogenic
inputs, is also poorly understood.
132
CHAPTER 7
The levels of
210
Po in surface water in freshwater systems are influenced by
in situ decay, surface runoff, fluvial inputs and atmospheric deposition. Within
a standing water body,
210
Po is removed through the settling of particulates, and
its behaviour in the sediment is influenced by the cycling of iron and manganese
as the redox conditions change in the sediment. This is also an important process
if there are seasonal anoxic zones within the water body. More research is still
needed on the partitioning of
210
Po onto particulates in the surface water column
to fully understand the cycling of
210
Po and how it relates to manganese, iron and
sulphur. A more comprehensive database of information needs to be developed to
better understand the dynamics of
210
Po in the freshwater food web.
REFERENCES TO CHAPTER 7
[7.1] MONTE, L., et al., Review and assessment of models used to predict the fate of
radionuclides in lakes, J. Environ. Radioact.
69 (2003) 177–205.
[7.2] INTERNATIONAL ATOMIC ENERGY AGENCY, Quantification of Radionuclide
Transfer in Terrestrial and Freshwater Environments for Radiological Assessments,
IAEA-TECDOC-1616, IAEA, Vienna
(2009).
[7.3] PORCELLI, D., A method for determining the extent of bulk
210
Po and
210
Pb adsorption
and retardation in aquifers, Chem. Geol. 382 (2014) 132–139.
[7.4] PORCELLI, D., “Investigating groundwater processes using U- and Th-series
nuclides”, U/Th Series Radionuclides in Aquatic Systems (KRISHNASWAMI, S.,
COCHRAN, J.K., Eds), Radiation in the Environment, Vol.
13, Elsevier, Amsterdam
(2008)
105–153.
[7.5] KIGOSHI, K., Alpha-recoil thorium-234: Dissolution into water and the uranium-234/
uranium-238 disequilibrium in nature, Science 173 (1971) 47–48.
[7.6] FLEISCHER, R.L., RAABE, O.G., Recoiling alpha-emitting nuclei: Mechanisms for
uranium-series disequilibrium, Geochim. Cosmochim. Acta
42 (1978) 973–978.
[7.7] FOCAZIO, M.J., et al., Occurrence of Selected Radionuclides in Ground Water Used
for Drinking Water in the United States: A Reconnaissance Survey, 1998,
Water-Resources Investigations Report
00-4273, United States Geological Survey,
Reston, VA
(2001).
[7.8] RUBERU, S.R., LIU, Y.-G., KUSUM PERERA, S., Occurrence and distribution of
210
Pb and
210
Po in selected California groundwater wells, Health Phys. 92
(2007)
432–441.
[7.9] SALONEN, L., HUIKURI, P., “Elevated levels of uranium series radionuclides in
private water supplies in Finland”, High Levels of Natural Radiation and Radon Areas:
Radiation Dose and Health Effects (Proc. 5th Int. Conf. Munich, 2000), Vol.
II: Poster
Presentations (2002)
87–91.
[7.10] DICKSON, B.L., HERCZEG, A.L., Naturally occurring radionuclides in acid: Saline
groundwaters around Lake Tyrrell, Victoria, Australia, Chem. Geol.
96 (1992) 95–114.
133
FRESHWATER AND GROUNDWATER SYSTEMS
[7.11] VAARAMAA, K., LEHTO, J., ERVANNE, H., Soluble and particle-bound
234,238
U,
226
Ra and
210
Po in ground waters, Radiochim. Acta 91 (2003) 21–28.
[7.12] LEHTO, J., KELOKASKI, P., VAARAMAA, K., JAAKKOLA, T., Soluble and
particle-bound Po-210 and Pb-210 in groundwaters, Radiochim. Acta
85
(1999)
149–155.
[7.13] VESTERBACKA, P., HÄMÄLÄINEN, K., LEHTO, J., The effect of water treatment
on the presence of particle-bound
210
Po and
210
Pb in groundwater, Radiochim. Acta 93
(2005)
291–296.
[7.14] MORROW, P.E., DELLA ROSA, R.J., CASARETT, L.J., MILLER, G.J.,
Investigations of the colloidal properties of polonium-210 solutions by using molecular
filters, Radiat. Res. Suppl.
5 (1964) 1–15.
[7.15] BURNETT, W.C., COWART, J.B., CHIN, P.A., “Polonium in the surficial aquifer of
West Central Florida”, Radon, Radium, and Other Radioactivity in Ground Water:
Hydrogeologic Impact and Application to Indoor Airborne Contamination (Proc.
NWWA Conf. Somerset, NJ, 1987) (GRAVES, B., Ed.), Lewis Publishers, Chelsea, MI
(1987)
251–269.
[7.16] SEILER, R.L.,
210
Po in Nevada groundwater and its relation to gross alpha radioactivity,
Ground Water
49 (2011) 160–171.
[7.17] COWART, J.B., BURNETT, W.C., CHIN, P.A., HARADA, K., “Occurrence of
210
Po
in natural waters in Florida”, Trace Substances in Environmental Health: XXI (Proc.
Univ. Missouri’s 21st Conf. St. Louis, MO, 1987) (HEMPHILL, D.D., Ed.), University
of Missouri Press, Columbia, MO (1987) 172–185.
[7.18] KRISHNASWAMI, S., GRAUSTEIN, W.C., TUREKIAN, K.K., DOWD, J.F.,
Radium, thorium and radioactive lead isotopes in groundwaters: Application to the
in
situ determination of adsorption–desorption rate constants and retardation factors,
Water Resour. Res.
18 (1982) 1663–1675.
[7.19] DAVIDSON, M.R., DICKSON, B.L., A porous flow model for steady state transport
of radium in groundwater, Water Resour. Res.
22 (1986) 34–44.
[7.20] KU, T.-L., LUO, S., LESLIEE, B.W., HAMMOND, D.E., “Decay-series disequilibria
applied to the study of rock-water interaction and geothermal systems”, Uranium-
series Disequilibrium: Applications to Earth, Marine, and Environmental Sciences
(IVANOVICH, M., HARMON, R.S., Eds), Clarendon Press, Oxford (1992)
631–668.
[7.21] LUO, S.D., KU, T.L., ROBACK, R., MURRELL, M., McLING, T.L., In-situ
radionuclide transport and preferential groundwater flows at INEEL (Idaho):
Decay-series disequilibrium studies, Geochim. Cosmochim. Acta
64 (2000) 867–881.
[7.22] TRICCA, A., PORCELLI, D., WASSERBURG, G.J., Factors controlling the
groundwater transport of U, Th, Ra, and Rn, J. Earth Syst. Sci.
109 (2000) 95–108.
[7.23] TRICCA, A., WASSERBURG, G.J., PORCELLI, D., BASKARAN, M., The transport
of U- and Th-series nuclides in a sandy unconfined aquifer, Geochim. Cosmochim.
Acta
65 (2001) 1187–1210.
[7.24] PORCELLI, D., SWARZENSKI, P.W., The behavior of U- and Th-series nuclides in
groundwater, Rev. Mineral. Geochem.
52 (2003) 317–361.
134
CHAPTER 7
[7.25] INTERNATIONAL UNION OF RADIOECOLOGY, Recommendations for Improving
Predictions of the Long-term Behaviour of
14
C,
36
Cl,
99
Tc,
237
Np and
238
U, IUR
Report
6, IUR, Warrington (2006).
[7.26] KIM, G., KIM, S.-J, HARADA, K., SCHULTZ, M.K., BURNETT, W.C., Enrichment
of excess
210
Po in anoxic ponds, Environ. Sci. Technol. 39 (2005) 4894–4899.
[7.27] COPPIN, F., ROUSSEL-DEBET, S., Comportement du
210
Po en milieu terrestre: revue
bibliographique, Radioprot. 39 (2004) 39–58.
[7.28] SHAHEED, K., SOMASUNDARAM, S.S.N., SHAHUL HAMEED, P., IYENGAR,
M.A.R., A study of polonium-210 distribution aspects in the riverine ecosystem of
Kaveri, Tiruchirappalli, India, Environ. Pollut. 95 (1997) 371–377.
[7.29] SHAHUL HAMEED, P., SHAHEED, K., SOMASUNDARAM, S.S.N., A study on
distribution of natural radionuclide polonium-210 in a pond ecosystem, J. Biosci.
22
(1997)
627–634.
[7.30] LADINSKAYA, L.A., PARFENOV, Yu.D., POPOV, D.K., FEDOROVA, A.V.,
210
Pb
and
210
Po content in air, water, foodstuffs, and the human body, Arch. Environ.
Health
27 (1973) 254–258.
[7.31] SKWARZEC, B., JAHNZ, A., The inflow of polonium
210
Po from Vistula river
catchments area, J. Environ. Sci. Health A
42 (2007) 2117–2122.
[7.32] SKWARZEC, B., TUSZKOWSKA, A., Inflow of
210
Po from the Odra River catchment
area to the Baltic Sea, Chem. Anal.
53 (2008) 809–820.
[7.33] THOMAS, P., LIBER, K., An estimation of radiation doses to benthic invertebrates
from sediments collected near a Canadian uranium mine, Environ. Int.
27
(2001)
341–353.
[7.34] ENVIRONMENT CANADA, Priority Substances List Assessment Report: Releases
of Radionuclides from Nuclear Facilities (Impact on Non-Human Biota), Government
of Canada, Ottawa
(2003).
[7.35] TALBOT, R.W., ANDREN, A.W., Seasonal variations of
210
Pb and
210
Po concentrations
in an oligotrophic lake, Geochim. Cosmochim. Acta
48 (1984) 2053–2063.
[7.36] BENOIT, G., HEMOND, H.F., Polonium-210 and lead-210 remobilization from lake
sediments in relation to iron and manganese cycling, Environ. Sci. Technol.
24
(1990)
1224–1234.
[7.37] GJELSVIK, R., BROWN, J. (Eds), Po-210 and Other Radionuclides in Terrestrial and
Freshwater Environments, NKS-181, Nordic Nuclear Safety Research,
Roskilde (2009).
[7.38] RYAN, B., BOLLHÖFER, A., MARTIN, P., Radionuclides and metals in freshwater
mussels of the upper South Alligator River, Australia, J. Environ. Radioact.
99
(2008)
509–526.
[7.39] STEPNOWSKI, P., SKWARZEC, B., A comparison of
210
Po accumulation in molluscs
from the southern Baltic, the coast of Spitsbergen and Sasek Wielki Lake in Poland, J.
Environ. Radioact.
49 (2000) 201–208.
[7.40] PARFENOV, Yu.D., Polonium-210 in the environment and in the human organism, At.
Energy Rev.
12 (1974) 75–143.
[7.41] LaROCK, P., HYUN, J.-H., BOUTELLE, S., BURNETT, W.C., HULL, C.D., Bacterial
mobilization of polonium, Geochim. Cosmochim. Acta 60 (1996) 4321–4328.
135
FRESHWATER AND GROUNDWATER SYSTEMS
[7.42] SKIPPERUD, L., JØRGENSEN, A.G., HEIER, L.S., SALBU, B., ROSSELAND,
B.O., Po-210 and Pb-210 in water and fish from Taboshar uranium mining Pit Lake,
Tajikistan, J. Environ. Radioact.
123 (2013) 82–89.
[7.43] MOMOSHIMA, N., SONG, L.-X., OSAKI, S., MAEDA, Y., Formation and emission
of volatile polonium compound by microbial activity and polonium methylation with
methylcobalamin, Environ. Sci. Technol.
35 (2001) 2956–2960.
[7.44] MOMOSHIMA, N., SONG, L.-X., OSAKI, S., MAEDA, Y., Biologically induced Po
emission from fresh water, J. Environ. Radioact.
63 (2002) 187–197.
[7.45] FIGGINS, P.E., The Radiochemistry of Polonium, NAS-NS 3037, National Academy
of Sciences, Washington, DC (1961).
[7.46] BALISTRIERI, L.S., MURRAY, J.W., PAUL, B., The geochemical cycling of stable
Pb,
210
Pb, and
210
Po in seasonally anoxic Lake Sammamish, Washington, USA,
Geochim. Cosmochim. Acta
59 (1995) 4845–4861.
[7.47] MONTE, L., PERIAÑEZ, R., BOYER, P., SMITH, J.T., BRITTAIN, J.E., “Physical
processes in freshwater ecosystems”, Quantification of Radionuclide Transfer in
Terrestrial and Freshwater Environments for Radiological Assessments,
IAEA-TECDOC-1616, IAEA, Vienna (2009)
419–434.
[7.48] CIFFROY, P., DURRIEU, G., GARNIER, J.-M., “Distribution of radionuclides
between solid and liquid phases in freshwaters”, ibid., pp
441–471.
[7.49] CARVALHO, F.P., OLIVEIRA, J.M., LOPES, I., BATISTA, A., Radionuclides from
past uranium mining in rivers of Portugal, J. Environ. Radioact.
98 (2007) 298–314.
[7.50] YANKOVICH, T.L., et al., Whole-body to tissue concentration ratios for use in biota
dose assessments for animals, Radiat. Environ. Biophys.
49 (2010) 549–565.
[7.51] HOWARD, B.J., et al., The IAEA handbook on radionuclide transfer to wildlife, J.
Environ. Radioact.
121 (2013) 55–74.
[7.52] YANKOVICH, T.L., Compilation of Concentration Ratios for Aquatic Non-human
Biota Collected by the Canadian Power Reactors Sector, CANDU Owners Group
Inc.
(2010).
[7.53] MARTIN, P., HANCOCK, G.J., JOHNSTON, A., MURRAY, A.S., Bioaccumulation
of Radionuclides in Traditional Aboriginal Foods from the Magela and Cooper Creek
Systems, Research Report
11, Supervising Scientist for the Alligator Rivers Region,
Australian Government Publishing Services, Canberra
(1995).
[7.54] BROWN, J.E., GJELSVIK, R., HOLM, E., ROOS, P., SAXEN, R., OUTOLA, I.
(Eds), Filling Knowledge Gaps in Radiation Protection Methodologies for Non-human
biota: Final Summary Report, NKS-187, Nordic Nuclear Safety Research,
Roskilde (2009).
[7.55] AHMAD, M.K., ISLAM, S., RAHMAN, M.S., HAQUE, M.R., ISLAM, M.M., Heavy
metals in water, sediment and some fishes of Buriganga River, Bangladesh, Int. J.
Environ. Res.
4 (2010) 321–332.
[7.56] MALIK, N., BISWAS, A.K., QURESHI, T.A., BORANA, K., VIRHA, R.,
Bioaccumulation of heavy metals in fish tissues of a freshwater lake of Bhopal,
Environ. Monit. Assess.
160 (2010) 267–276.
136
CHAPTER 7
[7.57] MOHAMED, F.A.S., Bioaccumulation of selected metals and histopathological
alterations in tissues of Oreochromis niloticus and Lates niloticus from Lake Nasser,
Egypt, Glob. Vet.
2 (2008) 205–218.
[7.58] ÖZTÜRK, M., ÖZÖZEN, G., MINARECI, O., MINARECI, E., Determination of
heavy metals in fish, water and sediments of Avsar Dam Lake in Turkey, Iran. J.
Environ. Health Sci. Eng.
6 (2009) 73–80.
JOHNSTON, A., MURRAY, A., MARTEN, R., MARTIN, P., PETTERSON, H.,
“Uranium series radionuclide concentrations in significant Aboriginal foods”, Alligator
Rivers Region Research Institute, Research Report
1983–84, Supervising Scientist for
the Alligator Rivers Region, Australian Government Publishing Services, Canberra
(1984)
43–44.
[7.59] ENGDAHL, A., TERNSELL, A., HANNU, S., Oskarshamn Site Investigation:
Chemical Characterisation of Deposits and Biota, SKB
P-06-320, Swedish Nuclear
Fuel and Waste Management, Stockholm
(2006).
[7.60] WOOD, M.D., BERESFORD, N.A., SEMENOV, D.V., YANKOVICH, T.L.,
COPPLESTONE, D., Radionuclide transfer to reptiles, Radiat. Environ. Biophys.
49
(2010)
509–530.
[7.61] SHAHUL HAMEED, P., ASOKAN, R., IYENGAR, M.A.R., KANNAN, V., The
freshwater mussel Parreysia favidens (Benson) as a biological indicator of
polonium-210 in a riverine system, Chem. Ecol.
8 (1993) 11–18.
[7.62] CANADA NORTH ENVIRONMENTAL SERVICES, Shea Creek Project Area,
Environmental Baseline Investigation 2007–2009, Draft Report (2010).
[7.63] JOHNSTON, A., Radiation Exposure of Members of the Public Resulting from
Pperation of the Ranger Uranium Mine, Technical Memorandum
20, Supervising
Scientist for the Alligator Rivers Region, Australian Government Publishing Services,
Canberra
(1987).
[7.64] INTERNATIONAL ATOMIC ENERGY AGENCY, Handbook of Parameter Values
for the Prediction of Radionuclide Transfer in Temperate Environments, Technical
Reports Series No.
364, IAEA, Vienna (1994).
[7.65] INTERNATIONAL ATOMIC ENERGY AGENCY, Handbook of Parameter Values
for the Prediction of Radionuclide Transfer in Terrestrial and Freshwater Environments,
Technical Reports Series No.
472, IAEA, Vienna (2010).
[7.66] INTERNATIONAL ATOMIC ENERGY AGENCY, Handbook of Parameter Values
for the Prediction of Radionuclide Transfer to Wildlife, Technical Reports Series
No. 479, IAEA, Vienna (2014).
137
Chapter 8
210
Pb AND
210
Po IN MARINE SYSTEMS
8.1. INTRODUCTION TO THE MARINE ENVIRONMENT
Two thirds of the planet is covered with sea water. Near the continents
(neritic province), waters located between the shoreline and the shelf break, or
continental margin, are called seas and are fringes of the immense blue waters
beyond continental margins that make up the oceans (oceanic province). Oceans
spread over all latitudes and longitudes, and their depths range from the shoreline
to about 11
000 m in a few hadal trenches. The average depth of the North
Atlantic, for example, is about 4500
m.
Despite the common chemical composition of sea water, oceans are by no
means a uniform system. The combination of water temperature, salinity, sunlight
and dissolved nutrients, such as chloride, sulphate, nitrate and other salts, creates
different biotopes that are home to many species in marine ecosystems.
Biota species and biomass are unevenly distributed in the oceans. On the
sea-floor, the biomass of benthic organisms decreases from the coastal waters to
abyssal and hadal regions. In the pelagic domain, biomass over the continental
shelf is much higher than in the open ocean. In specific areas of the continental
margin, the upwelling of nutrient loaded deep water enhances phytoplankton
primary productivity and contributes to the abundance of fishing resources.
8.2. POLONIUM SOURCES IN THE MARINE ENVIRONMENT
In sea water, polonium is present as a naturally occurring radionuclide and
it is a companion of many other essential and non-essential dissolved elements.
Polonium enters the marine environment from atmospheric deposition of
210
Pb
and
210
Po at the ocean surface, from the in situ radioactive decay of
226
Ra
dissolved in sea water, from the decay of
222
Rn gas exhaled from the sea-floor,
and from river and anthropogenic discharges. Dissolved polonium ions rapidly
adsorb onto suspended particles and are accumulated by marine organisms. The
downward movement of those particles and planktonic faecal pellets leads to
the depletion of polonium in surface waters, particularly in areas of enhanced
primary and secondary productivity.
The atmospheric depositions of
210
Po originates from the decay of
atmospheric radon, exhaled predominantly from landmasses and from
138
CHAPTER 8
resuspended dust. Most of the
210
Po measurements in surface air and in
atmospheric depositions were made simultaneously for
210
Pb and
210
Po, and
conclude that
210
Po concentrations in aerosols are minor and usually about
0.1
times
210
Pb concentrations (see Chapter 5). Average concentrations of
210
Pb
and
210
Po in the air, as well as atmospheric deposition fluxes, vary with latitude
and rainfall and are largely controlled by radon, exhalation and atmospheric
concentrations, which are dependent on land mass distributions, and are
modulated by global atmospheric circulation (see Chapter
5). Although direct
measurements of
210
Pb and
210
Po deposited at the ocean surface have not been
made,
210
Pb transport by global atmospheric circulation has been modelled, and
210
Pb deposition at the ocean surface has been estimated for specific areas to a
reasonable degree of accuracy
[8.1–8.3].
Ingrowth of
210
Po from
210
Pb–
210
Bi radioactive decay in sea water takes
place everywhere in the ocean and depends on
222
Rn concentration in sea
water, which in turn is the sum of
222
Rn from in situ decay of dissolved
226
Ra
and
222
Rn diffusing from the sea-floor following
226
Ra decay in sediments. The
resulting
210
Po concentrations in sea water also greatly depend on
210
Po binding
to suspended particulate matter and particle scavenging in the water column.
Polonium-210 also enters coastal areas with river discharges which feed the
seas with chemical elements, including radionuclides of the natural radioactive
series, in dissolved and solid phases. Owing to the low water solubility of
polonium and its high partitioning into the solid phase (K
d
), most polonium
discharged by rivers is associated with suspended particulate matter and bottom
sediment discharges
[8.4].
The most relevant, direct
210
Pb and
210
Po anthropogenic discharges into the
sea relate to non-nuclear industries, such as phosphate ore processing, oil and
gas exploitation, and heavy mineral sands mining
[8.5]. The better investigated
case studies relate to phosphate ore processing for phosphoric acid and phosphate
fertilizer manufacturing. Research conducted in several countries has shown
that naturally occurring concentrations of
210
Po in estuaries and coastal waters
receiving such phosphatic discharges were locally enhanced by factors in the
range of 10–1000
[8.2, 8.6–8.10]. Often neglected but locally important, past
radioactive waste dumping in the sea contained non-negligible amounts of
210
Po
and
226
Ra [8.11, 8.12].
8.3. POLONIUM IN THE OCEANS
Polonium concentrations in dissolved and particulate phases have been
reported for coastal waters and for the open ocean (see Refs [8.13–8.15]).
Generally, polonium in coastal waters is largely associated with suspended

139
MARINE SYSTEMS
particulate matter, with a minor fraction in the dissolved phase (<35%), probably
due to the higher suspended particle loads and more intensive mixing than in open
ocean waters, where polonium is mostly in the dissolved phase (see Table
8.1). In
coastal sea water,
210
Pb is also mainly associated with suspended matter, although
to a lesser extent than
210
Po. Neither concentration shows significant seasonal
variation; thus, atmospheric depositions do not seem substantially to modify their
concentrations in sea water throughout the year.
In coastal waters, concentrations of dissolved
210
Po are around
0.5
Bq/m
3
, and usually the Po:Pb ratio is near one in the dissolved phase. In
suspended particulate matter, however,
210
Po concentrations are generally higher
than
210
Pb. Partitioning coefficients (K
d
) for
210
Pb and
210
Po between suspended
particulate matter and water (soluble fraction) have been reported for a few
environments, and indicate high K
d
values for
210
Po in the range of (2–7) × 10
5
,
which are on average five
times higher than the K
d
values for
210
Pb. The strong
partitioning of these two radionuclides into the particulate phase, higher for
TABLE 8.1. PARTITIONING COEFFICIENT (K
d
) OF
210
Pb AND
210
Po IN THE
MARINE ENVIRONMENT BASED ON IN SITU DETERMINATIONS
Region
Dissolved
(Bq/m
3
)
Particulate
(Bq/m
3
)
%
dissolved
K
d
Ref.
210
Pb
NW Mediterranean
Sea, off Spain
1.27 ± 0.31 0.049 ± 0.011 96 (1.0 ± 0.57) × 10
5
[8.16]
NW Mediterranean
Sea, off Monaco
1.62 ± 0.07 0.27 ± 0.02 86 (6.4 ± 0.5) × 10
4
[8.17]
NE Atlantic Ocean,
Portuguese surf
zone
0.54 ± 0.28 1.11 ± 0.73 33 (1.7 ± 1.0) × 10
5
[8.15]
210
Po
NW Mediterranean
Sea, off Spain
1.09 ± 0.37 0.27 ± 0.083 79 (7.8 ± 5.6) × 10
5
[8.16]
NW Mediterranean
Sea, off Monaco
0.62 ± 0.03 0.25 ± 0.03 71 (1.6 ± 0.2) × 10
5
[8.17]
NE Atlantic Ocean,
Portuguese surf
zone
0.57 ± 0.13 1.87 ± 0.73 23 (2.8 ± 0.9) × 10
5
[8.15]
NE Atlantic Ocean,
French surf zone
0.47 ± 0.21 0.94 ± 0.37 33 (1.2 ± 0.62) × 10
5
[8.13]
140
CHAPTER 8
210
Po than
210
Pb, contributes to shaping the distribution of
210
Po in the marine
environment.
In the open ocean, the water column is stratified and vertical mixing is
reduced and mostly occurs in the epipelagic layer. Water stratification allows for
the formation of gradients of dissolved salts and relatively stable vertical profiles
of their concentrations. In the oceanic water column,
210
Po is produced from
radon decay in the atmosphere, the decay of dissolved radon and radon exhaled
from the ocean floor. Radon concentrations in the water column are lowest in the
surface layer and greatest in the deep-sea water layer. Radon distribution mainly
depends on diffusion in the water column and marine currents.
The isotopes
210
Pb and
210
Po are also introduced to the ocean from
atmospheric depositions, and can be detected in the upper layer of the ocean,
where a measurable excess of
210
Pb and
210
Po over dissolved
226
Ra concentrations
are reported
[8.16, 8.18]. Adsorption of
210
Pb and
210
Po onto particulates,
followed by uptake by phytoplankton and zooplankton, allows for the removal
of these radionuclides from the epipelagic layer with the downward flux of
biogenic particles. Some of the particles falling from the upper oceanic layer are
consumed in intermediate ocean layers and the chemical elements are recycled,
contributing to supporting the abundant marine life in the oceanic mesopelagic
layer. Particles, in their settling path through the deeper ocean layers, may further
adsorb and remove soluble
210
Pb and
210
Po from the water column, creating an
imbalance between
226
Ra (not adsorbed onto particles) and its particle reactive
daughters, and thus prevent the formation of secular radioactive equilibrium.
As a consequence of this radionuclide scavenging in the deep-sea layers,
210
Pb
and
210
Po are deficient in comparison to
226
Ra activity concentrations in all
oceans
[8.19–8.22]. Although very fine particles (Stokes particles, such as clay
particles) may settle with very low velocities of about 900
m/a, some of the
biogenic particulate materials generated in the upper layer of the ocean, especially
larger faecal pellets and carcasses of zooplankton organisms, sink rapidly and
may reach the abyssal sea-floor in days or weeks, adding
210
Pb and
210
Po to the
top sediment layer (see Fig.
8.1) [8.16, 8.23–8.25].
Figure 8.2 displays the vertical profiles of
210
Po along with those of
210
Pb
and
226
Ra in the north-eastern Atlantic Ocean water column. Similar profiles have
also been reported for other oceans
[8.17, 8.26, 8.27]. While the concentration
of dissolved
210
Pb can reach around 2.5 Bq/m
3
in the upper layer of the ocean,
dissolved
210
Pb in deeper layers barely reaches 1 Bq/m
3
. Dissolved
210
Po in
the upper layer is around 1
Bq/m
3
; it increases in the mesopelagic layer and
decreases again, and remains at concentrations much lower than those of
210
Pb all
the way down to the abyssal sea-floor. In particulate matter, the
210
Po:
210
Pb ratio
is generally consistently greater than 1.

141
MARINE SYSTEMS
The mean residence time of
210
Po in ocean water layers was calculated for
several oceans and values are about 0.5–1
years in the upper layer and somewhat
longer, around 2 years, in the soluble phase of deep-sea layers
[8.16–8.18,
8.23,
8.25]. Accumulation of
210
Po deposited onto the oceanic bottom sediments
has generally not been measured, as all available reports focus on
210
Pb
measurements
[8.15]. The
210
Po flux arriving at the abyssal sea-floor in the
north-eastern Atlantic Ocean was estimated as twice that of the
210
Pb flux and,
in turn, the settling
210
Pb flux at the abyssal sea-floor was computed as twice that
of the
210
Pb atmospheric flux entering at the surface of the ocean. Furthermore,
Source: Figure 2 of Ref. [8.23].
Note: d — dissolved phase; Fd — deposition flux on the sea-floor; Ia — atmospheric input
of
210
Pb (Bq·m
−2
·a
−1
); T — mean residence time (years); p — particulate phase;
Part. — radionuclide flux associated to the particle flux (Bq·m
−2
·a
−1
).
FIG. 8.1. Box model of the water column in the north-eastern Atlantic Ocean, with inventories
and mean residence times of
210
Pb and
210
Po, and deposition fluxes at the sea-floor.

142
CHAPTER 8
while the
210
Po:
210
Pb ratio in atmospheric particle depositions at the surface of
the ocean is around 0.1, in the sediment depositions onto the abyssal sea-floor of
the north-eastern Atlantic Ocean, the
210
Po:
210
Pb ratio is around 2 (see Fig. 8.1).
8.4. ACCUMULATION AND TURNOVER OF
210
Pb AND
210
Po
IN MARINE ORGANISMS
Carvalho
[8.28] reports that:
“Polonium (
210
Po) and radioactive lead (
210
Pb) in marine organisms
have attracted the attention of scientists because of their relatively high
concentrations in comparison with those in terrestrial organisms and also
because
210
Po concentrations in marine biota often are much enhanced
in comparison to those of
210
Pb, its radioactive grandparent [8.29, 8.30].
The first determinations of
210
Po in marine biota had shown high
concentrations of largely unsupported
210
Po [i.e. above the
210
Pb
concentration] in plankton, 110
Bq·kg
−1
(dry weight), and soon other
reports added information on unsupported
210
Po in marine fish, crustacean
and whales
[Refs [8.29, 8.31–8.33]]. Early measurements did record
also high
210
Po concentrations in some marine species, such as the
lanternfish, and in certain internal organs such as the pyloric caecca of
tuna fish
[Refs [8.34, 8.35]]. Pioneer data were reviewed by Cherry and
Shannon
[Ref. [8.29]] who highlighted the importance of
210
Po as a major
Source: Based on Ref. [8.23].
FIG. 8.2. Profiles of
226
Ra,
210
Pb and
210
Po in the water column of the north-eastern Atlantic
Ocean.

143
MARINE SYSTEMS
internal source of the radiation dose received in the tissues of marine
organisms.
“Further work revealed the distribution of
210
Po in crustacean tissues,
including the euphausiid Meganicthyphanes norvegica, and the
role of zooplankton in the removal of
210
Po from the ocean surface
layer
[Refs [8.36–8.38]].”
Early work on polonium distribution in marine crustaceans highlighted
elevated concentrations in the hepatopancreas and suggested that food could be
the main source of polonium in invertebrates and the release of faecal pellets its
main excretion route
[8.36, 8.39–8.43].
The understanding of accumulation mechanisms and the biokinetics of
many radionuclides in marine biota has been the goal of extensive experimental
work carried out over decades in many radioecology laboratories. However, in
contrast to, for example, fission products and transuranium elements, not much
experimental work has been performed on the biokinetics of polonium in marine
species and aquatic species in general (see the early works in Refs
[8.44, 8.45]).
This is understandable because, as a pure alpha emitter, polonium is not
easily amenable to measurement by direct spectrometric techniques and even
its measurement by total alpha counting is not possible without some type of
relatively expensive and time consuming method. This difficulty was likely
the main reason for the long persistent gap in knowledge on polonium uptake
pathways, metabolism and turnover rates in marine biota, leaving polonium
biokinetics open to guess work or assumptions by inference from indirect
evidence.
The development of alpha spectrometry equipment and the production of
artificial polonium isotopes allowed the use of double tracer techniques, involving
three polonium isotopes, to investigate the role of the food ingestion pathway
(food labelled with
208
Po) and sea water (labelled with naturally occurring
and added
210
Po), and, using
209
Po as an isotopic tracer, for determination of
radiochemical yield and internal analytical quality control of every sample
[8.46].
Using such a double tracer technique, it is possible to quantify food and
water as polonium sources, as well as polonium elimination by excretion and
radioactive decay. This allows a mathematical description of the kinetics
of polonium uptake and elimination in marine organisms. Carvalho and
Fowler [8.46] model the polonium biokinetics using the following mass balance
equation:
( )
ww f
d
d
t
t
Q
I WC AFC k Q
t
l= + -+
(8.1)

144
CHAPTER 8
where
Q
t
is the activity of polonium in the organism at time t (Bq);
I
w
is the uptake rate constant fom water (mL/g organism d
−1
) by all processes,
namely drinking and surface adsorption;
W
is the weight of the organism (g);
C
w
is the polonium concentration in water (Bq/mL);
A is the digestive assimilation efficiency or absorption efficiency of polonium
from food;
F
is the amount of food ingested by an organism per day (g/d);
C
f
is the concentration of polonium in food (mBq/g);
K is the elimination rate constant of polonium (d
−1
) from the organism;
and λ is the radioactive disintegration rate constant of
210
Po (λ
210Po
= 0.005 01 d
−1
);
λ
208Po
= 0.000 656 d
–1
). Each term in this equation can be considered separately
for assessing and describing the kinetics of polonium accumulation in the
organism from water and food only.
Expressed in terms of activity concentration, the polonium concentration in
an organism in steady state equilibrium of exchange with the environment (C
ss
) is
described by
[8.46]:
( )
f
ss
AC
F
C
kWl
æö
×
÷
ç
÷
ç
=
÷
ç
÷
÷
ç
+
èø
(8.2)
In a set of laboratory experiments using shrimp, prawn and fish, Carvalho
and Fowler [8.47] show that polonium absorbed in the internal tissues of biota
is almost exclusively taken up from the ingested food and absorbed through the
digestive pathway.
In shrimp, it was experimentally verified that, in spite of the presence
of dissolved
210
Po in sea water, there was only minor absorption of
210
Po ions
from water. Furthermore, this minor absorption did not take place through gill
epithelium, as it does for many other ionic elements, but rather the tiny amounts
of
210
Po absorbed from sea water were due to the drinking water reflex of marine
organisms for osmotic balance, and so this polonium was absorbed through the
gut wall in the same manner as polonium from food
[8.46, 8.47].
Furthermore, it was demonstrated that crustaceans, such as shrimp and
prawn, in sea water containing dissolved
210
Pb and
210
Po, easily accumulated
210
Po from ingested food into their internal organs, while
210
Pb absorption from
food was minor. In addition, dissolved
210
Pb in sea water was adsorbed onto
exposed surfaces, such as the exoskeleton and gills, without significant absorption
into internal organs. This differentiated uptake, facilitating gut absorption of
145
MARINE SYSTEMS
food-borne
210
Po in comparison to food-borne
210
Pb, explains the increased Po:Pb
ratio (>1) systematically found in internal tissues of marine biota.
Moreover, Carvalho and Fowler [8.46] show that the ingestion pathway
accounted for over 97% of
210
Po intake in shrimp, and for over 99% in fish
internal tissues. The absorption of polonium from
208
Po labelled food closely
matches the gut absorption efficiency of protein from diet in prawn and fish.
Once absorbed, this
208
Po is rapidly distributed in internal tissues according to
the distribution pattern of naturally occurring
210
Po. Further experiments show
that polonium absorbed though the digestive pathway and accumulated in the
liver of teleost fish binds to proteins, such as ferritin and metallothioneins, that
strongly bind
210
Po and are able to retain significant amounts of this radioelement
in internal tissues
[8.48].
Experiments carried out with phytoplankton cells verify their rapid uptake
of
210
Po from liquid culture media. Using these polonium labelled cells to feed
mussels, it can be shown that
210
Po is rapidly absorbed and accumulates in the
soft tissue of mussels. It can be concluded that filter feeding molluscs, such as
mussels, in spite of filtering large volumes of sea water containing dissolved
210
Po, accumulate
210
Po in internal tissues mainly from ingested food [8.49].
Experiments using the same polonium double tracer technique also show
that
210
Po dissolved in the liquid media of microalgal cultures can be rapidly
adsorbed onto and absorbed into phytoplankton cells. In addition, through
ingestion of these cells,
210
Po was transferred to herbivorous (planktivorous)
zooplankton
[8.50–8.52].
From kinetic studies on
210
Pb and
210
Po in marine shrimp, Carvalho and
Fowler [8.47] conclude that
210
Po has a biological half-life of 10–11 days
in whole body shrimp (T
1/2
= 7 days in the hepatopancreas; T
1/2
= 28 days in
muscle). At 4–11 days,
210
Pb has a shorter half-life in whole body shrimp and
a much shorter half-life in internal organs (T
1/2
= 4 days in the hepatopancreas;
T
1/2
= 2 days in muscle), which may further contribute to higher
210
Po:
210
Pb ratios
in marine biota.
The distribution of polonium in internal organs and tissues has been reported
for many marine organisms, including molluscs, crustaceans, fish and mammals.
Higher
210
Po concentrations are systematically found in the liver and digestive
tract, and lower ones in muscle tissue. For example,
210
Po concentrations in fish
tissues vary enormously from species to species (see Fig. 8.3), but there is a
general pattern of distribution: lower concentrations are consistently measured in
muscle tissue and higher concentrations are found in the gut and liver, mostly in
relation to the digestive organs (see Section 8.5.2).
This distribution pattern is common to teleost (bony fish) and
elasmobranchs (cartilaginous fish), and cartilaginous fish generally display
lower
210
Po concentrations than teleost fish — most probably on account of

146
CHAPTER 8
Source: See Ref. [8.53].
FIG. 8.3. Polonium-210 in fish organs of a teleost fish (above) and a cartilagineous fish
(below).
their physiology and the biochemical composition of their tissues [8.54, 8.55].
In several species of teleost fish and marine mammals,
210
Po variations in the
muscle tissue were correlated with red muscle content
[8.56]. As red muscle has
a higher myoglobin content, this association with
210
Po was considered a likely
biochemical association.
High
210
Po concentrations were reported in 1982 for the hepatopancreas
of some mid-water pelagic shrimp, giving rise to high absorbed radiation
doses
[8.57]. Surprisingly high
210
Po concentrations were also measured in
147
MARINE SYSTEMS
the gonads of several fish species [8.54]. This was assumed to relate to the
accumulation of proteins in the egg, but high
210
Po in eggs may expose the
genetic material to relatively high radiation doses. For example, in the case
of the common sardine, Carvalho [8.54] estimate that effective absorbed dose
from
210
Po could reach 170 mSv/a in the gonads and even higher in some other
tissues. It has been suggested that this could merit further investigation, as this
dose might have biological effects at a genetic level and might play a role in the
genetic variability of organisms.
The search for genotoxic effects (micronuclei and DNA strand breaks)
induced by
210
Po was undertaken in mussels from the Brazilian coast, with
naturally occurring
210
Po concentrations averaging 155.6 ± 7.9 Bq/kg (FW) in
their soft tissue. At this level of
210
Po concentrations, the internal dose rate was
0.02
mGy/d (7.3 mGy/a) and no genotoxic effects were observed. However, this
dose rate was lower than the suggested dose threshold for inducing effects —
10
mGy/d [8.58]. High
210
Po concentrations are also reported in marine mammals
at levels that are unusual by human standards (see Appendix
III).
8.5. POLONIUM IN MARINE BIOTA IN SEVERAL OCEAN REGIONS
The
210
Pb and
210
Po activity concentrations reported in marine biota are
spread over several orders of magnitude. The radionuclide levels do not relate
to seawater depths, ecosystems or geographical locations
[8.28]. This section
reviews
210
Po data pertinent to several ocean regions and many taxonomic
groups, with comments on ecology and trophic relationships in order to aid data
interpretation.
8.5.1. Intertidal zone
Carvalho
[8.28] reports that:
“The intertidal rocky shore and also soft bottom areas (e.g., in estuaries)
of the Atlantic Ocean and of the Mediterranean Sea are zones of dense
biological habitats with high biomass and typical populations such as
those of seaweeds, mussel and oyster beds, and characteristic fish species
(e.g., Blenniidae). Similar dense populations may be found on the rocky
shores of most temperate regions [Refs [8.59–8.61]].”
In the intertidal zone,
210
Po accumulated in macrophytic algae comes
from
210
Po dissolved in sea water. The first likely uptake mechanism is surface
adsorption by simple contact. Adsorption of dissolved radionuclides onto the
148
CHAPTER 8
surface of algal blades by diffusion mechanisms across the blade surface boundary
layer also apply to transuranic elements, such as plutonium and americium, with
similar ‘piston velocities’, and it is not considered an active biological uptake
mechanism (for further information, see the early work in Refs [8.62–8.64]).
Carvalho [8.28] reports that the
210
Po:
210
Pb ratios in macrophytic algae are
consistently higher than unity, often ranging from three to ten. This seems to be
due to preferential adsorption of
210
Po onto the algal surface. Analyses of
210
Pb
and
210
Po distribution in seaweed in the 1980s confirmed that most
210
Po is bound
or adsorbed onto external surfaces
[8.65]. Later work also confirmed passive
adsorption of metals to microalgal cell walls and fast kinetics of
210
Po and
241
Pu
sorption in marine phytoplankton and zooplankton cells
[8.66–8.68].
Detailed studies report
210
Po concentrations in seaweed averaging
4.8
Bq/kg (FW) (range: 1.6–9.1 Bq/kg) in the coastal area of Portugal in the
north-eastern Atlantic Ocean
[8.28, 8.54]. Carvalho [8.28] reports that differences
among algae classes are small, and Fucus sp. (Phaeophyceae), often used as
a bioindicator species, is the seaweed with the highest
210
Po concentration
(9.1
Bq/kg, FW). Concentrations of
210
Po in Fucus vesiculosus at Pirou, the
French Channel, are 13.7
Bq/kg (FW) [8.13]. Concentrations in the range
of 11–26
Bq/kg (FW) are reported for several seaweed species on the Tamil
Nadu coast, India, in a tropical environment
[8.69]. Since
210
Po accumulation
in seaweed is operated by sorption mechanisms, those slight variations in
210
Po
concentrations in seaweed might be due to the sorption characteristics of algal
coatings. Furthermore, adsorption of radionuclides might also be affected
by water temperature, water mixing and suspended particulate matter load,
especially in the surf zone.
Polonium-210 accumulation in animal species is very different to that in
seaweed. Different mollusc species living in the same intertidal rocky shore can
display very different
210
Po concentrations. For example, Carvalho [8.28] finds
the following concentrations in molluscs at the north-eastern Atlantic coast:
— Limpet (Patella aspera): A herbivore mollusc feeding on seaweed grasped
from the rock surface — 11.6
Bq/kg (FW);
— Mediterranean mussel (Mytilus galloprovincialis): A filter feeding mussel
whose diet is organic suspended particles — 132
Bq/kg (FW);
— Winkle (Littorina littorea): A carnivore gastropod — 283 Bq/kg (FW).
Although these molluscs all live in the same zone,
210
Po is concentrated
in their soft tissue at different levels, which reflects that these species occupy
distinct biotopes and trophic levels although they live together in the same
zone
[8.28]. Comparing and interpreting results for organisms from rocky shores
149
MARINE SYSTEMS
is generally straightforward. However, this might not be the case for results from
soft bottom organisms.
Intertidal, sediment dwelling bivalve molluscs which live in soft
bottoms at the temperate north-eastern Atlantic coast, such as the clams Tapes
decussatus and Ensis siliqua, exhibit dry weight concentrations of 760
Bq/kg and
225
Bq/kg, respectively. Another clam (Anadara granosa) from a similar
biotope in the tropical environment of Mumbai Harbour, India, exhibited
313
Bq/kg (DW) and another burrowing bivalve (Tonna dolium) on the Tamil
Nadu coast, India, exhibited 132
Bq/kg (FW) [8.69, 8.70]. Although living in
similar environments, these different species might have different feeding
strategies and diets, which would account for the differences. It should be
recognized that results of radionuclide analysis in sediment burrowing molluscs
may be affected by incidental parameters, such as the presence of sediment in the
gut that is sometimes difficult to remove. While some researchers remove the gut
sediment prior to analysis by dissection, washing or depuration, others do not.
Thus without a common methodology in sample preparation, comparing results
and interpretation can be problematic.
This difficulty is not present in every case, especially when studying
organisms that do not ingest bulk sediment to process organic matter as food.
Although all crab species inhabit sandy and muddy substrates, their feeding
strategies involve food selection instead of ingestion of bulk sediment
[8.71].
Extensive analysis of
210
Po in ten species of brachyuran crabs from the intertidal
zone of the Gulf of Mannar, on the Indian coast of the Bay of Bengal, show
that
210
Po concentration (FW) in crabs varies according to their food habits: it
is low in herbivores, 45.9
Bq/kg; and significantly higher in carnivores, up to
221.2
Bq/kg.
Heyraud et al. [8.72] examine whether mussel samples from different
latitudes might reflect the differences in atmospheric depositions of
210
Pb and
210
Po (see Refs [8.73–8.80] and Table 8.2.). As depositions in the southern
hemisphere are up to ten times lower than those in the northern hemisphere (see
Section
5.3), they hypothesize that
210
Pb and
210
Po in mussels might reflect that
difference. Their results show that mussels from European and South African
coasts display similar average radionuclide concentrations and no noticeable
latitude effect can be identified. Furthermore, mussels from the coasts of
Denmark and the United Kingdom with low seawater temperature and strong
seasonal temperature fluctuation, when analysed for
210
Pb and
210
Po, display an
average and range of
210
Po concentrations comparable to mussels from tropical
zones on the coast of Brazil and from the coast of the Mediterranean Sea.
Charmasson et al. [8.73] compare
210
Pb and
210
Po accumulated in filter
feeding mussels from intertidal areas of the Mediterranean coast and mussels
from deep-sea hydrothermal vents of the Menez Gwen field, in the Mid-Atlantic

150
CHAPTER 8
Ridge, the latter exposed to sulphide rich vent waters, and report similar
concentrations despite all the environmental differences. Results for coastal
clams and deep-sea clams from soft bottoms of the north-eastern Atlantic Ocean,
both sediment burrowing, suspension feeding bivalves, also reveal similar
210
Pb
and
210
Po concentrations [8.28]. These results suggest that, in spite of different
TABLE 8.2. ACTIVITY CONCENTRATIONS (AND RANGE) OF
210
Po AND
210
Pb IN MUSSEL (Mytilus spp.) SOFT TISSUES
Region DW:FW
Concentration (Bq/kg, DW)
Po:Pb
(range)
Ref.
210
Po
210
Pb
Adriatic Sea, Italy 0.16 112 (50–142) —
a
—
a
[8.75]
Aegean Sea, Turkey 0.19 305 (52–1344) 28.0 (6–167) 12.1
(3.1–25.0)
[8.76]
English Channel
Pirou, French coast
0.17
296
12.2
25.4
[8.13]
Irish Sea, Ireland 0.12 222 (80–468) 11.9 (4–25) 15.9 [8.14]
Mediterranean Sea
Croatia
Toulon, France
—
a
0.17
54–460
b
203.1
—
a
41.3
—
a
8.6
[8.77]
[8.73]
NE Atlantic
Portugal open coast
(oligotrophic)
Portugal Tejo Estuary
(mesotrophic)
Ré Island, France
0.13
0.13
—
a
759 (460–1470 )
210
289–589
45 (23–96)
1
—
a
17
21
—
a
[8.80]
[8.80]
[8.74]
North Sea
United Kingdom
—
a
104–3124
2.8–284
—
a
[8.79]
South Atlantic
Brazil
Brazil
Cape of Good Hope,
South Africa
—
a
—
a
—
a
1995
156
b
(54–460)
409 (170–1290)
—
a
—
a
5.7 (1.2–16.2)
—
a
—
a
86 (22–218)
[8.78]
[8.57]
[8.72]
a
—: data not available.
b
Fresh weight.
151
MARINE SYSTEMS
environmental conditions in distinct ecosystems, similar organisms with similar
feeding strategies and at the same trophic level in the food chain accumulate
210
Po up to comparable concentrations.
Coastal mussels, often of the family Mytillidae, are used worldwide
as sentinel organisms to monitor contamination in coastal areas following
the concept of the pilot International Mussel Watch programme of the United
Nations Educational, Scientific and Cultural Organization to report
210
Po in
mussels in many regions. In the Mediterranean Sea, for example,
210
Po has
been determined in mussels from the coast to assess the radiological risk from
radioactive atmospheric depositions (mainly
137
Cs) from the Chernobyl accident
compared to
210
Po. It was concluded that, in spite of widespread deposition of
137
Cs in the Mediterranean Sea, the radiation dose to mussels and to mussel
consumers would be a minor fraction of that from naturally occurring
210
Po. A
similar finding was deteremined in 1995 by the then IAEA Marine Environment
Laboratory, Morocco
[8.81]. However,
210
Po concentrations for mussels also
vary widely
[8.82].
Mussels have also been used as sentinel organisms to monitor the impact
of local discharges of radionuclides which include
210
Po, for example from
phosphate industries. In some coastal areas subject to these effluents,
210
Po
enhancement can be observed in mussels and other marine molluscs, while in
other cases, surprisingly no
210
Po enhancement could be detected [8.8, 8.79].
The
210
Po concentrations in mussels in different regions around the world vary
across a wide range of values, and results from specific areas with anthropogenic
discharges often cannot be considered significantly different on a statistical basis
(see Table
8.2). An examination of published regional
210
Po datasets reveals
wide ranges of concentration in mussels from the same coastal region, collected
at the same time of the year and by the same research team. For example, the
concentrations of naturally occurring
210
Po in mussels from 27 locations around
Ireland were in the range of 80–468
Bq/kg (DW) [8.14], which indicates that
wide variations truly do exist among samples and are not artefacts. An interesting
finding is that
210
Po concentrations in mussels from the most pristine areas are
sometimes even higher than those in mussels from areas subject to anthropogenic
discharges of
210
Po, thus rendering coastal monitoring results difficult to interpret
(see Refs [8.13, 8.14, 8.83]).
This interest in the use of mussels as biomonitors led to a detailed
investigation of
210
Pb and
210
Po bioaccumulation in mussels, the effects of
environmental parameters and the physiological condition index of mussels on
210
Po concentration [8.84], and the assessment of the allometric effect on
210
Po
concentration
[8.85]. One important finding is that the fluctuation of
210
Po in
mussel soft tissue throughout the year seems at first glance to be a consequence of
the seasonal
210
Pb–Po atmospheric deposition flux. Instead, Carvalho et al. [8.85]
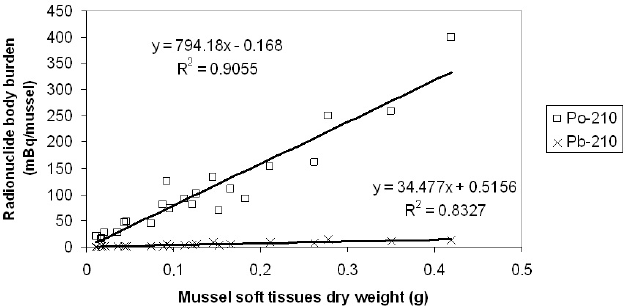
152
CHAPTER 8
Note: Based on the data presented in Ref. [8.85].
FIG. 8.4. Variation of
210
Pb and
210
Po body burden in mussel soft tissue as a function of shell
length.
verify that it is an effect of the seasonal fluctuation of the physiological condition
of mussels. They report that during winter, when mussels have less fat, the
210
Po
concentration per kilogram in the soft tissue is higher than in summer. In summer,
when the accumulation of lipids and ripe gonads increase the physiological
condition index of mussels,
210
Po activity concentration expressed on a mass
basis exhibit lower values. However, when
210
Po concentration is expressed per
mussel (
210
Po body burden),
210
Po values are nearly stable over the year, and the
main seasonal fluctuation is of lipid (fat) reserves, not of
210
Po [8.15]. Carvalho et
al. [8.85] describe the allometric effect on
210
Po concentration as very pronounced
in mussels and, for example, an increase in mussel shell length from 2.5
cm to
5.0
cm can correspond to a decrease of 50% in
210
Po concentration in mussel soft
tissue (see Figs
8.4 and 8.5).
These findings on mussels, based on the determinations of
210
Pb and
210
Po, lead Carvalho et al. [8.85] to recommendations that can be applied to all
contaminants and include the following:
(a) To select carefully mussels of the same size class to avoid allometric effects
on
210
Po concentrations;
(b) To determine the physiological condition index of mussels to correct for the
fat content;
(c) To take and pool a minimum number of individuals in each sample to
minimize inter-individual variation;
(d) To allow for statistically valid comparison of results from different places.

153
MARINE SYSTEMS
An improved sampling methodology can achieve comparable results in
coastal monitoring using molluscs.
8.5.2. Coastal seas
Carvalho
[8.28] reports that:
“Amongst the large diversity of fish species in the waters above continental
shelf, the Clupeidae (sardine, herring, and sprats), the Scombridae
(mackerel) and Thunidae (tuna), have a special importance due to their
abundance compared with other fish and to their major place in the human
diet. While these are all pelagic fishes, other also very common fish species
are demersal [i.e. live near the sea-floor and feed on benthic organisms],
such as the sea hake (Merlucius merlucius), the pouting (Trisopterus luscus)
and the small benthic sharks, such as the spotted catshark (Scyliorhinus
canicula).”
With regard to
210
Po transfer from the marine environment to human
populations, the consumption of seafood from coastal seas is the main pathway
and therefore has received much attention in the assessment of radiation exposure
(see Refs [8.70, 8.86–8.90]).
Fish species from many coastal regions around the world have been
analysed, either on a whole body basis or in segregated organs and tissues, and
the
210
Po concentrations are spread over a very wide range of values which has
challenged interpretation (see Table
8.3). Attempts have been made to find an
y = - 209.87x + 1589.8
R
2
= 0.386
y
= - 8.5992x + 70.912
R
2
= 0.335
1
10
100
1000
10000
0
1 234 5
Shell length (cm)
Radionuclide conc (Bq/kg)
Po210
Pb210
Note: Based on the data presented in Ref. [8.85].
FIG. 8.5. Allometry of
210
Pb and
210
Po concentrations in mussels as a function of shell length.

154
CHAPTER 8
TABLE 8.3. ACTIVITY CONCENTRATIONS OF
210
Po AND
210
Pb IN FISH
TISSUE FROM COASTAL AREAS OF THE NORTH ATLANTIC
Species and region Tissue DW:FW
210
Po
(Bq/kg)
210
Pb
(Bq/kg)
Po:Pb
Sardine
(Sardina pilchardus)
Madeira Island, Portugal
Muscle 0.23 66 1.0 66
Liver 0.28 2 140 6.2 345
Gonad 0.27 275 1.8 156
Bone 0.39 197 26 7.5
Stomach 0.27 510 2.5 199
Caecca 0.23 2 490 138 18
Intestine 0.24 28 000 100 281
Chub mackerel
(Scomber japonicas)
Madeira Island, Portugal
Muscle 0.26 19 0.63 30
Liver 0.56 1 035 7.3 141
Gonad 0.25 183 22 8
Bone 0.39 42 11 3.7
Blue jack mackerel
(Trachurus picturatus)
Madeira Island, Portugal
Muscle 0.28 8.9 0.7 13
Liver 0.28 615 134 4.6
Gonad 0.17 56 1.1 51
Bone 0.38 43 15 2.8
White sea bream
(Diplodus sargus)
Lisbon, Portugal
Muscle 0.27 0.52 0.20 2.6
Liver 0.31 28 14.5 1.9
Gut 0.28
39 1.13 34
Caecca 0.25 69 5.0 14
Bone 0.53 25 31 0.8
Cod
(Gadus morhua)
Newfoundland, Canada
Muscle 0.19 0.628 0.040 16
Liver —
a
6.59 0.08 81
Gonad —
a
1.87 0.014 14
Bone —
a
0.41 0.61 0.7
European hake
(Merlucius merlucius)
Sesimbra, Portugal
Muscle 0.19 6.4 0.15 43
Liver 0.64 10.8 1.51 7
Gonad 0.17 52.0 1.26 41
Bone 0.30 7.8 0.77 10
Source: See Refs [8.28, 8.83].
a
—: data not available.
155
MARINE SYSTEMS
explanation for
210
Po variation based on allometry (body size and metabolic
rates of species), diet composition, water depth, latitude and other environmental
parameters.
For example, Dahlgaard [8.83] attempts to relate
210
Po concentration levels
in fish with variation of environmental parameters in the Baltic Sea. Average
210
Po concentrations (FW) in frozen fish fillets are 0.35 Bq/kg (cod), 0.65 Bq/kg
(herring) and 0.96 Bq/kg (plaice). The differences in
210
Po between species are
statistically significant. However, an attempt to find a correlation between
210
Po
concentrations in fish and the salinity gradient, which in the Baltic–North Sea
region is in the range of 8–35%, shows no statistically significant effect either on
fish or on mussels.
A 1988 study of herring, cod and flounder in the Baltic Sea (Gulf of
Gdansk, Poland) found that the highest concentrations were measured in the
digestive organs, in particular the intestine
[8.91]. Furthermore, the contribution
of the digestive organs to the total
210
Po accumulated in the fish body was
positively correlated with the degree of fullness of the stomach, while the
210
Po
concentration in fish decreased if food was lacking in the stomach.
The
210
Po concentration (
210
Po whole body burden or
210
Po concentration
in muscle) can allow for ranking species into different
210
Po concentration levels.
Heyraud and Cherry [8.40] tentatively interpret this as being dependent on
the diet of each species. For example,
210
Po concentrations are much lower in
herbivorous fish (e.g. white sea bream, Diplodus sargus) than in planktivorous
fish (e.g. sardine, Sardina pilchardus)
[8.28, 8.54]. Research was also published
in 1989 on the effect of diet composition on
210
Po concentrations in fish, in an
attempt to apply
210
Po as an indicator of diet, at least in clupeoid fish [8.92].
While some parameters play a role in constraining
210
Po concentrations, none
is sufficient to provide a key explanation for such a wide range of concentrations
determined in fish and other biota living in the same environment. A different
approach, rooted in previous findings, has been proposed for interpreting
210
Po concentrations in marine biota on the basis of food chains in the ocean
and the trophic position of organisms (see Refs [8.28, 8.46, 8.48, 8.54]). This
interpretation is based on evidence provided by a comparison of
210
Po levels in
species that are taxonomically related, often with very similar feeding strategies
and especially occupying the same trophic level in the food chain
[8.28].
One example is that the
210
Po concentration level in the muscle of
planktivorous sardines (Sardina pilchardus) from the north-eastern Atlantic
Ocean (see Table 8.3) is comparable to that in similar fish of the tropical sea
environment off the Tamil Nadu coast, India, where
210
Po concentrations (FW) in
planktivorous fish muscle are 190
Bq/kg for Sardinella longiceps and 116Bq/kg
for Eleuteronema tetradactylum
[8.28, 8.69]. Similarly high
210
Po concentrations
are reported for the anchovy (Engraulis encrasicolous) in the Adriatic Sea, and
156
CHAPTER 8
for the anchovy and the sprat (Sprattus sprattus) in the Black Sea — all of which
are planktivorous fish species that consistently exhibit
210
Po concentrations
higher than all other fish from the same areas
[8.75, 8.93].
Another example is the similar
210
Po concentration levels in mesopelagic
fish species, such as the lantern fish and hatchet fish, from the Pacific Ocean
and the North Atlantic Ocean, but with a similar diet based on small pelagic
zooplankton crustaceans and occupying the same trophic level (see Refs [8.28,
8.54, 8.94]).
A last example, reported by Carvalho
[8.28], is the
210
Po concentration in
large predators of coastal seas, such as dolphins (Delphinus delphis), with average
210
Po concentrations of 49 Bq/kg in the dorsal muscle and 107 Bq/kg in the liver,
which are generally higher than in fish
[8.95, 8.96]. The diet of these marine
mammals (Delphinidae) is exclusively based on seafood and they are at the top of
food chains in coastal seas. Dolphins concentrate
210
Po exclusively from the diet
(i.e.
210
Po transferred along the food chain) — there is practically no absorption
from water. Interestingly, other Delphinidae from the tropical waters of Brazil
in the southern hemisphere and from temperate waters off the Portuguese coast
exhibit very similar
210
Po concentrations, reinforcing the absence of an influence
from geographical and climatic regions and the key role of trophic level and diet
on
210
Po accumulation (see Appendix III).
Some trophic relationships and
210
Po concentration levels are easy to
interpret and correlate, such as
210
Po transfer from phytoplankton to herbivorous
zooplankton, from plankton to planktivorous fish, or from fish to marine
mammals, as described above; while others might be more challenging. For
example, Hassona et al. [8.97] report
210
Po concentrations (FW) for coral reef
fish species from the Red
Sea, a tropical environment with complex food webs in
the sublittoral zone, according to their general dietary habits:
— Carnivorous species: 1.8 Bq/kg (range: 0.25–6.42 Bq/kg);
— Herbivores: 2.2 Bq/kg (range: 1.52–3.80 Bq/kg);
— Omnivorous fish: 2.5 Bq/kg (range: 0.72–5.04 Bq/kg).
Although Hassona et al. [8.97] interpret
210
Po concentrations as a reflection
of the dietary habit of the species, differences in
210
Po concentrations among
groups are minor.
Eventually, and particularly in reef ecosystems, one aspect that might need
refinement and further detailed work is the understanding and classification
of the dietary habits of fish and a proper relationship with
210
Po accumulation.
There are at least four types of alimentary canal in marine herbivorous fish which
can be modelled using analogies with chemical reactors
[8.98]. Herbivores can
consume food of different qualities, with different digestion rates and different
157
MARINE SYSTEMS
transit times; and they can process food in non-acidic to highly acidic stomachs,
which can modify
210
Po uptake. Furthermore, carnivorous fish in coral reef
ecosystems involuntarily ingest large amounts of coral fragments while capturing
their prey. Studies have shown that sea-birds that find food in the sea exhibit
210
Po concentrations that reflect the food habits and
210
Po concentrations in their
prey
[8.99, 8.100].
8.5.3. Epipelagic zone
In the oceanic province,
210
Pb and
210
Po concentrations in the upper layer
of the ocean are controlled mainly by atmospheric deposition and, to a much
lesser extent, by the decay of
226
Ra dissolved in sea water [8.28]. A 1988 study
found the average
210
Po concentration in this oceanic upper layer to be around
1 mBq/L [8.22]. The upper layer of the ocean, or epipelagic zone, contains a
very significant biomass of phytoplankton and zooplankton
[8.59, 8.61].
Phytoplankton exhibits
210
Po concentration factors of around 10
4
–10
6
and Po:Pb
ratios of around 3, which are lower than those observed in some zooplankton
groups (see Refs [8.101–8.103]).
Investigations made of
210
Po accumulation in zooplankton report a high
concentration in euphausiids (krill) and that
210
Po concentration increases from
phytoplankton to macrozooplankton to mesozoopklankton
[8.39, 8.54, 8.102].
The concentrations of
210
Pb and
210
Po in zooplankton faecal pellets and their
role in the rapid removal of these radionuclides from the epipelagic zone have
triggered research on the vertical transport of elements carried by relatively large
biogenic particles in the water column (see Refs [8.24, 8.26, 8.27, 8.36–8.38]).
However, studies on plankton always face the difficulty that plankton samples
obtained with towed nets are based on the size of organisms, being a mixture
of species rather than plankton organisms sorted by phylogenetic group,
ecological niche or trophic level. Organism size is not an indication of trophic
level. Furthermore, even small planktonic organisms have diverse strategies of
food selection and may occupy different trophic levels regardless of their similar
size
[8.104].
There are data obtained by analysing plankton sorted by species or genus
rather than by analysing bulk plankton samples. Results on
210
Po based on species
separation has increased the understanding of
210
Po accumulation from sea water
to phytoplankton to mesozooplankton to krill (Eupasia superba) in the Antarctic
Ocean
[8.105]. Furthermore, a comparison of
210
Po datasets from Antarctica,
the Mediterranean and the north-eastern Atlantic Ocean reveals that the range
of
210
Po concentrations are similar and unrelated to seawater temperature or
geographical region (see Refs [8.54, 8.101, 8.102, 8.105]).
158
CHAPTER 8
Reporting on the north-eastern Atlantic Ocean, Carvalho [8.28] finds that:
“The groups of small zooplanktonic crustaceans such as ostracods,
amphipods, copepods, euphausiids and mysids, displayed the highest
210
Po
activity concentrations in marine organisms ranging from 84
Bq·kg
−1
in
euphausiids [generally carnivores] to 2900
Bq·kg
−1
in amphipods [mostly
planktivorous herbivores]. Other important zooplanktonic groups, such as
the chaetognaths (Sagitta spp.), pteropods (Creseis spp., Diacria trispinosa)
and pelagic polychaetes contained
210
Po concentrations in that range and
Po:Pb ratios also higher than jellyfish, generally between 5 and 10 and
occasionally higher.
“Planktonic fish larvae (Mycthopidae, Alepochephalidae) and cephalopod
larvae (Loligonodae, Ommastrephidae) displayed
210
Po concentrations
similar to other zooplankton taxa as reported above, but with Po:Pb around
6 and not different from their adult fish stages while this ratio went up to
157 in crustaceans (mysids).”
Carvalho
[8.28] reports that large predator teleosts of the north-eastern
Atlantic Ocean epipelagic zone, such as tuna (Thynnus obesus and other tuna
species), feed upon clupeoid fish, squid and zooplankton, while the blue marlin
(Makaira nigricans) feeds upon clupeoid fish, and the oil-fish (Ruvettus pretiosus)
eats several pelagic fish species (see Fig.
8.6). Polonium-210 concentration in
tuna muscle is 3.0
± 0.1 Bq/kg, with a Po:Pb ratio of 6.6, in blue marlin muscle
0.4
± 0.2 Bq/kg and in oil-fish 0.7 ± 0.02 Bq/kg, thus also much lower than in
tuna.
Carvalho
[8.28] finds that:
“Other oceanic large predators on the top of food chains, such as the sperm
whale (Physeter catodon) feeding upon deep water cephalopods, contained
210
Po and
210
Pb concentrations in muscle tissue roughly comparable to
tuna.”
One interesting question is raised by
210
Po concentrations in the same or
closely related species, occupying the same trophic level in different oceans.
For example,
210
Po concentrations in tuna muscle from the Atlantic Ocean, the
Pacific Ocean and the Mediterranean Sea are within one order of magnitude.
However, taking into account the uncertainty of determinations, they are
significantly different and are in the range of 3–53.3
Bq/kg (FW) (see Table 8.4).
As tuna are at the top of marine food chains and do not absorb
210
Po from water,
different
210
Po concentrations in tuna must be a consequence of different
210
Po

159
MARINE SYSTEMS
Source: See Fig. 2 of Ref. [8.28].
FIG. 8.6. Polonium-210 in pelagic food chains in the ocean (reproduced with permission
courtesy of Elsevier).
TABLE 8.4. ACTIVITY CONCENTRATIONS (AND RANGES) OF
210
Po IN
TISSUES OF TUNA FISH FROM SEVERAL OCEANS
Region Species Tissue
210
Po concentration
(Bq/kg, FW)
Ref.
NE Atlantic Ocean
Azores Islands
Several
Muscle
Liver
Bone
5 (3–8)
288 (278–297)
20 (5–51)
[8.53]
Pacific Ocean,
S. California
Several
Muscle
Liver
Gonad
11–48
144–777
85–278
[8.34]
Japan
Thunnus thynnus
Muscle 24.4 ± 1.6 [8.85]
Marshall Islands Bonito Muscle 36.9 (21.5–53.3) [8.86]
160
CHAPTER 8
concentrations in the first trophic levels of the food chain (i.e. in phytoplankton
and zooplankton in their environment). The
210
Po analyses of plankton from the
open ocean, especially from oligotrophic waters in the Pacific Ocean, actually
show that
210
Po concentrations in zooplankton are very much affected by plankton
density (see Ref. [8.101] for a model to describe the relationship). Low biomass
prompts higher concentration levels in phytoplankton, which is transferred
to zooplankton, and so forth. Polonium-210 concentrations in zooplankton in
oligotrophic regions of the Pacific Ocean are indeed much higher than
210
Po
concentrations in zooplankton of the North Atlantic Ocean. This is propagated in
the food chain and reflected by
210
Po concentrations in muscle tissue of tuna fish
of both oceans
[8.5, 8.87].
8.5.4. Mesopelagic and bathypelagic zones
Zooplankton from the deep waters of the north-eastern Atlantic Ocean
display very different concentrations according to the type of organism.
Carvalho
[8.28] reports that deep-sea medusa (Atolla sp. and Crossota sp.), salpes
(Salpa spp.) and Pyrosoma sp. — all gelatinous organisms — contain the lowest
210
Po concentrations measured in marine organisms at 0.7–35 Bq/kg (with Po:Pb
ratios generally <
5). Their
210
Po concentrations are similar to those determined
in epipelagic jellyfish (i.e. Hydromedusae Sarsia spp. and Obelia spp.).
Research on mesopelagic decapod shrimp shows the existence of
specialized diets, with the consumption of
210
Po rich organic particles in the
oceanic water column, leading in some species to very high concentrations of
210
Po. High
210
Po concentrations are reported, especially in the hepatopancreas
of some shrimp species
[8.106] and in the liver and gut of some mesopelagic fish
feeding on particles and zooplankton
[8.54, 8.94]. This accumulation leads to
unusually high internal radiation doses from the alpha emission of
210
Po, reaching
values of 3–5
Sv/a, reported in the 1980s, in the hepatopancreas of shrimp and
liver and other internal organs of small mesopelagic fish (Argyropelecus and
Myctophum)
[8.54, 8.57].
Carvalho
[8.28] analyses more than 50 fish species living in the mid-water
column and sampled near Madeira Island, at the Great Meteor East Seamountin of
the Madeira Abyssal Basin, and in the Porcupine Abyssal Basin. Generally, most
mesopelagic and bathypelagic fish species are small (0.5–60
g adult weight),
but there are large differences in their
210
Po concentrations, suggesting feeding
regimes based on different prey and different trophic levels (see Fig.
8.6).
Large predators in mesopelagic and bathypelagic domains include
teleosts, such as Aphanopus carbo and Mora mora, and black sharks, such as
Scynodesmus sp., all with low
210
Po concentrations, around 1 Bq/kg in muscle
161
MARINE SYSTEMS
tissue, and comparable to concentrations in other fish in other ocean layers
occupying similar trophic levels
[8.28].
8.5.5. Abyssal zone
The amount of data available on abyssal species is much less than for
other oceanic regions. Nevertheless, the available data show that
210
Pb and
210
Po
concentrations determined in ascidians, polychaetes, Ophiuridae, cephalopods,
crustaceans and fish are similar to concentrations reported for organisms at other
oceanic depths (see Table
8.5).
Carvalho
[8.28] finds that:
“Also
210
Po concentrations measured in the giant abyssal mysid
Gnatophausia ingens and Eryonidae crabs, 18
± 2 and 22 ± 2 Bq kg
−1
respectively, were close to the
210
Po concentration measured in the coastal
shrimp Leander serratus, 25
± 0.8 Bq kg
−1
”.
In Carvalho
[8.28], a small bivalve mollusc from the abyssal plain
(Sillicula fragilis) displays
210
Po concentrations in soft tissue comparable
to the north-eastern Atlantic clam (Tapes decussatus) and the North Sea clam
(Mya arenaria
) (see also Ref. [8.84]). Unusually high
210
Po concentrations,
1.6
× 10
3
Bq/kg (DW) were reported in the 1980s for infaunal xenophytophores,
large protozoans that live in sediment and are closely related to foraminifera, from
a hadal trench in the Pacific Ocean, which might be exposed to radiation doses
comparable to the dose in the hepatopancreas of mid-water shrimp
[8.57, 8.107].
Carvalho
[8.28] concludes that:
“One singularity of abyssal fauna shall be highlighted. In 3 out of the
15 abyssal species analyzed the Po:Pb ratios were around the unity. In
the abyssal fish Nematonurus (Coryphaenoides) armatus, a rattail fish
characteristic of the abyssal zone, the
210
Po:
210
Pb ratio in muscle was 0.5,
which was uncommon amongst all fish analyzed
[8.84]. These low Po:Pb
ratio values are probably linked to the long fasting periods of abyssal
fauna
[Ref. [8.108]].”

162
CHAPTER 8
TABLE 8.5. ACTIVITY CONCENTRATIONS (AND RANGES) OF
210
Po AND
210
Pb (FW) IN BENTHIC FAUNA OF THE PORCUPINE ABYSSAL PLAIN,
THE NORTH-EASTERN ATLANTIC OCEAN AND HYDROTHERMAL
VENTS IN THE MID-ATLANTIC RIDGE
Species and tissue N DW:FW
210
Po
(Bq/kg ± SD)
210
Pb
(Bq/kg ± SD)
Po:Pb
Ascidian
Chitonanthus abyssorum
4 0.15 38 ± 2 38 ± 2 1
Polychaetes 3
Gut 0.22 27 ± 2 11 ± 0.5 2.5
Remainder 0.18 52 ± 2 13 ± 0.5 4
Asteridae
Astropecten sp., wt = 0.6 g
3 0.38 50 ± 3 7.6 ± 0.4 6.4
Astropecten sp., wt = 13 g
1 0.23 171 ± 13 25 ± 2 6.7
Bivalve mollusc (soft tissue)
Silicula fragilis
1 0.16 124 ± 8 123 ± 4 1
Bathymodiolus azoricus
a
—
b
0.19
123.9 ± 21.4
—
b
—
b
Isopods 2
Whole body 0.23 27 ± 1
1.91 ± 0.09
14
Gut 0.41 94 ± 3 11.4 ± 0.3 8.2
Exoskeleton 0.68 194 ± 5 8.4
± 0.2 23
Amphipods
Eurythenes grillus
3 0.20 32 ± 10 28 ± 11 1.1
Eurythenes grillus
a
26 0.28 104.1 (62.4–285.9) 26.5 (11.8–67.4) 3.9
Cephalopods
Vampiroteuthis infernalis
1 0.07 39 ± 3 6.9 ± 0.3 5.6
Fish
Nematonurus (C.) armatus
Muscle 9 0.17 0.31 ± 0.28 0.60 ± 0.55 0.5
Liver 9 —
b
3.58 ± 2.29 2.50 ± 1.64 1.4
Skin 4 —
b
1.94 ± 0.73 0.68 ± 0.33 2.8
Bone 4 —
b
3.25 ± 1.96 0.85 ± 0.24 3.8
Source: See Ref. [8.83] unless otherwise indicated.
Note: SD — standard deviation.
a
Source is Ref. [8.73].
b
—: data not available.
163
MARINE SYSTEMS
8.6. POLONIUM AND LEAD CONCENTRATION RATIOS IN
MARINE ORGANISMS
Concentration ratios (CR) are defined as the ratio of activity concentration
in the organism (Bq/kg, FW) to the activity concentration in filtered sea water
(Bq/L). Determinations of CRs are frequently performed when comparing
bioaccumulation of radionuclides in different locations and seas and are used
in modelling. As this ratio uses the concentration of radionuclide dissolved
in sea water, it allows for an easy identification of organisms that concentrate
more of the radionuclide, which might be of interest for further study and use in
environmental radiological monitoring programmes as biomonitors. Organisms
that concentrate a particular radionuclide might also be the main transfer route of
that radionuclide to humans.
However, computation of CRs does not imply that sea water is the source
of radionuclide to the organism. Indeed, it is food and not sea water that is the
source of internally accumulated
210
Po. Nevertheless, the information on CRs
for biota groups is useful to model the transfer of radionuclides in food chains
and to predict potential radiation exposures in specified ecosystems and food
webs. This is the main purpose of the data compilation and recommended
CRs in IAEA publications such as the Handbook of Parameter Values for the
Prediction of Radionuclide Transfer to Wildlife
[8.109]. Furthermore, CRs of
210
Po concentrations in sea water are not different from those of similar species in
coastal areas (see Fig.
8.7).
8.7. TRANSFER OF
210
Pb AND
210
Po IN MARINE FOOD CHAINS
In the past, the interpretation that
210
Po concentration in tissues of marine
biota could be a reflection of
210
Po content in their diet was gaining credence, but
the relative importance of water and food as
210
Po sources remained unknown.
The use of radioactive tracers clarified that diet is the almost exclusive source of
210
Po accumulated in the internal organs of marine organisms [8.46, 8.47]. Since
then, several studies have adopted this point of view and interpreted
210
Po data in
marine biota in the light of food chains and trophic levels (see Refs [8.28, 8.52,
8.110, 8.111]).
A consistent view of
210
Po transfer in marine food chains in the pelagic
environment is depicted in Fig.
8.7. Whole body
210
Po CRs are depicted, relating
210
Po concentrations in biota to
210
Po concentrations in sea water. Furthermore,
210
Po transfer factors (TFs) relating
210
Po concentrations in predator tissues to
210
Po concentrations in the diet of each species are indicated.

164
CHAPTER 8
Although these are simplified trophic chains and crude representations
of real food webs in the oceans, they are based on the known diet of species
and predator–prey relationships
[8.28]. The advantage is that it clearly shows
that in the same oceanic ecosystem there are organisms often in the same
zoological group, such as fish, that display very different concentration factors
and thus highlight that water cannot be the main source of
210
Po to organisms.
Furthermore, organisms with similar
210
Po CRs can be found in different regions
and ecosystems without any relationship between them.
Carvalho [8.28] reports that
210
Po TFs from prey to predator are often
around 0.1, with interesting deviations such as 0.7 in the trophic link from
phytoplankton to copepods, 0.3 in the trophic link from small pelagic fish
and euphausiids to tuna, and 0.7 in the transfer of
210
Po from zooplankton to
the lantern fish (Myctophidae). Carvalho
[8.28] proposes that these
210
Po TFs
are similar to energy transfer in marine food chains, known as eco-trophic
coefficients, and that this strongly suggests an association between
210
Po transfer
and energy transfer in marine food chains. Furthermore [8.28]:
Source: Figure 1 of Ref. [8.84].
Note: Concentration ratios are equivalent to concentration factors (CFs).
FIG. 8.7. Transfer factors (TFs) and concentration ratios for
210
Po for the demersal food chain
at the abyssal north-eastern Atlantic Ocean (reproduced with permission courtesy of Oxford
Journals).
165
MARINE SYSTEMS
“Based on
210
Po concentration data in marine biota combined with
210
Po
behaviour in biological systems, such as
210
Po binding to amino acids
and proteins
[Ref. [8.48]], and experimental research on
210
Po and
210
Pb
assimilation in marine species
[Refs [8.46, 8.47, 8.49]], we interpret
210
Po
transfer in marine food chains as a tracer of protein transfer from prey to
predator tissues. In every ecosystem of the ocean, the accumulation of
210
Po in the marine biota thus reflects the trophic position of the species
in the food web rather than the geographic location, water depth, or other
environmental parameter.
“The behaviour of
210
Pb in the food chains seems slightly different.
210
Pb
concentrations do not increase from phytoplankton to copepods as much as
210
Po, and the
210
Pb transfer to planktivorous fish, such as sardines, is less
efficient than
210
Po transfer.”
At higher trophic levels, such as in marine mammals,
210
Po levels are high,
while
210
Pb levels are as low as in lower trophic levels. The Po:Pb ratio in marine
food webs seem to increase with trophic level, from around 10 in phytoplankton
and zooplankton, 3–10 in herbivorous fish and 50–100 in piscivores (carnivorous
fish), rising to 200 in the muscle tissue of marine mammals
[8.96].
In summary, the concentrations of dissolved
210
Pb and
210
Po in the oceans
vary within a relatively narrow range. Differences between regions and ocean
layers do exist, and there are geochemical and geographical reasons for this, such
as
210
Pb and
210
Po concentrations in surface water of the oceans and land mass
distribution. However, these differences are relatively small and are not sufficient
to explain the wide range of
210
Po concentrations in biota, especially when
species coexist in the same water body or region. Carvalho [8.28] finds that:
“Po-210 and
210
Pb concentrations were determined in marine species from
all the ecological zones in the ocean, from the shoreline to abyssal depths. In
the tissues of all species the
210
Po concentration values spread across 5 orders
of magnitude, from 0.5
Bq kg
−1
in jellyfish to (2.8 ±0.2) × 10
4
Bq kg
−1
in the gut wall of the common sardine [Sardina pilchardus] and even to
(3.33
± 0.17) × 10
4
Bq kg
−1
in the gut walls of the pelagic shrimp Plesionika
edwardsi.”
Furthermore,
210
Po concentrations seem to be similar in the tissues of a
coastal bivalve mollusc and in a deep-sea mollusc, and in the muscle tissue of
tuna fish in the Pacific Ocean and the Atlantic Ocean, provided that those species
occupy a similar position in the food chain. Conversely,
210
Po concentrations in
marine biota might be very different in species living in the same water mass, and
166
CHAPTER 8
therefore exposed to the same
210
Po concentrations in water, as reviewed in this
appendix for many species in marine ecosystems.
The concentration of dissolved
210
Po in suspended particulate matter,
including living microorganisms, such as bacteria and phytoplanton cells,
probably starts with surface adsorption on ultra fine particles and accumulation
of polonium in bacteria and phytoplankton as an element similar to sulphur in
the building blocks of proteins — the amino acids. According to Carvalho [8.28],
the enhancement of
210
Po concentrations is very pronounced in biota feeding
on bacteria and phytoplankton at the base of marine food chains, such as
observed in small zooplankton organisms (e.g. copepods and mysids), but also
in large planktivorous organisms, such as mussels, sardines and blue whales,
and it is transferred to carnivores, such as marlins, dolphins and sperm whales.
Carvalho [8.28] concludes that:
“Actually, the
210
Po activity concentration levels recorded in marine
organisms and
210
Po:
210
Pb ratios may depend upon the number of trophic
levels in the food chain. The less trophic links exist in the food chain, the
higher will be the
210
Po concentration in the top predator tissues.”
Furthermore, in oligotrophic regions of the oceans, more
210
Po is
concentrated per unit of mass of phytoplankton and zooplankton, and this
210
Po
is transferred to upper trophic levels in the food chain, while in coastal seas with
high plankton biomass, the reverse is observed (i.e. plankton display lower
210
Po
concentrations) [8.101]. In marine organisms [8.28]:
“
210
Po:
210
Pb ratios mostly ranged from near 1 up to 100, and occasionally
higher, showing the enhancement of
210
Po concentrations comparatively to
210
Pb in internal organs, especially in those related to digestive functions
and the gonad.
.......
“Regarding the
210
Po transfer in marine food chains,
210
Po concentrations
in trophic levels and in characteristic species highlighted the similarities of
210
Po transfer factors with energy transfer rates in marine food chains.”
This has been experimentally verified in a few species using a double tracer
technique
[8.46, 8.47]. Carvalho [8.28] suggests that:
167
MARINE SYSTEMS
“
210
Po follows protein transfer in food chains and may thus give a surrogate
measure of the fraction of prey tissues that is incorporated in predator’s
tissues in marine food webs.”
With regard to
210
Po transfer from the marine environment to human
populations, the consumption of seafood from coastal seas is the main pathway
and deserves much more attention in radiation exposure assessment. Humans,
as consumers of seafood, are also elements of sea-borne food chains and,
according to their dietary habits, will have different intakes of
210
Po and thus
different internal radiation doses. This was pointed out in early studies on
seafood consumers and documented in dietary studies for several countries [8.70,
8.86, 8.88]. This exposure of humans to
210
Po is not constant and, at present
and due to overfishing, humans are increasingly fishing down the marine food
chains, which implies that current trends of wild seafood consumption are likely
to increase
210
Po ingestion and the collective radiation dose in seafood prone
populations
[8.28].
REFERENCES TO CHAPTER 8
[8.1] TUREKIAN, K.K., NOZAKI, Y., BENNINGER, L.K., Geochemistry of atmospheric
radon and radon products, Annu. Rev. Earth Planet. Sci.
5 (1977) 227–255.
[8.2] CARVALHO, F.P., Origins and concentrations of
222
Rn,
210
Pb,
210
Bi and
210
Po in the
surface air at Lisbon, Portugal, at the Atlantic edge of the European continental
landmass, Atmos. Environ.
29 (1995) 1809–1819.
[8.3] HEINRICH, P., JAMELOT, A., Atmospheric transport simulation of
210
Pb and
7
Be by
the LMDz general circulation model and sensitivity to convection and scavenging
parameterization, Atmos. Res.
101 (2011) 54–66.
[8.4] CARVALHO, F.P., Distribution, cycling and mean residence time of
226
Ra,
210
Pb and
210
Po in the Tagus estuary, Sci. Total Environ. 196 (1997) 151–161.
[8.5] “Summary”, Naturally Occurring Radioactive Material (NORM V) (Proc. Int. Symp.
Seville, 2007), IAEA, Vienna (2008)
1–26.
[8.6] BOLIVAR, J.P., GARCIA-TENORIO, R., VACA, F., Radioecological study of an
estuarine system located in the south of Spain, Water Res.
34 (2000) 2941–2950.
[8.7] GERMAIN, P., LECLERC, G., SIMON, S., Distribution of
210
Po in Mytilus edulis
and Fucus vesiculosus along the Channel coast of France: Influence of industrial
releases in the Seine River and Estuary, Radiat. Prot. Dosim.
45 (1992) 257–260.
[8.8] CAMPLIN, W.C., BAXTER, A.J., ROUND, G.D., The radiological impact of
discharges of natural radionuclides from a phosphate plant in the United Kingdom,
Environ. Int.
22 (1996) 259–270.
[8.9] ROLLO, S.F.N., CAMPLIN, W.C., ALLINGTON, D.J., YOUNG, A.K., Natural
radionuclides in the UK marine environment, Radiat. Prot. Dosim.
45 (1992) 203–209.
168
CHAPTER 8
[8.10] EL SAMAD, O., AOUN, M., NSOULI, B., KHALAF, G., HAMZE, M., Investigation
of the radiological impact on the coastal environment surrounding a fertilizer plant,
J. Environ. Radioact. 133 (2014) 69–74.
[8.11] OECD NUCLEAR ENERGY AGENCY, Co-ordinated Research and Environmental
Surveillance Programme Related to Sea Disposal of Radioactive Waste, CRESP Final
Report
1981–1955, OECD, Paris (1996).
[8.12] INTERNATIONAL ATOMIC ENERGY AGENCY, Inventory of Radioactive Waste
Disposals at Sea, IAEA-TECDOC-1105, IAEA, Vienna
(1999).
[8.13] GERMAIN, P., LECLERC, G., SIMON, S., Transfer of polonium-210 into Mytilus
edulis (L.) and Fucus vesiculosus (L.) from the baie de Seine (Channel coast of
France), Sci. Total Environ.
164 (1995) 109–123.
[8.14] RYAN, T.P., DOWDALL, A.M., McGARRY, A.T., POLLARD, D., CUNNINGHAM,
J.D.,
210
Po in Mytilus edulis in the Irish marine environment, J. Environ. Radioact. 43
(1999)
325–342.
[8.15] CARVALHO, F.P., OLIVEIRA, J.M., SOARES, A.M.M, Sediment accumulation and
bioturbation rates in the deep Northeast Atlantic determined by radiometric
techniques, ICES J. Mar. Sci.
68 (2011) 427–435.
[8.16] MASQUÉ, P., SANCHEZ-CABEZA, J.A., BRUACH, J.M., PALACIOS, E.,
CANALS, M., Balance and residence times of
210
Pb and
210
Po in surface waters of
the northwestern Mediterranean Sea, Cont. Shelf Res.
22 (2002) 2127–2146.
[8.17] TATEDA, Y., CARVALHO, F.P., FOWLER, S.W., MIQUEL, J.-C., Fractionation of
210
Po and
210
Pb in coastal waters of the NW Mediterranean continental margin, Cont.
Shelf Res.
23 (2003) 295–316.
[8.18] BACON, M.P., BELASTOCK, R.A., TECOTZKY, M., TUREKIAN, K.K.,
SPENCER, D.W., Lead-210 and polonium-210 in ocean water profiles of the
continental shelf and slope south of New England, Cont. Shelf Res.
8 (1988) 841–853.
[8.19] SPENCER, D.W., BACON, M.P., BREWER, P.G., The Distribution of
210
Pb and
210
Po in the North Sea, Center for Marine Research (1980).
[8.20] CHUNG, Y.,
210
Pb in the western Indian Ocean: Distribution, disequilibrium, and
partitioning between dissolved and particulate phases, Earth Planet. Sci. Lett.
85
(1987)
28–40.
[8.21] CHUNG, Y., FINKEL, R.,
210
Po in the western Indian Ocean: Distributions,
disequilibria and partitioning between the dissolved and particulate phases, Earth
Planet. Sci. Lett.
88 (1988) 232–240.
[8.22] CHERRY, R.D., HEYRAUD, M., “Lead-210 and polonium-210 in the world’s
oceans”, Inventories of Selected Radionuclides in the Oceans, IAEA-TECDOC-481,
IAEA, Vienna (1988)
139–158.
[8.23] CARVALHO, F.P., “Uranium series radionuclides in the water column of the
northeast Atlantic Ocean”, Isotopes in Hydrology, Marine Ecosystems and Climate
Change Studies (Proc. Int. Symp. Monaco, 2011), Vol.
2, IAEA, Vienna
(2013)
427–441.
[8.24] FOWLER, S.W., KNAUER, G.A., Role of large particles in the transport of elements
and organic compounds through the oceanic water column, Prog. Oceanogr.
16
(1986)
147–194.
169
MARINE SYSTEMS
[8.25] STEWART, G.M., MORAN, S.B., LOMAS, M.W., Seasonal POC fluxes at BATS
estimated from
210
Po deficits, Deep Sea Res. Part I 57 (2010) 113–124.
[8.26] VERDENY, E., et al., POC export from ocean surface waters by means of
234
Th/
238
U
and
210
Po/
210
Pb disequilibria: A review of the use of two radiotracer pairs, Deep Sea
Res. Part
II 56 (2009) 1502–1518.
[8.27] LE MOIGNE, F.A.C., et al., Export of organic carbon and biominerals derived from
234
Th and
210
Po at the Porcupine Abyssal Plain, Deep Sea Res. Part I 72
(2013)
88–101.
[8.28] CARVALHO, F.P., Polonium (
210
Po) and lead (
210
Pb) in marine organisms and their
transfer in marine food chains, J. Environ. Radioact. 102 (2011) 462–472.
[8.29] CHERRY, R.D., SHANNON, L.V., The alpha radioactivity of marine organisms, At.
Energy Rev. 12 (1974) 3–45.
[8.30] PARFENOV, Yu.D., Polonium-210 in the environment and in the human organism,
At. Energy Rev. 12
(1974) 75–143.
[8.31] SHANNON, L.V., CHERRY, R.D., Polonium-210 in marine plankton, Nature 216
(1967) 352–353.
[8.32] HOLTZMAN, R.B., “Concentrations of the naturally occurring radionuclides
226
Ra,
210
Pb and
210
Po in aquatic fauna”, paper presented at 2nd Nat. Symp. Radioecology,
Ann Arbor, MI (1967).
[8.33] SCHELL, W.R., JOKELA, T., EAGLE, R., “Natural
210
Pb and
210
Po in a marine
environment”, Radioactive Contamination of the Marine Environment (Proc. Symp.
Seattle, 1972), IAEA, Vienna (1973) 701–724.
[8.34] FOLSOM, T.R., BEASLEY, T.M., “Contributions from the alpha emitter,
polonium-210, to the natural radiation environment of the marine organisms”, ibid.,
pp
625–632.
[8.35] HOFFMAN, F.L., HODGE, V.F., FOLSOM, T.R.,
210
Po radioactivity in organs of
selected tunas and other marine fish, J. Radiat. Res.
15 (1974) 103–106.
[8.36] BEASLEY, T.M., HEYRAUD, M., HIGGO, J.J.W., CHERRY, R.D., FOWLER,
S.W.,
210
Po and
210
Pb in zooplankton fecal pellets, Mar. Biol. 44 (1978) 325–328.
[8.37] CHERRY, R.D., FOWLER, S.W., BEASLEY, T.M., HEYRAUD, M., Polonium-210:
Its vertical oceanic transport by zooplankton metabolic activity, Mar. Chem.
3
(1975)
105–110.
[8.38] JEFFREE, R.A., SZYMCZAK, R., Enhancing effect of marine oligotrophy on
environmental concentrations of particle-reactive trace elements, Environ. Sci.
Technol.
34 (2000) 1966–1969.
[8.39] HEYRAUD, M., FOWLER, S.W., BEASLEY, T.M., CHERRY, R.D., Polonium-210
in euphausiids: A detailed study, Mar. Biol.
34 (1976) 127–136.
[8.40] HEYRAUD, M., CHERRY, R.D., Polonium-210 and lead-210 in marine food chains,
Mar. Biol.
52 (1979) 227–236.
[8.41] CHERRY, R.D., HEYRAUD, M., HIGGO, J.J.W., Polonium-210: Its relative
enrichment in the hepatopancreas of marine invertebrates, Mar. Ecol. Prog. Ser.
13
(1983)
229–236.
170
CHAPTER 8
[8.42] HIGGO, J.J.W., CHERRY, R.D., HEYRAUD, M., FOWLER, S.W., BEASLEY,
T.M., “Vertical oceanic transport of alpha-radioactive nuclides by zooplankton fecal
pellets”, Natural Radiation Environment
III (Proc. Symp. Houston, 1978), Vol. 1
(GESELL, T.F., LOWDER, W.M., Eds), United States Department of Energy, Oak
Ridge, TN (1980) 502–513.
[8.43] HEYRAUD, M., DOMANSKI, P., CHERRY, R.D., FASHAM, M.J.R., Natural
tracers in dietary studies: Data for
210
Po and
210
Pb in decapod shrimp and other
pelagic organisms in the Northeast Atlantic Ocean, Mar. Biol.
97 (1988) 507–519.
[8.44] PENTREATH, R.J., Radionuclides in marine fish, Oceanogr. Mar. Biol. Annu.
Rev.
15 (1977) 365–460.
[8.45] RYAN, T.P., Transuranic biokinetic parameters for marine invertebrates: A review,
Environ. Int.
28 (2002) 83–96.
[8.46] CARVALHO, F.P., FOWLER, S.W., A double-tracer technique to determine the
relative importance of water and food as sources of polonium-210 to marine prawns
and fish, Mar. Ecol. Prog. Ser.
103 (1994) 251–264.
[8.47] CARVALHO, F.P., FOWLER, S.W., An experimental study on the bioaccumulation
and turnover of polonium-210 and lead-210 in marine shrimp, Mar. Ecol. Prog.
Ser.
102 (1993) 125–133.
[8.48] DURAND, J.P., et al.,
210
Po binding to metallothioneins and ferritin in the liver of
teleost marine fish, Mar. Ecol. Prog. Ser.
177 (1999) 189–196.
[8.49] WILDGUST, M.A., McDONALD, P., WHITE, K.N., Assimilation of
210
Po by the
mussel Mytilus edulis from the alga Isochrysis galbana, Mar. Biol.
136 (2000) 49–53.
[8.50] REINFELDER, J.R., FISHER, N.S., The assimilation of elements ingested by marine
copepods, Science
251 (1991) 794–796.
[8.51] FISHER, N.S., REINFELDER, J., “The trophic transfer of metals in marine systems”,
Metal Speciation and Bioavailabliity in Aquatic Systems (TESSIER, A., TURNER,
D.R., Eds), John Wiley and Sons, Chichester (1995)
363–406.
[8.52] STEWART, G.M., et al., Contrasting transfer of polonium-210 and lead-210 across
three trophic levels in marine plankton, Mar. Ecol. Prog. Ser.
290 (2005) 27–33.
[8.53] CARVALHO, F.P., Contribution a l’étude du cycle du polonium-210 et du plomb-210
dans l’environnement, PhD thesis, Univ. Nice Sophia Antipolis, Nice (1990).
[8.54] CARVALHO, F.P.,
210
Po in marine organisms: A wide range of natural radiation dose
domains, Radiat. Prot. Dosim.
24 (1988) 113–117.
[8.55] SMITH, D.J., TOWLER, H., Polonium-210 in cartilaginous fishes (Chondrichthyes)
from south-eastern Australian waters, Aust. J. Mar. Freshwater Res.
44
(1993)
727–733.
[8.56] CHERRY, R.D., HEYRAUD, M., RINDFUSS, R., Polonium-210 in teleost fish and
in marine mammals: Interfamily differences and a possible association between
polonium-210 and red muscle content, J. Environ. Radioact.
24 (1994) 273–291.
[8.57] CHERRY, R.D., HEYRAUD, M., Evidence of high natural radiation doses in certain
mid-water oceanic organisms, Science
218 (1982) 54–56.
[8.58] GODOY, J.M., et al.,
210
Po concentration in Perna perna mussels: Looking for
radiation effects, J. Environ. Radioact.
99 (2008) 631–640.
171
MARINE SYSTEMS
[8.59] PÉRÈS, J.M., Précis d’océanographie biologique, Presses Universitaires de France,
Paris (1976).
[8.60] NEWELL, R.C., Biology of Intertidal Animals, Marine Ecological Surveys,
Faversham
(1979).
[8.61] KAISER, M.J., et al., Marine Ecology: Processes, Systems, and Impacts, Oxford
University Press
(2005).
[8.62] WONG, K.M., HODGE, V.F., FOLSOM, T.R., Plutonium and polonium inside giant
brown algae, Nature
237 (1972) 460–462.
[8.63] FOLSOM, T.R., HODGE, V.F., Experiments suggesting some first steps in the
dispersal and disposal of plutonium and other alpha emitters in the ocean, Mar. Sci.
Commun.
1 (1975) 213–247.
[8.64] CARVALHO, F.P., FOWLER, S.W., Americium adsorption on the surface of
macrophytic algae, J. Environ. Radioact.
2 (1985) 311–317.
[8.65] FISHER, N.S., BURNS, K.A., CHERRY, R.D., HEYRAUD, M., Accumulation and
cellular distribution of
241
Am,
210
Po and
210
Pb in two marine algae, Mar. Ecol. Prog.
Ser.
11 (1983) 233–237.
[8.66] XUE, H.-B., STUMM, W., SIGG, L., The binding of heavy metals to algal surfaces,
Water Res.
22 (1998) 917–926.
[8.67] DAVIS, T.A., VOLESKY, B., MUCCI, A., A review of the biochemistry of heavy
metal biosorption by brown algae, Water Res.
37 (2003) 4311–4330.
[8.68] WILSON, R.C., WATTS, S.J., VIVES i BATLLE, J., McDONALD, P., Laboratory
and field studies of polonium and plutonium in marine plankton, J. Environ.
Radioact.
100 (2009) 665–669.
[8.69] SURIYANARAYANAN, S., BRAHMANANDHAN, G.M., SAMIVEL, K.,
RAVIKUMAR, S., SHAHUL HAMEED, P., Assessment of
210
Po and
210
Pb in marine
biota of the Mallipattinam ecosystem of Tamil Nadu, India, J. Environ. Radioact.
101
(2010)
1007–1010.
[8.70] CARVALHO, F.P.,
210
Po and
210
Pb intake by the Portuguese population: The
contribution of seafood in the dietary intake of
210
Po and
210
Pb, Health Phys. 69
(1995)
469–480.
[8.71] FEROZ KHAN, M., UMARAJESWARI, S., GODWIN WESLEY, S., Biomonitoring
210
Po and
210
Pb in marine brachyuran crabs collected along the coast of Kudankulam,
Gulf of Mannar (GOM), India, J. Environ. Monit.
13 (2011) 553–562.
[8.72] HEYRAUD, M., et al., Polonium-210 and lead-210 in edible molluscs from near the
Cape of Good Hope: Sources of variability in polonium-210 concentrations,
J. Environ. Radioact. 24 (1994) 253–272.
[8.73] CHARMASSON, S., FAOUDER, A., LOYEN, J., COSSON, R.P., SARRADIN,
P.M.,
210
Po and
210
Pb in the tissues of the deep-sea hydrothermal vent mussel
Bathymodiolus azoricus from the Menez Gwen field (Mid-Atlantic Ridge), Sci. Total
Environ.
409 (2011) 771–777.
[8.74] BUSTAMANTE, P., GERMAIN, P., LECLERC, G., MIRAMAND, P., Concentration
and distribution of
210
Po in the tissues of the scallop Chlamys varia and the mussel
Mytilus edulis from the coasts of Charente-Maritime (France), Mar. Pollut. Bull.
44
(2002)
997–1002.
172
CHAPTER 8
[8.75] DESIDERI, D., MELI, M.A., ROSELLI, C., A biomonitoring study:
210
Po and heavy
metals in marine organisms from the Adriatic Sea (Italy), J. Radioanal. Nucl.
Chem.
285 (2010) 373–382.
[8.76] UĞUR, A., YENER, G., BAŞSARI, A., Trace metals and
210
Po (
210
Pb) concentrations
in mussels (Mytilus galloprovincialis) consumed at western Anatolia, Appl. Radiat.
Isot.
57 (2002) 565–571.
[8.77] ROŽMARIĆ, M., et al.,
210
Po and
210
Pb activity concentrations in Mytilus
galloprovincialis from Croatian Adriatic coast with the related dose assessment to the
coastal population, Chemosphere
87 (2012) 1295–1300.
[8.78] GOUVEA, R.C., SANTOS, P.L., DUTRA, I.R., Lead-210 and polonium-210
concentrations in some species of marine molluscs, Sci. Total Environ.
112
(1992)
263–267.
[8.79] McDONALD, P., BAXTER, M.S., SCOTT, E.M., Technological enhancement of
natural radionuclides in the marine environment, J. Environ. Radioact.
32
(1996)
67–90.
[8.80] CARVALHO, F.P., OLIVEIRA, J.M., ALBERTO, G., Factors affecting
210
Po and
210
Pb activity concentrations in mussels and implications for environmental
bio-monitoring programmes, J. Environ. Radioact.
102 (2011) 128–137.
[8.81] INTERNATIONAL ATOMIC ENERGY AGENCY, Sources of Radioactivity in the
Marine Environment and their Relative Contributions to Overall Dose Assessment
from Marine Radioactivity, IAEA-TECDOC-838, IAEA, Vienna (1995).
[8.82] THEBAULT, H., RODRIGUEZ Y BAENA, A.M., Mediterranean Mussel Watch: A
regional program for detecting radionuclides, trace- and emerging-contaminants,
Rapp. Comm. Int. Mer Médit.
38 (2007) 41.
[8.83] DAHLGAARD, H., Polonium-210 in mussels and fish from the Baltic–North Sea
estuary, J. Environ. Radioact.
32 (1996) 91–96.
[8.84] CARVALHO, F.P., OLIVEIRA, J.M., MALTA, M., Radionuclides in deep-sea fish
and other organisms from the North Atlantic Ocean, ICES J. Mar. Sci.
68
(2011)
333–340.
[8.85] CARVALHO, F.P., OLIVEIRA, J.M., ALBERTO, G., VIVES i BATLLE, J.,
Allometric relationships of
210
Po and
210
Pb in mussels and their application to
environmental monitoring, Mar. Pollut. Bull.
60 (2010) 1734–1742.
[8.86] YAMAMOTO, M., et al., Polonium-210 and lead-210 in marine organisms: Intake
levels for Japanese, J. Radioanal. Nucl. Chem.
178 (1994) 81–90.
[8.87] NOSHKIN, V.E., ROBISON, W.L., WONG, K.M., Concentration of
210
Po and
210
Pb
in the diet at the Marshall Islands, Sci. Total Environ.
155 (1994) 87–104.
[8.88] POLLARD, D., RYAN, T.P., DOWDALL, A., The dose to Irish seafood consumers
from
210
Po, Radiat. Prot. Dosim. 75 (1998) 139–142.
[8.89] KANNAN, V., IYENGAR, M.A.R., RAMESH, R., Dose estimates to the public from
210
Po ingestion via dietary sources at Kalpakkam (India), Appl. Radiat. Isot. 54
(2001)
663–674.
[8.90] MISHRA, S., BHALKE, S., PANDIT, G.G., PURANIK, V.D., Estimation of
210
Po
and its risk to human beings due to consumption of marine species at Mumbai, India,
Chemosphere
76 (2009) 402–406.
173
MARINE SYSTEMS
[8.91] SKWARZEC, B., Accumulation of
210
Po in selected species of Baltic fish, J. Environ.
Radioact. 8 (1988) 111–118.
[8.92] CHERRY, R.D., HEYRAUD, M., JAMES, A.G., Diet prediction in common clupeoid
fish using polonium-210 data, J. Environ. Radioact. 10 (1989) 47–65.
[8.93] LAZORENKO, G.E., POLIKARPOV, G.G., BOLTACHEV, A.R., Natural
radioelement polonium in primary ecological groups of Black Sea fishes, Russ. J.
Mar. Biol. 28 (2002) 52–56.
[8.94] HOFFMAN, F.L., HODGE, V.F., FOLSOM, T.R., Polonium radioactivity in certain
mid-water fish of the Eastern Temporal Pacific, Health Phys. 26 (1974) 65–70.
[8.95] MALTA, M., CARVALHO, F.P., Radionuclides in marine mammals off the
Portuguese coast, J. Environ. Radioact.
102 (2011) 473–478.
[8.96] GODOY, J.M., SICILIANO, S., DE CARVALHO, Z.L., DE MOURA, J.F., GODOY,
M.L.D.P.,
210
Polonium content of small cetaceans from Southeastern Brazil, J.
Environ. Radioact. 106 (2012) 35–39.
[8.97] HASSONA, R.K., SAM, A.K., OSMAN, O.I., SIRELKHATIM, D.A., LAROSA, J.,
Assessment of committed effective dose due to consumption of Red Sea coral reef
fishes collected from the local market (Sudan), Sci. Total Environ. 393
(2008) 214–218.
[8.98] HORN, M.H., MESSER, K.S., Fish guts as chemical reactors: A model of the
alimentary canals of marine herbivorous fishes, Mar. Biol. 113 (1992) 527–535.
[8.99] SKWARZEC, B., FABISIAK, J., Bioaccumulation of polonium
210
Po in marine
birds, J. Environ. Radioact. 93 (2007) 119–126.
[8.100] GWYNN, J.P., GABRIELSEN, G.W., JÆGER, I., LIND, B., “Bioaccumulation of
210
Po and
210
Pb in Arctic seabirds”, Radioecology and Environmental Radioactivity,
(Poster Proc. Int. Conf. Bergen, 2008), Part I (STRAND, P., BROWN, J., JOLLE, T.,
Eds), Bergen (2008) 101–104.
[8.101] JEFFREE, R.A., CARVALHO, F., FOWLER, S.W., FARBER-LORDA, J.,
Mechanism for enhanced uptake of radionuclides by zooplankton in French
Polynesian oligotrophic waters, Environ. Sci. Technol. 31 (1997) 2584–2588.
[8.102] SKWARZEC, B., BOJANOWSKI, R.,
210
Po content in sea water and its accumulation
in southern Baltic plankton, Mar. Biol. 97 (1988) 301–307.
[8.103] STEWART, G.M., FISHER, N.S., Experimental studies on the accumulation of
polonium-210 by marine phytoplankton, Limnol. Oceanogr. 48 (2003) 1193–1201.
[8.104] COWLES, T.J., OLSON, R.J., CHISHOLM, S.W., Food selection by copepods:
Discrimination on the basis of food quality, Mar. Biol. 100 (1988) 41–49.
[8.105] CHERRY, M.I., CHERRY, R.D., HEYRAUD, M., Polonium-210 and lead-210 in
Antarctic marine biota and sea water, Mar. Biol. 96 (1987) 441–449.
[8.106] CHERRY, R.D., HEYRAUD, M., Polonium-210 content of marine shrimp: Variation
with biological and environmental factors, Mar. Biol. 65 (1981) 165–175.
[8.107] SWINBANKS, D.D., SHIRAYAMA, Y., High levels of natural radionuclides in a
deep-sea infaunal xenophyophore, Nature 320 (1986) 354–358.
[8.108] MARSHALL, N.B., Developments in Deep-Sea Biology, Blandford Press,
Dorset (1979).
174
CHAPTER 8
[8.109] INTERNATIONAL ATOMIC ENERGY AGENCY, Handbook of Parameter Values
for the Prediction of Radionuclide Transfer to Wildlife, Technical Reports Series
No. 479, IAEA, Vienna (2014).
[8.110] ALAM, L., MOHAMED, C.A.R., A mini review on bioaccumulation of
210
Po by
marine organisms, Int. Food Res. J. 18 (2011) 1–10.
[8.111] FOWLER, S.W.,
210
Po in the marine environment with emphasis on its behaviour
within the biosphere, J. Environ. Radioact. 102 (2011) 448–461.
175
Chapter 9
POLONIUM IN HUMANS
9.1. INTRODUCTION
Polonium-210 is one of the most radiotoxic natural radioactive isotopes
known to humans due to its relatively long half-life (138 days) compared to
many radon progeny in the uranium series, its high specific activity (166 TBq/g)
and its emission of high linear energy transfer alpha radiation (5.304 MeV) [9.1].
On account of its high specific activity, it is also one of the rarest elements in
nature. In addition to occurring naturally, it can also be produced by neutron
activation of
209
Bi. The fractional intestinal uptake was given as 10–90% by
Hill [9.2] in 1965 and as 50% by the International Commission on Radiological
Protection (ICRP) in 1993 [9.3]. The daily intake is in the range of 37–370 mBq
for a European diet [9.2], but higher daily intake occurs in seafood rich countries,
such as Japan (480–690 mBq) [9.4]. The biological half-life is in the range of
26–100 days (see Table 9.1). There is a prompt excretion of about 3% within a
period of 9 days [9.6].
With a half-life of 22.3 years,
210
Pb is the parent nuclide of
210
Po and builds
up from the decay of
222
Rn in the uranium series. The inert noble gas
222
Rn
is generated in all soils and rocks during the decay of
226
Ra and is subject to
exhalation from the ground to the atmosphere. The daughter radionuclides of
222
Ra are then deposited by rain, wind and gravitational settling onto terrestrial
vegetation. Hence, humans are exposed to radioactive polonium as a consequence
of natural processes, and its estimated contribution to the annual background
effective dose is 120 μSv/a [9.1].
Polonium accumulates readily in biota, mainly owing to its affinity
for protein
[9.2], and hence easily passes through the food chain to humans.
For example,
210
Po has been found to bind to unidentified proteins with high
molecular weight (70
kilodaltons), which could be haemocyanin or metal binding
enzymes
[9.15]. Protein rich foods, such as crustaceans and other shellfish,
contain the highest activity concentrations of
210
Po. Elevated body burdens of
210
Po are also observed in humans that consume large amounts of reindeer meat,
for example subarctic native populations
[9.16, 9.17].
Most polonium enters the body through the consumption of food and then
reaches the gastrointestinal tract, from which much of it is excreted with faeces.
Polonium and lead absorbed into plasma are distributed throughout the soft tissue
of the body and accumulate in the liver and kidneys
[9.6]. The accumulation

176
CHAPTER 9
of
210
Pb in the skeleton provides another source of
210
Po over time. Since
210
Po
delivers the main absorbed dose of the Pb–Bi–Po decay chain, due to its alpha
emission (E
α
= 5.3 MeV), the behaviour of this isotope is of great interest.
Human biomonitoring is conducted mainly by urine and faecal sampling.
Blood sampling, however, has the advantage of being an integrated indicator
of polonium deposits across the metabolically active parts of the body;
hence, changes in the polonium body burden should be reflected in the blood
concentration. There is an absence, however, of suitable analogues (i.e. elements
with similar biochemical behaviour to polonium) to help to understand the
metabolic pathways in the human body which influence
210
Po biokinetics.
Polonium is a chalcogen element and, hence, it has chemical similarities to the
elements of the oxygen group in the periodic table (Group
VIA). According to
Waska et al.
[9.15],
210
Po has similar pathway characteristics in biological matter
as another chalcogen, selenium.
Most of the data for polonium activity concentrations in human tissues
and other matrices are from the 1960s and 1970s. The analytical procedures at
TABLE 9.1. A SURVEY OF AVAILABLE DATA FOR FRACTIONAL UPTAKE
AND BIOLOGICAL HALF-LIFE OF
210
Po IN HUMANS
Matrix N
Gastrointestinal
uptake fraction
Biological half-life
(days)
Ref.
Crab meat 7 0.60–0.94 —
a
[9.5]
Caribou meat 14 0.31–0.70 100 [9.6]
Normal food 15–63
7
—
a
—
a
—
a
0.35
0.1–0.3
0.05–0.45
b
0.1
—
a
83
—
a
50
30–60
73 (Inuit), 114 (Caucasians)
[9.7]
[9.8]
[9.9]
[9.10]
[9.11]
Po in water 5 0.38–0.78 31 [9.12]
Puncture wound
(accident)
1 —
a
26 [9.13]
Shellfish 7
—
a
0.44–0.52
0.50
28–51
50
[9.14]
[9.3]
a
—: data not available.
b
Depending on the organ.
177
POLONIUM IN HUMANS
that time did not undergo any inter-calibration or quality control [9.18]. Yield
determinants were often not used for the analytical procedure and a recovery of
100% was assumed, meaning that the true activity concentrations of
210
Po in the
samples were often underestimated. Furthermore, the results might be affected
by in
vitro buildup of
210
Po from
210
Pb. Samples need to be analysed shortly after
collection, otherwise
210
Pb
would also have to be determined to correlate the
result to the day of collection.
9.2. INGESTION AND INHALATION
The average daily intake of naturally occurring
210
Po for people following a
typical European diet is estimated to be 37–370 mBq [9.2, 9.19]. Populations that
consume marine food, such as crustaceans and shellfish, can have a much higher
intake, and this can be exacerbated for people living in areas of low oceanic
primary productivity, such as on the islands of the central Pacific Ocean [9.20]
(see also Chapter 8). Certain critical groups, such as radium dial painters, exhibit
exceptionally high levels [9.2, 9.21]. It is also clear that populations that consume
caribou and reindeer have higher concentrations in their body [9.11, 9.16].
A health risk evaluation for ingestion of
210
Po has been performed by Scott [9.10]
and an internal dose assessment using a biokinetic model is reported by Li et
al. [9.9].
The main sources of
210
Po (and its progenitor
210
Pb) in humans are food,
water and inhalation, with additional in
vivo contributions from
222
Rn dissolved
in the body as well as from
210
Pb and
226
Ra in bones. A 1966 study shows
indications of higher levels of
210
Po in the organs of smokers [9.22]. The intake
via inhalation is much smaller than that from food [9.7], even at uranium milling
facilities [9.23].
9.3. DISTRIBUTION IN TISSUES
The highest activity concentrations of
210
Po in humans are found in the
liver, kidneys and bone. The content in the skeleton and muscle tissue comprises
about 70% of the total body burden. For bone, a significant proportion is due to
buildup from
210
Pb [9.24]. Activity concentrations generally increase with age by
accumulation of
226
Ra and
210
Pb. For other animals, the concentrations are also
highest in the liver and kidneys, but the fraction of body content is different due
to a large portion being contained in the fur [9.25]. Table 9.2 shows the results
of a survey of
210
Po activity concentrations and the
210
Po:
210
Pb activity ratio in
different human organs. Almost all of the data are from the 1960s and 1970s,

178
CHAPTER 9
TABLE 9.2. POLONIUM IN HUMAN ORGANS
Matrix
Activity
concentration
(mBq/kg, FW)
Provenance Ref.
Lung 326 Former USSR
[9.7]; bb = 1.7%;
210
Po:
210
Pb = 1.4
318 USA, smokers [9.2]
118 USA, non-smokers [9.2];
210
Po:
210
Pb = 1.7
200 United Kingdom [9.16]
189 USA [9.17];
210
Po:
210
Pb = 0.75
Lung
parenchyma
290 USA, smokers [9.22]
93 USA, non-smokers [9.22]
Lung bronchi 470 USA, smokers [9.22]
370 USA, non-smokers [9.22]
Lung nodes 4 000 USA, smokers [9.22]
1 400 USA, non-smokers [9.22]
Liver 970 Former USSR
[9.7]; bb = 8.7%;
210
Po:
210
Pb = 2.2
400 USA [9.24]; bb = 1.7%
625 United Kingdom [9.16]
7 000 Radium dial painters [9.2];
210
Po:
210
Pb = 1.0
740 USA, smokers [9.2]
550 USA, non-smokers [9.2];
210
Po:
210
Pb = 2.1
Liver 529 USA [9.17];
210
Po:
210
Pb = 2.1
503 Children, AK, USA [9.17];
210
Po:
210
Pb = 2.1
530 USA, smokers [9.22]
220 USA, non-smokers [9.22]
750 Former USSR [9.26]
Bone 1 070 United Kingdom [9.16]
55 000 Radium dial painters [9.2];
210
Po:
210
Pb = 1.0
8 700
a
USA [9.24]; bb = 63%
1 070 Former USSR [9.7]; bb = 50.2%
1 700 —
b
[9.26]

179
POLONIUM IN HUMANS
TABLE 9.2. POLONIUM IN HUMAN ORGANS (cont.)
Matrix
Activity
concentration
(mBq/kg, FW)
Provenance Ref.
Bone (femur) 1 006 Children, AK, USA [9.17];
210
Po:
210
Pb = 0.82
Blood 7 900 Radium dial painters [9.21];
210
Po:
210
Pb = 0.7
292
c
Lapland, Finland [9.27]
38
c
Helsinki, Finland [9.27]
370
c
Sami, male, Sweden [9.28];
210
Po:
210
Pb = 3.3
180
c
Sami, female, Sweden [9.28];
210
Po:
210
Pb = 2.6
Muscle 111 Former USSR [9.7]; bb = 17.6%;
210
Po:
210
Pb = 0.8
222 USA [9.24]; bb = 17%
22 USA, smokers [9.22]
37 USA, non-smokers [9.22]
Hair
2 000–11 000
d
Sweden [9.29];
210
Po:
210
Pb = 2.2
900 000 Radium dial painters [9.2];
210
Po:
210
Pb = 1.0
3 300 Former USSR [9.7];
210
Po:
210
Pb = 2.2
9 000–22 000
d
India, non-smokers [9.30]
23 000
d
India, smokers [9.30]
800–14 000
d
USA, non-smokers [9.31]
1 200–12 000
d
USA, smokers [9.31]
5 300 Former USSR [9.26]
Kidney 640 United Kingdom [9.16]
970 Former USSR
[9.7]; bb = 1.2%;
210
Po:
210
Pb = 2.8
9 300 Radium dial painters [9.2];
210
Po:
210
Pb = 9
758 USA, smokers [9.2]
555 USA, non-smokers [9.2];
210
Po:
210
Pb = 3.2
384 USA [9.17];
210
Po:
210
Pb = 2.6
277 Children, AK, USA [9.17];
210
Po:
210
Pb = 2.6
330 USA, smokers [9.22]
210 USA, non-smokers [9.22]

180
CHAPTER 9
TABLE 9.2. POLONIUM IN HUMAN ORGANS (cont.)
Matrix
Activity
concentration
(mBq/kg, FW)
Provenance Ref.
Placenta 122 United Kingdom [9.16]
740–4 000 Reindeer and caribou
consumers, Canada
[9.16];
210
Po:
210
Pb = 6.3
400 N. Canada [9.11]
Gonads 144 USA, smokers [9.2]
104 USA, non-smokers [9.2]
255 USA [9.17];
210
Po:
210
Pb = 1.1
Testis 144 United Kingdom [9.16]
Spleen 117 USA [9.17];
210
Po:
210
Pb = 1.8
22 Children, AK, USA [9.17];
210
Po:
210
Pb = 0.6
220 Former USSR [9.7]; bb = 0.8%;
210
Po:
210
Pb = 0.2
Pancreas 107 USA [9.17];
210
Po:
210
Pb = 1.8
Thyroid 189 USA [9.17]
Heart 19 USA [9.17]
70 USA, smokers [9.22]
37 USA, non-smokers [9.22]
Eye, choroid
and iris
1 000–10 000 United Kingdom [9.32]
Bone marrow 24 United Kingdom [9.33]
Note: bb — body burden.
a
mBq/kg, ash.
b
—: data not available.
c
mBq/L.
d
mBq/kg (DW).
when access to autopsy samples was permitted. However, estimation of body
content can be achieved by analysing urine and faeces. Hair also seems to be a
good indicator of polonium contamination [9.12].

181
POLONIUM IN HUMANS
Polonium has an affinity for proteins. For example, gelatin is reported to
contain 1500 mBq/kg of
210
Po and insulin 23 000–70 000 mBq/kg [9.2]. In the
past, people with diabetes may have been exposed to elevated levels of
210
Po in
natural insulin. Insulin is now made artificially; it is no longer extracted from
the pancreas and does not contain any
210
Po. However, another critical group
might be people undergoing dialysis. If polonium became associated with protein
molecules in the body too large to pass through the membrane during dialysis,
the radionuclide might build up in the body. This has not yet been investigated.
9.4. RETENTION AND BIOLOGICAL HALF-LIVES
The kinetics of polonium are rather complex (see Fig. 9.1) and are
influenced by its wide variety of potential oxidation states (−2, +2, +4 and +6;
see Section 3.2). Polonium can be released from one organ and recirculated.
Source: Figure 5 of Ref. [9.34].
FIG. 9.1. Compartments of the systemic model, paths of movement of polonium between
compartments, and connections between the systemic model and user-supplied models of the
respiratory tract, gastrointestinal tract and wounds (reproduced with permission courtesy of
Elsevier).

182
CHAPTER 9
The in vivo concentration of
210
Pb causes buildup of
210
Po, especially in bone
where the biological half-life of
210
Pb is long. In addition,
226
Ra plays a role as
a precursor of
210
Po. Several studies report values between 10 and 60 days for
the effective biological half-life of
210
Po in humans (see Fig. 9.2), and fractional
uptake following ingestion ranges from a few percent to 90%. The dose factor is
thereby quite uncertain. All those factors probably depend, to a large degree, on
the composition of the foodstuff with which polonium is ingested.
The highest activity concentrations in humans are found in the liver and, to
a lesser extent, in the kidneys. It has also been found that hair is a good indicator
of polonium contamination and that hair is an important excretion route in
addition to faecal and urinary routes
[9.11]. Due to its complex biokinetics and its
array of exposure and excretion pathways, the biological and effective half-lives
of polonium are highly variable (see Fig.
9.2).
Leggett and Eckerman [9.34] find that:
“Although there is a relatively large database on the biological behavior
of polonium in man and laboratory animals, some important aspects of the
biokinetics of polonium in man have not been characterized with much
certainty. A central problem is that a substantial portion of the data on
urinary excretion of
210
Po by human subjects may not be reliable. Although
relatively detailed data are available from a controlled study on human
subjects, these data are limited not only by potential errors in the urinary
excretion data but also by the fact that the state of health of the subjects
could have affected the biokinetics of polonium.”
Source: Figure 2 of Ref. [9.34].
FIG. 9.2. Distribution of effective half-lives derived from urinary excretion records for a large
number of workers using polonium (reproduced with permission courtesy of Elsevier).
183
POLONIUM IN HUMANS
Hence, the results might be poorly representative of the general population.
Conclusions about data on laboratory animals are complicated by the variation in
biokinetics of polonium due to differences between “species, route of exposure,
and the chemical form of polonium taken into the body” [9.34].
The human gastrointestinal uptake fraction f
1
has been established by a
number of different studies, with a wide range of results (for fractional uptake and
biological half-life for
210
Po, see Table 9.1). Thomas et al. [9.6] show that a total
ingestion of 20
Bq with caribou meat resulted in a maximum of 3.2 Bq/d in faecal
excretion over 4–6
days after intake and a maximum of 0.32 Bq/d of urinary
excretion from 5 to 10
days after ingestion. From these results, a gastrointestinal
uptake fraction of 0.56
± 0.04 was established. In the 1950s, Silberstein et al. [9.8]
published gastrointestinal uptake fractions as low as 0.1–0.3. The ICRP
[9.3] has
increased its reported gastrointestinal uptake fraction from 10% to 50%, which,
according to other studies, still seems too small. Hunt and Allington
[9.5] report
gastrointestinal uptake fractions in the range of 0.6–0.94, with a mean of 0.76,
after analysing a study in 1991 in which seven volunteers ingested crab meat
with a total
210
Po activity intake of up to 44.2 Bq. A range of 10–25% for daily
faecal excretion and a maximum daily urine excretion of 0.2–0.3% is reported.
Additional data from the 1970s are provided by Landinskaya et
al. [9.7], who
analysed tissue from humans and estimated their intake from normal food, and in
Ref. [9.11], which reports on the retention of
210
Po in Inuit and Caucasians.
In a 2012 study on radioactive polonium biokinetics by Henricsson [9.12],
volunteers ingested
209
Po in a PoCl
4
solution. The excretion via faeces and urine
of four of the subjects is shown in Fig.
9.3. It is well known that there are some
critical groups, such as those who eat reindeer or caribou and those who consume
large quantities of marine food (particularly molluscs and crustaceans).
In conclusion, there have been limited data generated on the kinetics and
organ distribution of polonium in humans since the 1970s. Only about eight
studies have been performed and, of these, only three experimental studies were
conducted after the 1970s. Considering that
210
Po is the largest contributor of
radiological dose to humans (from alpha particle emission), this is surprising.
There have been some critical population groups identified, such as those
who consume large amounts of seafood or consume caribou and reindeer.
However, the large variations in fractional uptake and biological residence time
results in a large uncertainty in the estimated dose factor for oral intake of
210
Po.
The fractional intake probably depends on the food composition with which
polonium is consumed, which will vary between regions and lifestyles
[9.16]. It
would be of interest to conduct a survey of the main food for ingestion of
210
Po in
different countries, perhaps by analysing hair, which could be a good indicator of
internal contamination
[9.11, 9.30].

184
CHAPTER 9
REFERENCES TO CHAPTER 9
[9.1] UNITED NATIONS SCIENTIFIC COMMITTEE ON THE EFFECTS OF ATOMIC
RADIATION, Sources and Effects of Ionizing Radiation UNSCEAR 2000 Report to
the General Assembly, with Scientific Annexes, Vol. I: Sources, United Nations,
New York (2000) Annex B.
[9.2] HILL, C.R.,
210
Po in man, letter to K. Liden, Lund University, personal communication,
1965.
[9.3] INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION,
Age-dependent Doses to Members of the Public from Intake of Radionuclides —
Part
2: Ingestion Dose Coefficients, Publication 67, Pergamon Press, Oxford and
New
York (1993).
[9.4] YAMAMOTO, M., et al., Polonium-210 and lead-210 in marine organisms: Intake
levels for Japanese, J. Radioanal. Nucl. Chem.
178 (1994) 81–90.
[9.5] HUNT, G.J., ALLINGTON, D.J., Absorption of environmental polonium-210 by the
human gut, J. Radiol. Prot.
13 (1993) 119–126.
[9.6] THOMAS, P.A., FISENNE, I., CHORNEY, D., BAWEJA, A.S., TRACY, B.L., Human
absorption and retention of polonium-210 from caribou meat, Radiat. Prot. Dosim.
97
(2001)
241–250.
[9.7] LADINSKAYA, L.A., PARFENOV, Yu.D., POPOV, D.K., FEDOROVA, A.V.,
210
Pb
and
210
Po content in air, water, foodstuffs, and the human body, Arch. Environ.
Health
27 (1973) 254–258.
0.0%
0.5%
1.0%
1.5%
2.0%
2.5%
3.0%
3.5%
4.0%
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
0 200 400 600 800 1000 1200 1400 1600
Accumulatedfr actionofintake
Hoursfromintake
Source: Data from Ref. [9.12].
FIG. 9.3. Accumulated excretion of
209
Po via faeces.
185
POLONIUM IN HUMANS
[9.8] SILBERSTEIN, H.E., VALENTINE, W.N., MINTO, W.L., LAWRENCE, J.S., FINK,
R.M., “Studies of polonium metabolism in human subjects”, Biological Studies with
Polonium, Radium and Plutonium (FINK, R.M., Ed.), McGraw-Hill, New
York (1950).
[9.9] LI, W.B., GERSTMANN, U., GIUSSANI, A., OEH, U., PARETZKE, H.G., Internal
dose assessment of
210
Po using biokinetic modeling and urinary excretion measurement,
Radiat. Environ. Biophys.
47 (2008) 101–110.
[9.10] SCOTT, B.R., Health risk evaluations for ingestion exposure of humans to
polonium-210, Dose Response
5 (2007) 94–122.
[9.11] ARGONNE NATIONAL LABORATORY, Renal Excretion Rates of
137
Cs,
210
Pb,
210
Po
and Stable Lead by Northern Canadians, ANL-7860, Part II, Argonne Natl Lab.,
IL (1971).
[9.12] HENRICSSON, C.F., RANEBO, Y., HANSSON, M., RÄÄF, C.L., HOLM, E., A
biokinetic study of
209
Po in man, Sci. Total Environ. 437 (2012) 384–389.
[9.13] WRAIGHT, J.C., STRONG, R., “A case study on the retention and excretion of
internally deposited polonium”, Radiation Protection: Theory and Practice (Proc. 4th
Int. Symp. Malvern, 1989), Society for Radiological Protection, Berkeley
(GOLDFINCH, E.P., Ed.) (1989)
227–230.
[9.14] HUNT, G.J., RUMNEY, H.S., The human alimentary tract transfer and body retention
of environmental polonium-210, J. Radiol. Prot.
27 (2007) 405–426.
[9.15] WASKA, H., KIM, S., KIM, G., KANG, M.R., KIM, G.B., Distribution patterns of
chalcogens (S, Se, Te, and
210
Po) in various tissues of a squid, Todarodes pacificus, Sci.
Total Environ.
392 (2008) 218–224.
[9.16] HILL, C.R., Polonium-210 content of human tissues in relation to dietary habit,
Science 152 (1966)
1261–1262.
[9.17] BLANCHARD, R.L., “Relationship between
210
Po and
210
Pb in man and his
environment”, Radioecological Concentration Processes (Proc. Int. Symp. Stockholm,
1966) (ÅBERG, B., HUNGATE, F.P., Eds), Pergamon Press, Oxford (1967)
275–280.
[9.18] HENRICSSON, F., RANEBO, Y., HOLM, E., ROOS, P., Aspects on the analysis of
210
Po, J. Environ. Radioact. 102 (2011) 415–419.
[9.19] BEOWULF, G., HERMANN, M., Intake and excretion of natural radionuclides
210
Pb
and
210
Po by humans, Strahlenther. 131 (1966) 281–296.
[9.20] JEFFREE, R.A., SZYMCZAK, R., Enhancing effect of marine oligotrophy on
environmental concentrations of particle-reactive trace elements, Environ. Sci.
Technol.
34 (2000) 1966–1969.
[9.21] HOLTZMAN, R.B., “
210
Pb and
210
Po metabolism in radium-dial painters”, paper
presented at the Meeting on Biology and Ecology of Polonium and Radiolead,
Surrey,
1970.
[9.22] FERRI, E.S., BARATTA, E.J., Polonium 210 in tobacco, cigarette smoke, and selected
human organs, Public Health Rep.
81 (1966) 121–127.
[9.23] NUCLEAR REGULATORY COMMISSION, Final Generic Environmental Impact
Statement on Uranium Milling, Project M-25, NUREG-0706, Office of Nuclear
Material Safety and Safeguards, NRC, Washington, DC (1980).
186
CHAPTER 9
[9.24] HOLTZMAN, R.B., Measurement of the natural content of RaD (
210
Pb) and RaF
(
210
Po) in human bone estimates of whole-body burdens, Health Phys. 9
(1963)
385–400.
[9.25] SCHRECKHISE, R.G., WALTERS, R.L., Internal distribution and milk secretion of
polonium-210 after oral administration to a lactating goat, J. Dairy Sci.
52
(1969)
1867–1869.
[9.26] ANTONOVA, V.A., Po-210 in the hair, bone tissue and liver of man, Gig. Sanit. 10
(1971) 52–55 (in
Russian).
[9.27] KAURANEN, P., MIETTINEN, J.K.,
210
Po and
210
Pb in the arctic food chain and the
natural radiation exposure of Lapps, Health Phys.
16 (1969) 287–295.
[9.28] PERSSON, B.R., “Lead-210, polonium-210 and stable lead in the food-chain lichen,
reindeer and man”, The Natural Radiation Environment
II (Proc. 2nd Int. Symp.
Houston, 1972) (ADAMS, J.A.S., LOWDER, W.M., GESELL, T.F., Eds), United
States Department of Commerce, Springfield, VA (1972)
347–367.
[9.29] HOLM, E., et al., Hair and Feathers as Indicator of Internal Contamination of
210
Po
and
210
Pb, NKS-217, Nordic Nuclear Safety Research, Roskilde (2010).
[9.30] RATHI, C.R., ROSS, E.M., WESLEY, S.G., Polonium-210 activity in human hair
samples and factors affecting its accumulation, Iran. J. Radiat. Res.
9 (2011) 41–47.
[9.31] LYKKEN, G.I., ALKHATIB, H.A., “Analysis of hair for polonium-210 α-particle
emissions”, Communicating the Radon Issue (Proc. Int. Radon Conf. Colorado, 1993)
(1993)
347–367.
[9.32] HUNT, V.R., “Concentrations of
210
Po,
226
Ra, and
228
Th in the choroid of the eye,
particularly in cattle”, Radioecological Concentration Processes (Proc. Int. Symp.
Stockholm, 1966) (ÅBERG, B., HUNGATE, F.P., Eds), Pergamon Press, Oxford
(1967)
303–311.
[9.33] SALMON, P.L., CLAYTON, R.F., HENSHAW, D.L., Measurement of
210
Po in
biopsied human red bone marrow, Radiat. Prot. Dosim.
78 (1998) 127–131.
[9.34] LEGGETT, R.W., ECKERMAN, K.F., A systemic biokinetic model for polonium, Sci.
Total Environ.
275 (2001) 109–125.
187
Chapter 10
RADIOLOGICAL DOSE ASSESSMENT FOR
210
Po
TO HUMANS AND OTHER BIOTA
10.1. MODELS AND DATA FOR ESTIMATING INTERNAL EXPOSURES
TO HUMANS
Dosimetric models to estimate internal exposures in humans, subsequent to
incorporation of radionuclides via ingestion and inhalation, are developed by the
International Commission on Radiological Protection (ICRP). The most recent
models for
210
Po are described in ICRP Publications 67 [10.1] and 72 [10.2] for
ingestion, and in ICRP Publications 71 [10.3] and 72 [10.2] for inhalation. The end
points of those models are dose coefficients for ingestion and inhalation, which
are calculated for six age groups (3 month old children; 1, 5, 10 and 15 year old
children; and adults). The dose coefficients are based on the equivalent dose rates
received until the age of 70 years following an acute intake at the age of intake:
for a one year old child, the dose contributions are integrated over a period of
69 years; for adults, the dose is integrated over a period of 50 years.
The key processes in the biokinetic models are absorption of the
radionuclides into the blood and the distribution and retention in the organism.
Hence, the time integrated concentrations in the organs and tissues and the
number of disintegrations during the integration period are calculated from these
quantities. For a specific organ, the integrated absorbed dose is calculated from
the following:
(a) The number of decays during the integration period;
(b) The energy per decay;
(c) The energy deposited in a specific organ from radionuclides within the
organ;
(d) The energy deposited in a specific organ from radionuclides decaying in
other organs and tissues.
The model parameters applied in the biokinetic models are derived from
all available and appropriate experiments and observations on the uptake and
distribution of radionuclides, many of which determined from animal studies.
Following ingestion, the transfer of
210
Po in the ICRP model from the gut
to the blood (resorption) is age dependent. The highest resorption is applied for
infants (3
months) with 100%, and 50% for all other age groups [10.1].
188
CHAPTER 10
Distribution and retention are described by complex model simulation of
the fluxes of
210
Po between different organs and tissues. The most important
process for long term retention is the exchange of
210
Po between the kidneys,
liver and skeleton.
To calculate dose per unit intake values, it is assumed that for
210
Po
entering the systemic circulation, fractions of 0.05, 0.1, 0.1, 0.3 and 0.45 are
deposited in the spleen, kidneys, red bone marrow, liver and the rest of the body,
respectively [10.1], where it is retained with a half-life of 50 days. For
210
Po
that has entered the excretory system, a urinary:faecal excretion ratio of 1:2 is
assumed.
The transfer to the blood from the lungs depends on the age and the
chemical form of
210
Po. In general, the ICRP takes into account three absorption
classes: slow (S), medium (M) and fast (F) retention. In Tables 10.1–10.4, the
dose coefficients for
210
Po are summarized for ingestion and inhalation. These are
given as effective dose per unit intake (Sv/Bq), and all of the organs considered
in Refs [10.1, 10.3] are listed. For ingestion of
210
Po, the organs with the highest
dose coefficients are the kidneys, liver, bone surface, red bone marrow and
spleen. For inhalation, the dose coefficients for the lung and the extrathoracic
airways are also important. There are large differences between the dose
coefficients for the different absorption classes, in particular when comparing
organ doses (see Tables 10.2–10.4). To estimate the effective dose from
210
Po,
the highest dose coefficients are calculated for the absorption class S, which are
about a factor of seven higher than for inhalation class F. This is because the less
soluble materials remain in the lungs longer and thus provide more exposure to
those tissues. The ranking of the organ doses also varies with the inhalation class.
10.2. DOSE CONVERSION FACTORS FOR WILDLIFE
10.2.1. General assumptions
Plants and animals can be exposed to ionizing radiation from radionuclides
in the environment by both external and internal exposure. The United Nations
Scientific Committee on the Effects of Atomic Radiation (UNSCEAR)
[10.4]
reports that:
“101. Radionuclides distributed in the environment lead to external
exposure of an organism living in or close to a medium that contains
radionuclides. The external exposure of biota is the result of complex and
non-linear interactions of various factors:

189
DOSE ASSESSMENT
TABLE 10.1. DOSE COEFFICIENTS FOR INGESTION OF
210
Po
Organ/tissue Dose coefficient (Sv/Bq)
Age at intake
(years)
<1 1–2 2–7 7–12 12–17 >17
f
1
value
1 0.5 0.5 0.5 0.5 0.5
Adrenals 5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.8 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Bladder 5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.8 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Bone surface 8.0 × 10
−5
2.3 × 10
−5
8.9 × 10
−6
4.8 × 10
−6
2.8 × 10
−6
1.6 × 10
−6
Brain 5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.8 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Breast 5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.8 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Gastrointestinal
tract
Oesophagus 5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.8 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Stomach 5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.8 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Small intestine 5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.9 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Upper large
intestine
5.8 × 10
−6
2.1 × 10
−6
1.0 × 10
−6
6.1 × 10
−7
3.6 × 10
−7
2.9 × 10
−7
Lower large
intestine
6.0 × 10
−6
2.3 × 10
−6
1.1 × 10
−6
6.7 × 10
−7
3.9 × 10
−7
3.2 × 10
−7
Colon 5.9 × 10
−6
2.1 × 10
−6
1.1 × 10
−6
6.4 × 10
−7
3.7 × 10
−7
3.0 × 10
−7
Kidney 1.8 × 10
−4
6.2 × 10
−5
3.4 × 10
−5
2.3 × 10
−5
1.6 × 10
−5
1.3 × 10
−5
Liver 1.1 × 10
−4
4.0 × 10
−5
2.0 × 10
−5
1.3 × 10
−5
8.5 × 10
−6
6.6 × 10
−6
Muscle 5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.8 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Ovaries 5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.8 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Pancreas 5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.8 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Red bone marrow 8.1 × 10
−5
2.6 × 10
−5
1.2 × 10
−5
6.4 × 10
−6
3.8 × 10
−6
2.6 × 10
−6
Respiratory tract
Extrathoracic
airways
5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.8 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Lungs 5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.8 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Skin 5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.8 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Spleen 2.2 × 10
−4
7.6 × 10
−5
4.1 × 10
−5
2.5 × 10
−5
1.6 × 10
−5
1.1 × 10
−5
Testes 5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.8 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Thymus 5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.8 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Thyroid 5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.8 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Uterus 5.7 × 10
−6
2.0 × 10
−6
9.8 × 10
−7
5.8 × 10
−7
3.4 × 10
−7
2.8 × 10
−7
Remainder 9.3 × 10
−5
3.9 × 10
−5
2.1 × 10
−5
1.3 × 10
−5
8.2 × 10
−6
6.6 × 10
−6
Effective dose 2.6 × 10
−5
8.8 × 10
−6
4.4 × 10
−6
2.6 × 10
−6
1.6 × 10
−6
1.2 × 10
−6
Source: See Ref. [10.1].

190
CHAPTER 10
TABLE 10.2. DOSE COEFFICIENTS FOR INHALATION OF
210
Po,
INHALATION CLASS F
Organ/tissue Dose coefficient (Sv/Bq)
Age at intake
(years)
<1 1–2 2–7 7–12 12–17 >17
f
1
value
0.2 0.1 0.1 0.1 0.1 0.1
Adrenals 1.6 × 10
−6
1.1 × 10
−6
4.9 × 10
−7
2.9 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Bladder 1.6 × 10
−6
1.1 × 10
−6
4.9 × 10
−7
2.9 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Bone surface 2.3 × 10
−5
1.3 × 10
−5
4.5 × 10
−6
2.4 × 10
−6
1.4 × 10
−6
8.0 × 10
−7
Brain 1.6 × 10
−6
1.1 × 10
−6
4.9 × 10
−7
2.9 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Breast 1.6 × 10
−6
1.1 × 10
−6
4.9 × 10
−7
2.9 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Gastrointestinal
tract
Oesophagus 1.6 × 10
−6
1.1 × 10
−6
4.9 × 10
−7
2.9 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Stomach 1.6 × 10
−6
1.1 × 10
−6
4.9 × 10
−7
2.9 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Small intestine 1.6 × 10
−6
1.1 × 10
−6
4.9 × 10
−7
2.9 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Upper large
intestine
1.7 × 10
−6
1.1 × 10
−6
5.0 × 10
−7
3.0 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Lower large
intestine
1.8 × 10
−6
1.2 × 10
−6
5.3 × 10
−7
3.2 × 10
−7
1.8 × 10
−7
1.5 × 10
−7
Colon 1.7 × 10
−6
1.1 × 10
−6
5.2 × 10
−7
3.1 × 10
−7
1.7 × 10
−7
1.6 × 10
−7
Kidney 5.1 × 10
−5
3.4 × 10
−5
1.7 × 10
−5
1.1 × 10
−5
7.7 × 10
−6
6.4 × 10
−6
Liver 3.1 × 10
−5
2.2 × 10
−5
1.0 × 10
−5
6.6 × 10
−6
4.1 × 10
−6
3.3 × 10
−6
Muscle 1.6 × 10
−6
1.1 × 10
−6
4.9 × 10
−7
2.9 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Ovaries 1.6 × 10
−6
1.1 × 10
−6
4.9 × 10
−7
2.9 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Pancreas 1.6 × 10
−6
1.1 × 10
−6
4.9 × 10
−7
2.9 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Red bone marrow 2.3 × 10
−5
1.4 × 10
−5
6.1 × 10
−6
3.2 × 10
−6
1.8 × 10
−6
1.3 × 10
−6
Respiratory Tract
Extrathoracic
airways
1.6 × 10
−6
1.1 × 10
−6
5.0 × 10
−7
3.0 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Lungs 1.7 × 10
−6
1.1 × 10
−6
5.2 × 10
−7
3.1 × 10
−7
1.9 × 10
−7
1.6 × 10
−7
Skin 1.6 × 10
−6
1.1 × 10
−6
4.9 × 10
−7
2.9 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Spleen 6.3 × 10
−5
4.2 × 10
−5
2.0 × 10
−5
1.3 × 10
−5
7.8 × 10
−6
5.5 × 10
−6
Testes 1.6 × 10
−6
1.1 × 10
−6
4.9 × 10
−7
2.9 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Thymus 1.6 × 10
−6
1.1 × 10
−6
4.9 × 10
−7
2.9 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Thyroid 1.6 × 10
−6
1.1 × 10
−6
4.9 × 10
−7
2.9 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Uterus 1.6 × 10
−6
1.1 × 10
−6
4.9 × 10
−7
2.9 × 10
−7
1.7 × 10
−7
1.4 × 10
−7
Remainder 3.3 × 10
−5
2.2 × 10
−5
1.0 × 10
−5
6.5 × 10
−6
4.0 × 10
−6
3.3 × 10
−6
Effective dose 7.4 × 10
−6
4.8 × 10
−6
2.2 × 10
−6
1.3 × 10
−6
7.7 × 10
−7
6.1 × 10
−7
Source: See Ref. [10.3].

191
DOSE ASSESSMENT
TABLE 10.3. DOSE COEFFICIENTS FOR INHALATION OF
210
Po,
INHALATION CLASS M
Organ/tissue Dose coefficient (Sv/Bq)
Age at intake
(years)
<1 1–2 2–7 7–12 12–17 >17
f
1
value
0.2 0.1 0.1 0.1 0.1 0.1
Adrenals 6.4 × 10
−7
3.5 × 10
−7
1.6 × 10
−7
9.7 × 10
−8
5.7 × 10
−8
4.9 × 10
−8
Bladder 6.4 × 10
−7
3.5 × 10
−7
1.6 × 10
−7
9.7 × 10
−8
5.7 × 10
−8
4.9 × 10
−8
Bone surface 8.9 × 10
−6
4.1 × 10
−6
1.5 × 10
−6
7.9 × 10
−7
4.6 × 10
−7
2.8 × 10
−7
Brain 6.4 × 10
−7
3.5 × 10
−7
1.6 × 10
−7
9.7 × 10
−8
5.7 × 10
−8
4.9 × 10
−8
Breast 6.4 × 10
−7
3.5 × 10
−7
1.6 × 10
−7
9.7 × 10
−8
5.7 × 10
−8
4.9 × 10
−8
Gastrointestinal
tract
Oesophagus 6.4 × 10
−7
3.5 × 10
−7
1.6 × 10
−7
9.7 × 10
−8
5.7 × 10
−8
4.9 × 10
−8
Stomach 6.5 × 10
−7
3.5 × 10
−7
1.6 × 10
−7
9.7 × 10
−8
5.7 × 10
−8
4.9 × 10
−8
Small intestine 6.5 × 10
−7
3.5 × 10
−7
1.7 × 10
−7
9.8 × 10
−8
5.8 × 10
−8
5.0 × 10
−8
Upper large
intestine
7.1 × 10
−7
3.9 × 10
−7
1.8 × 10
−7
1.1 × 10
−7
6.3 × 10
−8
5.4 × 10
−8
Lower large
intestine
8.2 × 10
−7
4.7 × 10
−7
2.2 × 10
−7
1.3 × 10
−7
7.3 × 10
−8
6.2 × 10
−8
Colon 7.6 × 10
−7
4.3 × 10
−7
2.0 × 10
−7
1.2 × 10
−7
6.7 × 10
−8
5.7 × 10
−8
Kidney 2.0 × 10
−5
1.1 × 10
−5
5.7 × 10
−6
3.8 × 10
−6
2.7 × 10
−6
2.2 × 10
−6
Liver 1.2 × 10
−5
7.1 × 10
−6
3.4 × 10
−6
2.2 × 10
−6
1.4 × 10
−6
1.2 × 10
−6
Muscle 6.4 × 10
−7
3.5 × 10
−7
1.6 × 10
−7
9.7 × 10
−8
5.7 × 10
−8
4.9 × 10
−8
Ovaries 6.4 × 10
−7
3.5 × 10
−7
1.6 × 10
−7
9.7 × 10
−8
5.7 × 10
−8
4.9 × 10
−8
Pancreas 6.4 × 10
−7
3.5 × 10
−7
1.6 × 10
−7
9.7 × 10
−8
5.7 × 10
−8
4.9 × 10
−8
Red bone marrow 9.1 × 10
−6
4.6 × 10
−6
2.1 × 10
−6
1.1 × 10
−6
6.3 × 10
−7
4.6 × 10
−7
Respiratory tract
Extrathoracic
airways
3.2 × 10
−5
2.4 × 10
−5
9.9 × 10
−6
6.6 × 10
−6
3.6 × 10
−6
3.5 × 10
−6
Lungs 1.1 × 10
−4
8.1 × 10
−5
5.1 × 10
−5
3.5 × 10
−5
3.1 × 10
−5
2.6 × 10
−5
Skin 6.4 × 10
−7
3.5 × 10
−7
1.6 × 10
−7
9.7 × 10
−8
5.7 × 10
−8
4.9 × 10
−8
Spleen 2.5 × 10
−5
1.4 × 10
−5
6.8 × 10
−6
4.2 × 10
−6
2.7 × 10
−6
1.9 × 10
−6
Testes 6.4 × 10
−7
3.5 × 10
−7
1.6 × 10
−7
9.7 × 10
−8
5.7 × 10
−8
4.9 × 10
−8
Thymus 6.4 × 10
−7
3.5 × 10
−7
1.6 × 10
−7
9.7 × 10
−8
5.7 × 10
−8
4.9 × 10
−8
Thyroid 6.4 × 10
−7
3.5 × 10
−7
1.6 × 10
−7
9.7 × 10
−8
5.7 × 10
−8
4.9 × 10
−8
Uterus 6.4 × 10
−7
3.5 × 10
−7
1.6 × 10
−7
9.7 × 10
−8
5.7 × 10
−8
4.9 × 10
−8
Remainder 1.0 × 10
−6
5.6 × 10
−7
2.7 × 10
−7
1.6 × 10
−7
1.0 × 10
−7
8.4 × 10
−8
Effective dose 1.5 × 10
−5
1.1 × 10
−5
6.7 × 10
−6
4.6 × 10
−6
4.0 × 10
−6
3.3 × 10
−6
Source: See Ref. [10.3].

192
CHAPTER 10
TABLE 10.4. DOSE COEFFICIENTS FOR INHALATION OF
210
Po,
INHALATION CLASS S
Organ/tissue Dose coefficient (Sv/Bq)
Age at intake
(years)
<1 1–2 2–7 7–12 12–17 >17
f
1
value 0.02 0.01 0.01 0.01 0.01 0.01
Adrenals 4.4 × 10
−8
1.9 × 10
−8
8.5 × 10
−9
5.0 × 10
−9
2.8 × 10
−9
2.4 × 10
−9
Bladder 4.4 × 10
−8
1.9 × 10
−8
8.5 × 10
−9
5.1 × 10
−9
2.9 × 10
−9
2.4 × 10
−9
Bone surface 6.1 × 10
−7
2.2 × 10
−7
7.7 × 10
−8
4.1 × 10
−8
2.3 × 10
−8
1.4 × 10
−8
Brain 4.4 × 10
−8
1.9 × 10
−8
8.5 × 10
−9
5.0 × 10
−9
2.8 × 10
−9
2.4 × 10
−9
Breast 4.4 × 10
−8
1.9 × 10
−8
8.5 × 10
−9
5.0 × 10
−9
2.8 × 10
−9
2.4 × 10
−9
Gastrointestinal
tract
Oesophagus 4.4 × 10
−8
1.9 × 10
−8
8.5 × 10
−9
5.0 × 10
−9
2.8 × 10
−9
2.4 × 10
−9
Stomach 4.9 × 10
−8
2.1 × 10
−8
9.6 × 10
−9
5.7 × 10
−9
3.2 × 10
−9
2.7 × 10
−9
Small intestine 5.5 × 10
−8
2.6 × 10
−8
1.2 × 10
−8
7.0 × 10
−9
3.8 × 10
−9
3.2 × 10
−9
Upper large
intestine
1.1 × 10
−7
6.5 × 10
−8
2.8 × 10
−8
1.7 × 10
−8
8.6 × 10
−9
7.2 × 10
−9
Lower large
intestine
2.4 × 10
−7
1.5 × 10
−7
6.4 × 10
−8
3.9 × 10
−8
2.0 × 10
−8
1.6 × 10
−8
Colon 1.7 × 10
−7
1.0 × 10
−7
4.4 × 10
−8
2.6 × 10
−8
1.3 × 10
−8
1.1 × 10
−8
Kidney 1.4 × 10
−6
5.9 × 10
−7
2.9 × 10
−7
2.0 × 10
−7
1.3 × 10
−7
1.1 × 10
−7
Liver 8.4 × 10
−7
3.8 × 10
−7
1.7 × 10
−7
1.2 × 10
−7
7.0 × 10
−8
5.7 × 10
−8
Muscle 4.4 × 10
−8
1.9 × 10
−8
8.5 × 10
−9
5.0 × 10
−9
2.8 × 10
−9
2.4 × 10
−9
Ovaries 4.4 × 10
−8
1.9 × 10
−8
8.5 × 10
−9
5.0 × 10
−9
2.8 × 10
−9
2.4 × 10
−9
Pancreas 4.4 × 10
−8
1.9 × 10
−8
8.5 × 10
−9
5.0 × 10
−9
2.8 × 10
−9
2.4 × 10
−9
Red bone marrow 6.2 × 10
−7
2.4 × 10
−7
1.1 × 10
−7
5.6 × 10
−8
3.1 × 10
−8
2.3 × 10
−8
Respiratory tract
Extrathoracic
airways
5.7 × 10
−5
4.4 × 10
−5
1.9 × 10
−5
1.3 × 10
−5
7.1 × 10
−6
6.9 × 10
−6
Lungs 1.5 × 10
−4
1.1 × 10
−4
7.2 × 10
−5
4.9 × 10
−5
4.3 × 10
−5
3.5 × 10
−5
Skin 4.4 × 10
−8
1.9 × 10
−8
8.5 × 10
−9
5.0 × 10
−9
2.8 × 10
−9
2.4 × 10
−9
Spleen 1.7 × 10
−6
7.2 × 10
−7
3.5 × 10
−7
2.2 × 10
−7
1.3 × 10
−7
9.6 × 10
−8
Testes 4.4 × 10
−8
1.9 × 10
−8
8.5 × 10
−9
5.0 × 10
−9
2.8 × 10
−9
2.4 × 10
−9
Thymus 4.4 × 10
−8
1.9 × 10
−8
8.5 × 10
−9
5.0 × 10
−9
2.8 × 10
−9
2.4 × 10
−9
Thyroid 4.4 × 10
−8
1.9 × 10
−8
8.5 × 10
−9
5.0 × 10
−9
2.8 × 10
−9
2.4 × 10
−9
Uterus 4.4 × 10
−8
1.9 × 10
−8
8.5 × 10
−9
5.0 × 10
−9
2.8 × 10
−9
2.4 × 10
−9
Remainder 1.0 × 10
−7
4.8 × 10
−8
2.2 × 10
−8
1.4 × 10
−8
8.3 × 10
−9
7.5 × 10
−9
Effective dose 1.8 × 10
−5
1.4 × 10
−5
8.6 × 10
−6
5.9 × 10
−6
5.1 × 10
−6
4.3 × 10
−6
Source: See Ref. [10.3].
193
DOSE ASSESSMENT
— The geometrical relation between the source of the radiation and the target;
— The activity levels of the radionuclides in the environment;
— The materials in the environment and their shielding properties;
— The radionuclide-specific decay properties characterized by the radiation
type, the energies emitted and their emission probabilities; and
— The habitat and size of the organism.”
For estimating exposures to wildlife, models have been developed for
deriving dose conversion coefficients (DCCs) for a set of plants and animals.
Since it is impossible to consider all species of flora and fauna explicitly in dose
assessment, DCCs are provided for a set of reference organisms that were selected
within the FASSET and ERICA project to represent typical members of common
ecosystems (see Refs
[10.5–10.7]). The reference organisms considered here
are summarized in Table
10.5. For calculating DCCs for internal and external
exposures, the following simplifying assumptions were made:
(a) The radionuclides are uniformly distributed within the body and the
supporting media.
(b) The shape of all organisms is approximated by spheres and ellipsoids.
10.2.2. Dose conversion factors for flora and fauna
UNSCEAR
[10.4] reports that (see also Refs [10.8–10.11]):
“103. The exposure due to radionuclides incorporated into the organism
is determined by the activity concentrations in the organism, the size of
the organism, and the type and the energy of the emitted radiation. A key
quantity for estimating internal doses is the absorbed fraction of energy
ϕ(E), which is defined as the fraction of energy emitted by a radiation
source that is absorbed within the target tissue, organ or organism. In the
simplest case, the organism is assumed to be in an infinite homogeneous
medium and to have a uniform activity concentration throughout its body.
The densities of the medium and the organism’s body are assumed to be
identical. Under these conditions, both internal (D
int
) and external (D
ext
)
dose conversion coefficients (DCCS; the DCC is defined as either the
absorbed dose or the absorbed dose rate, according to the circumstances,
per unit activity concentration of the relevant radionuclide in the organism
or medium) for monoenergetic radiation can be expressed as a function of
the absorbed fraction…:
D
int
= E·ϕ(E) and D
ext
= E·(1−ϕ(E)) (4)”

194
CHAPTER 10
TABLE 10.5. REFERENCE ORGANISMS DEFINED IN FASSET/ERICA,
AND REFERENCE ANIMALS AND PLANTS AS DEFINED BY THE
INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION
ERICA reference organisms (examples)
ICRP reference
organisms
Habitat
Mass
(kg)
Terrestrial environment
Detritivorous invertebrates (woodlouse) n.a.
a
On and in soil 1.7 × 10
−4
Insects Bee In air 5.9 × 10
−4
Lichen and bryophytes (bryophyte) n.a.
a
On soil 1.1 × 10
−4
Gastropods (snail) n.a.
a
On soil 1.4 × 10
−3
Grasses and herbs Wild grass On soil 2.6 × 10
−3
Soil invertebrates (earthworm) Earthworm In soil 5.2 × 10
−3
Amphibians (frog) Frog On soil 0.031
Bird eggs Duck egg On soil 0.050
Burrowing mammals (rat) Rat In soil 0.31
Reptiles (snake) n.a.
a
On soil 0.74
Wading birds (duck) Duck On soil 1.3
Large mammals (deer) Deer On soil 250
Trees Pine tree On soil 470
Shrubs n.a.
a
On soil —
b
Marine environment
Phytoplankton n.a.
a
In water 6.5 × 10
−11
Zooplankton n.a.
a
In water 6.1 × 10
−5
Sea anemones/true corals n.a.
a
In water 1.8 × 10
−3
Macroalgae Brown seaweed In water 6.5 × 10
−3
Benthic molluscs n.a.
a
In water 1.6 × 10
−2
Polychaete worms n.a.
a
In water 1.7 × 10
−2
Vascular plants n.a.
a
In water 2.6 × 10
−2

195
DOSE ASSESSMENT
TABLE 10.5. REFERENCE ORGANISMS DEFINED IN FASSET/ERICA,
AND REFERENCE ANIMALS AND PLANTS AS DEFINED BY THE
INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION (cont.)
ERICA reference organisms (examples)
ICRP reference
organisms
Habitat
Mass
(kg)
Pelagic fish n.a.
a
In water 0.57
Crustaceans Crab In water 0.75
Benthic fish Flat fish In water 1.3
Reptiles (marine turtle) n.a.
a
In water 140
Mammals n.a.
a
In water 180
Sea anemones/true corals (colony) n.a.
a
In water 200
Wading birds Duck In water 1.3
Freshwater environment
Phytoplankton n.a.
a
In water 2.1 × 10
−12
Zooplankton n.a.
a
In water 2.4 × 10
−6
Crustaceans n.a.
a
In water 1.6 × 10
−5
Insect larvae n.a.
a
In water 1.8 × 10
−5
Vascular plants n.a.
a
In water 1.1 × 10
−3
Gastropods n.a.
a
In water 3.5 × 10
−3
Bivalve molluscs n.a.
a
In water 7.1 × 10
−2
Pelagic fish Salmonid/trout In water 1.3
Benthic fish n.a.
a
In water 1.5
Mammals n.a.
a
In water 3.9
Birds Duck In water 1.3
Amphibians Frog In water 3.1 × 10
−2
Source: See Ref. [10.7].
a
n.a.: not applicable.
b
—: data not available.
196
CHAPTER 10
Ulanovsky et al. [10.11] find that:
“The second equation is an approximation that — in a strict sense — only
holds if the organism and the surrounding medium are of the same density
and elemental composition.”
UNSCEAR
[10.4] continues:
“104. Absorbed fractions for photon and electron sources uniformly
distributed in soft-tissue spherical bodies immersed in an infinite
water medium have been systematically calculated by Monte Carlo
simulation
[Ref. [10.10]]. The calculations covered a particle energy range
of 10
keV to 5 MeV, a range for the mass of the body from 10
−6
to 10
3
kg….
…….
“109. The approach was also applied to the calculation of the absorbed
fractions for non-aquatic animals and their internal exposures. With the
use of the absorbed fractions for spheres and the suggested re-scaling and
interpolation techniques, a set of internal DCCs has been calculated for all
reference animals and plants [Ref. [10.10]].”
In Ulanovsky and Pröhl [10.12], DCCs for external exposure are calculated
separately for aquatic and terrestrial reference animals and plants. For aquatic
organisms, which are immersed by water, external exposure is calculated
according to equations above.
For terrestrial reference animals and plants, the estimation of external
exposures is more complex, since soil, air and organic matter differ considerably
in composition and density [10.11, 10.13]. Therefore, the derivation of DCCs is
based on radiation transport simulated for monoenergetic photons by means of
Monte Carlo techniques [10.11, 10.13]. Owing to the complexity of the processes
and the variability of life forms, reference geometries, as defined by energy,
contaminated media, and organism size and shape, are considered in detail by
Taranenko et al. [10.13]. External exposures under conditions for which specific
calculations have not been performed, can be estimated with sufficient accuracy
by interpolation. The DCCs for
210
Po are summarized in Table 10.6 for terrestrial
organisms, and in Tables 10.7 and 10.8 for aquatic organisms.
The values for internal exposure are given in units of dose rate per unit
activity in the organisms (μGy/h per Bq/kg). The values are given for absorbed
dose (i.e. radiation weighting factors to account for the effectiveness of the

197
DOSE ASSESSMENT
TABLE 10.6. DOSE CONVERSION COEFFICIENTS FOR TERRESTRIAL
REFERENCE ORGANISMS FOR
210
Po FOR INTERNAL AND EXTERNAL
EXPOSURE
Reference organism
(example)
Internal
exposure
External
exposure
DCC
(μGy/h per
Bq/kg)
f
1
(%)
f
2
(%)
f
3
(%)
In soil/
infinite
(μGy/h per
Bq/kg )
On soil/
planar
(μGy/h per
Bq/m
2
)
On soil/
volume
(μGy/h per
Bq/kg)
Woodlouse 3.1 × 10
−3
100 0 0 4.6 × 10
−9
2.9 × 10
−11
1.7 × 10
−9
Bees 3.1 × 10
−3
100 0 0 —
a
2.9 × 10
−11
1.7 × 10
−9
Terrestrial lichens 3.1 × 10
−3
100 0 0 —
a
1.5 × 10
−11
9.1 × 10
−10
Terrestrial
gastropods (snail)
3.1 × 10
−3
100 0 0 4.6 × 10
−9
2.9 × 10
−11
1.7 × 10
−9
Terrestrial grasses
(wild grass)
3.1 × 10
−3
100 0 0 —
a
6.5 × 10
−11
1.7 × 10
−9
Soil invertebrates
(earthworm)
3.1 × 10
−3
100 0 0 4.5 × 10
−9
—
a
—
a
Terrestrial
amphibians (frog)
3.1 × 10
−3
100 0 0 4.5 × 10
−9
2.9 × 10
−11
1.7 × 10
−9
Bird eggs (duck
egg)
3.1 × 10
−3
100 0 0 —
a
2.8 × 10
−11
1.7 × 10
−9
Terrestrial
burrowing
mammals (rat)
3.1 × 10
−3
100 0 0 4.3 × 10
−9
2.8 × 10
−11
1.7 × 10
−9
Terrestrial reptiles
(snake)
3.1 × 10
−3
100 0 0 4.1 × 10
−9
2.7 × 10
−11
1.6 × 10
−9
Large terrestrial
mammals (deer)
3.1 × 10
−3
100 0 0 —
a
1.4 × 10
−11
8.6 × 10
−10
Trees (pine tree) 3.1 × 10
−3
100 0 0 —
a
2.0 × 10
−11
1.4 × 10
−9
Terrestrial shrubs —
a
—
a
—
a
—
a
—
a
3.1 × 10
−11
1.6 × 10
−9
Terrestrial wading
birds (duck)
3.1 × 10
−3
100 0 0 —
a
2.6 × 10
−11
1.6 × 10
−9
2.3 × 10
−11
1.5 × 10
−9
Source: See Ref. [10.13].
a
—: data not available.

198
CHAPTER 10
different radiation types in causing effects to biota are not applied, since the
discussion on this topic is ongoing [10.14]).
To estimate the possible impact of different radiation qualities, the
contributions of the different types of radiation to the DCCs for internal exposure
are given separately in the tables. These contributions are quantified through the
factors f
1
, f
2
and f
3
, respectively, where f
1
specifies densely ionizing radiation
(alpha particles and spontaneous fission fragments), f
2
represents the fraction
of low energy beta radiation (E
< 10 keV), and f
3
gives the contributions of
higher energy beta radiation (E
> 10 keV) and gamma radiation of all energies.
TABLE 10.7. DOSE CONVERSION COEFFICIENTS FOR MARINE
REFERENCE ORGANISMS FOR
210
Po FOR INTERNAL AND EXTERNAL
EXPOSURE
Reference organism
(example)
Internal
exposure
External
exposure
DCC
(
μGy/h per
Bq/kg)
f
1
(%)
f
2
(%)
f
3
(%)
DCC
(
μGy/h
per Bq/L)
Marine phytoplankton
2.0
× 10
−7
100 0 0
3.1
× 10
−3
Zooplankton
3.1
× 10
−3
100 0 0
4.9
× 10
−9
Sea anemones/true corals
3.1
× 10
−3
100 0 0
4.8
× 10
−9
Macroalgae (brown seaweed)
3.1
× 10
−3
100 0 0
4.9
× 10
−9
Benthic molluscs
3.1
× 10
−3
100 0 0
4.7
× 10
−9
Vascular plants
3.1
× 10
−3
100 0 0
4.7
× 10
−9
Marine worms (polychaete worm)
3.1
× 10
−3
100 0 0
4.8
× 10
−9
Marine pelagic fish
3.1
× 10
−3
100 0 0
4.4 × 10
−9
Marine crustaceans (crab)
3.1
× 10
−3
100 0 0
4.3
× 10
−9
Marine benthic fish (flatfish)
3.1
× 10
−3
100 0 0
4.5
× 10
−9
Marine reptiles
3.1
× 10
−3
100 0 0
2.3
× 10
−9
Marine mammals (dolphin)
3.1
× 10
−3
100 0 0
2.2
× 10
−9
Source: See Ref. [10.13].

199
DOSE ASSESSMENT
Consequently, any user-defined radiation weighting factors w
r
can be applied to
these fractions to calculate radiation weighted DCCs:
wt
int int rr
D D wf=×
å
(10.1)
where D
int
represents the DCC as provided in Tables 10.6–10.8.
The discussion on the derivation of radiation weighting to be applied in
dosimetry for flora and fauna is still ongoing. Radiation weighting factors
for alpha radiation are reviewed in Ref.
[10.14]. For screening models being
applied to obtain conservative results, radiation weighting factors of 10 and 20
for deterministic and stochastic end points, respectively, were considered to be
TABLE 10.8. DOSE CONVERSION COEFFICIENTS FOR FRESHWATER
TERRESTRIAL REFERENCE ORGANISMS FOR
210
Po FOR INTERNAL
AND EXTERNAL EXPOSURE
Reference organism
(example)
Internal
exposure
External
exposure
DCC
(
μGy/h per
Bq/kg)
f
1
(%)
f
2
(%)
f
3
(%)
DCC
(
μGy/h
per Bq/L)
Freshwater phytoplankton
6.3
× 10
−9
100 0 0
3.1
× 10
−3
Freshwater zooplankton
3.1
× 10
−3
100 0 0
4.9
× 10
−9
Freshwater crustaceans
3.1
× 10
−3
100 0 0
4.9
× 10
−9
Freshwater insect larvae
3.1
× 10
−3
100 0 0
4.9
× 10
−9
Freshwater plants (vascular plant)
3.1
× 10
−3
100 0 0
4.9
× 10
−9
Freshwater gastropods
3.1
× 10
−3
100 0 0
4.8
× 10
−9
Freshwater molluscs (bivalve mollusc)
3.1
× 10
−3
100 0 0
4.6
× 10
−9
Freshwater pelagic sh (trout)
3.1
× 10
−3
100 0 0
4.3 × 10
−9
Freshwater benthic sh
3.1
× 10
−3
100 0 0
4.3
× 10
−9
Freshwater mammals (muskrat)
3.1
× 10
−3
100 0 0
3.9
× 10
−9
Amphibians (frog)
3.1
× 10
−3
100 0 0
4.7
× 10
−9
Wading birds (duck)
3.1
× 10
−3
100 0 0
4.2
× 10
−9
Source: See Ref. [10.13].
200
CHAPTER 10
appropriate to obtain conservative results. To evaluate radiological impacts on
flora and fauna, deterministic end points are relevant [10.7].
Values for external exposures for aquatic organisms are given for a total
immersion in water in units of μGy/h per Bq/L. For terrestrial organisms, various
different source geometries are considered, depending on the habitat of the
organism considered
[10.13]:
(a) For on or above soil organisms, two geometries are considered:
— A planar source, the source being at a depth of 0.3 cm to account for
surface roughness (μGy/h per Bq/m
2
);
— A 10 cm thick volume source (soil density: 1.6 g/cm
3
)(μGy/h per
Bq/kg).
(b) For in soil organisms, the values are given for organisms living in the
middle of a volume source with a thickness of 50
cm (soil density:
1.6 g/cm
3
) (μGy/h per Bq/kg).
The decay properties are reflected in the contributions of alpha, beta and
gamma radiation as quantified in Tables 10.6–10.8 by f
1
, f
2
and f
3
, respectively.
Only alpha radiation contributes to the internal exposure. Due to the short
range of alpha particles, external exposure due to
210
Po in the environment is
negligible [10.13]. Only very small organisms, with dimensions in the order of
the range of alpha particles (ca. 100 μm), can be exposed externally [10.13]. The
short range of alpha particles means that all energy emitted is absorbed locally.
Therefore, the DCCs for internal exposure are the same for all organisms, with
the exception of very small organisms, with dimensions that are in the order of
the range of alpha particles [10.13].
10.2.3. Non-homogeneous distribution
The DCCs for internal absorbed dose rate are calculated assuming a
homogeneous distribution of radionuclides within the body
[10.15]. However,
this is often not the case: for example, iodine accumulates in the thyroid,
polonium in the liver and transuranic elements in the kidney. The influence of
inhomogeneous distributions on the average whole body dose is investigated by
Gómez-Ros et al. [10.15]. In the case of
210
Po, for which only alpha radiation is
relevant for internal exposure, the exposure due to
210
Po in tissues and organs is
directly proportional to the
210
Po activity in the organ.
When a radionuclide accumulates in a specific organ, the dose will be
higher than the whole body average. The dose in the specific organ is enhanced
by a factor that can approximately be estimated from the ratio of whole body
mass to organ mass.
201
DOSE ASSESSMENT
10.2.4. Application of dose conversion coefficients
The DCCs can be used to estimate external and internal exposures for
organisms living in different habitats. The total dose is calculated as the sum of
external and internal exposure. The internal exposure of a terrestrial or aquatic
organism (D
int
) is estimated from the activity concentration in soil or water
(C
s
, C
w
), concentration ratios (CR
soil-organism
, CR
water-organism
) and the DCC for
internal exposure according to:
Terrestrial organisms: D
int
= C
s
·CR
soil-organism
·DCC
int
(10.2)
Aquatic organisms: D
int
= C
w
·CR
water-organism
·DCC
int
(10.3)
Dose rates can also be estimated directly from measured radionuclide
concentrations in biota. External exposures are directly estimated from the
activity in relevant media and the appropriate DCC. Some organisms move
between different habitats owing to their lifestyle or in different stages of
development. This effect can be accounted for through a simple superposition of
the DCCs for isolated habitats.
Ulanovsky and Pröhl [10.12] provide a simple example: an aquatic
benthic organism is considered that lives on the interface of water and sediment.
Assuming that sediment density and composition are close to those of water, and
that the radionuclide concentrations in water C
w
and sediment C
s
are known, the
external dose to such an organism can be calculated according to:
D
ext
= (0.5C
w
+ 0.5C
p
) DCC
ext
(10.4)
where D
ext
is the DCC for the radionuclide in water. The factor of 0.5 is to account
for the habitat at the interface of water and sediment; the organism is exposed to
50% of the radionuclides in sediments and water, respectively.
A more complex exposure has to be considered, for example, for a wading
bird, such as a duck, that lives in three different habitats: on soil, above soil and
on the water surface
[10.12]. For assessing the total external exposure of a duck,
the time spent in these three habitats has to be known.
202
CHAPTER 10
REFERENCES TO CHAPTER 10
[10.1] INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION,
Age-dependent Doses to Members of the Public from Intake of Radionuclides —
Part
2: Ingestion Dose Coefficients, Publication 67, Pergamon Press, Oxford and
New York
(1992).
[10.2] INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION,
Age-dependent Doses to Members of the Public from Intake of Radionuclides —
Part
5: Compilation of Ingestion and Inhalation Coefficients, Publication 72,
Pergamon Press, Oxford and New York
(1995).
[10.3] INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION,
Age-dependent Doses to Members of the Public from Intake of Radionuclides —
Part
4: Inhalation Dose Coefficients, Publication 71, Pergamon Press, Oxford and
New York
(1995).
[10.4] UNITED NATIONS SCIENTIFIC COMMITTEE ON THE EFFECTS OF ATOMIC
RADIATION, Sources and Effects of Ionizing Radiation, UNSCEAR 2008 Report to
the General Assembly, with Scientific Annexes, Vol. II: Effects, United Nations,
New York (2008) Annex E.
[10.5] LARSSON, C.M., An overview of the ERICA Integrated Approach to the assessment
and management of environmental risks from ionising contaminants, J. Environ.
Radioact.
99 (2008) 1364–1370.
[10.6] BROWN, J.E., et al., The ERICA Tool, J. Environ. Radioact. 99 (2008) 1371–1383.
[10.7] INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION,
Environmental Protection: The Concept and Use of Reference Animals and Plants,
Publication
108, Amsterdam, Elsevier (2008).
[10.8] NATIONAL COUNCIL ON RADIATION PROTECTION AND MEASUREMENTS,
Effects of Ionizing Radiation on Aquatic Organisms, NCRP Report No.
109, NCRP,
Bethesda, MD
(1991).
[10.9] VIVES i BATLLE, J., JONES, S.R., GÓMEZ-ROS, J.M., A method for calculation
of dose per unit concentration values for aquatic biota, J. Radiol. Prot.
24
(2004)
A13–A34.
[10.10] ULANOVSKY, A., PRÖHL, G., A practical method for assessment of dose
conversion coefficients for aquatic biota, Radiat. Environ. Biophys.
45
(2006)
203–214.
[10.11] ULANOVSKY, A., PRÖHL, G., GÓMEZ-ROS, J.M., Methods for calculating dose
conversion coefficients for terrestrial and aquatic biota, J. Environ. Radioact. 99
(2008) 1440–1448.
[10.12] ULANOVSKY, A., PRÖHL, G., Tables of dose conversion coefficients for estimating
internal and external radiation exposures to terrestrial and aquatic biota, Radiat.
Environ. Biophys. 47 (2008) 195–203.
[10.13] TARANENKO, V., PRÖHL, G., GÓMEZ-ROS, J.M., Absorbed dose rate conversion
coefficients for reference terrestrial biota for external photon and internal exposures,
J. Radiol. Prot. 24 (2004) A35–A62.
203
DOSE ASSESSMENT
[10.14] CHAMBERS, D.B., OSBORNE, R.V., GARVA, A.L., Choosing an alpha radiation
weighting factor for doses to non-human biota, J. Environ. Radioact.
87 (2006) 1–14.
[10.15] GÓMEZ-ROS, J.M., PRÖHL, G., ULANOVSKY, A., LIS, M., Uncertainties of
internal dose assessment for animals and plants due to non-homogeneously
distributed radionuclides, J. Environ. Radioact.
99 (2008) 1449–1455.
205
Appendix I
GEOCHRONOLOGICAL APPLICATIONS
OF POLONIUM
I.1. INTRODUCTION
The systematics of the decay of
210
Pb to
210
Po (through the decay of
210
Bi)
can be used to determine ages and residence times over several half-lives of
210
Po, in the order of approximately 500 days. This can be applied to situations in
which
210
Po is strongly fractionated from
210
Pb so that substantial disequilibrium
is generated.
I.2. DATING VOLCANIC ROCKS
There have only been a few examples of dating using
210
Po. The method
was first developed for, and mostly applied to, volcanic rocks, especially
submarine basalts where volcanic events are not so readily observed. In a rock
that forms incorporating
210
Pb and
210
Po in disequilibrium, the
210
Po will evolve
according to the following equation [I.1]:
( ) ( ) ( )
( )
234 234
Po Po
210 210 210
0
Po Po e Pb 1 e
tt
ll--
= +-
(I.1)
For time periods substantially longer than the half-life of
210
Bi, the
ingrowth of this intermediate nuclide can be ignored. Samples of recent volcanics
are analysed for (
210
Po) soon after sample collection, and subsequent analyses
can be used to determine
210
Pb. An age can then be determined once an estimate
of (
210
Po)
0
has been made. This method was first adapted by Rubin et al. [I.1] to
determine the age of mid-ocean ridge basalts from a volcanically active region.
The method relies on disequilibrium within the basalt at the time of solidification
and no subsequent gains or losses of
210
Pb or
210
Po. Since
210
Po can be completely
volatilized at temperatures over 400°C, it might be expected that the samples
completely lose
210
Po during eruption. However, the retention of some
210
Po
cannot be discounted due to incomplete degassing as basalts are quickly cooled,
especially under increased pressures at large ocean depths.
Rubin et al. [I.1] measure the (
210
Po) in each sample over two years
after collection (see Fig. I.1). The simple observation of
210
Po ingrowth
demonstrates that the samples erupted within approximately two years. Through

206
APPENDIX I
repeated analyses of each sample, best-fit ingrowth curves can be determined
which defined the maximum value of (
210
Po) (equal to (
210
Pb)), and when
(
210
Po) = 0. This approach puts a greater constraint on
210
Po ingrowth than
a simple determination of (
210
Pb) once secular equilibrium is achieved. While
the time of eruption is constrained between when (
210
Po) = 0 and the time of
collection, the actual eruption time could be between these if the basalt did not
completely lose all
210
Po and (
210
Po)
0
> 0. The lowest measured value of (
210
Po)
is approximately 0.25 and represents the highest possible initial value. Age
windows, corresponding to (
210
Po)
0
= 0–0.25, of up to several months are then
obtained [I.1]. Rubin et al. [I.2] find that
210
Po–
210
Pb dates of fresh basalts at a
ridge section are consistent with eruption during recent recorded seismic events,
and Johnson et al. [I.3] find the age of a seamount sample to be approximately
five months. Basalt from Samoa is assumed to have completely lost
210
Po during
subaerial eruption, and an age of around 260 days was determined [I.4].
REFERENCES TO APPENDIX I
[I.1] RUBIN, K.H., MACDOUGALL, J.D., PERFIT, M.R.,
210
Po–
210
Pb dating of recent
volcanic eruptions on the sea floor, Nature 368 (1994) 841–844.
[I.2] RUBIN, K.H., SMITH, M.C., PERFIT, M.R., CHRISTIE, D.M., SACKS, L.F.,
Geochronology and geochemistry of lavas from the 1996 North Gorda Ridge eruption,
Deep Sea Res. Part II 45 (1998) 2571–2597.
[I.3] JOHNSON, K.T.M., et al., Boomerang Seamount: The active expression of the
Amsterdam–St. Paul hotspot, Southeast Indian Ridge, Earth Planet. Sci. Lett. 183
(2000) 245–259.
Source: Figure 1 of Ref. [I.1].
FIG.
I.1. Polonium-210 activities per gram in two samples of mid-ocean ridge basalts from
the East Pacific Rise (reproduced with permission courtesy of Nature).
207
GEOCHRONOLOGICAL APPLICATIONS
[I.4] SIMS, K.W.W., et al.,
238
U–
230
Th–
226
Ra–
210
Pb–
210
Po,
232
Th–
228
Ra, and
235
U–
231
Pa
constraints on the ages and petrogenesis of Vailulu’u and Malumalu Lavas, Samoa,
Geochem. Geophys. Geosyst. 9 (2008).
209
Appendix II
RADIOCAESIUM,
210
Po AND
210
Pb IN WOLV ES
II.1. INTRODUCTION
Since the Chernobyl accident,
137
Cs (T
1/2
= 30.07 years) activity deposition
in Sweden has varied in the range of 1000–100 000 Bq/m
2
, and more than half
of this activity has now decayed. The isotope
210
Po (T
1/2
= 138 days) originates
from
222
Rn exhaled from the ground and its intermediate
210
Pb. The more even
terrestrial distribution of
210
Po depends on the uranium content of the ground,
the topography of the surrounding land mass and the amount of wet deposition.
Persson [II.1] estimated the annual deposition of
210
Pb in central Sweden in
1970 to be around 63
Bq/m
2
. The integrated activity of
210
Pb, supposing that the
same amount is decaying as is introduced by precipitation, gives 2000
Bq/m
2
.
The
210
Po:
210
Pb activity ratio in precipitation is around 0.1–0.2. The integrated
deposition is then slightly lower for
210
Po than for
210
Pb, since it takes about two
years for
210
Po to come into radioactive equilibrium with
210
Pb.
Polonium-210 and
137
Cs can accumulate in the food chain, and especially in
reindeer, which can contain enhanced concentrations in soft tissue after feeding
on lichen during the winter. A study by Gjelsvik et al. [II.2] published in 2014
measured activity levels of
137
Cs,
210
Po, and the
210
Po:
210
Pb ratio in different
tissues of 28 wolves in Sweden to determine the aggregated transfer factor (TF)
and to calculate doses to the animals.
II.2. MATERIALS AND METHODS
Gjelsvik et al. [II.2] examined samples of muscle, blood, liver and kidney
28 wolves killed in Sweden in 2009 and 2010 (see Table II.1). The samples for
analysis were prepared by drying and grinding, and
137
Cs was measured with
high purity germanium gamma spectrometry. The
210
Po activity was determined
with alpha spectrometry (ion implanted silicon detectors) after wet ashing and
spontaneous deposition onto silver discs. The radiochemical yield determinant
was
209
Po; and
210
Pb was analysed with dry incineration of a larger amount where
Po evaporates. After six months, the buildup
210
Po from
210
Pb was analysed.
The ingrowth rate gives the contribution of
210
Pb, and the
137
Cs and
210
Po
concentrations were decay corrected to date of death.

210
APPENDIX II
TABLE II.1. DATA FOR SAMPLED WOLVES
Wolf County Community Sex Weight (kg) Age (years)
1 Jämtland Härjedalen F 32.1 1
2 Jämtland Härjedalen M 34.9 1
3 Dalarna Malung M 40 0
4 Dalarna Malung M 41 1
5 Dalarna Malung F 28 0
6 Dalarna Malung F 29 0
7 Örebro Degerfors M 48 1
8 Norrbotten Gällivare M 47 1
9 Jämtland Härjedalen F 32.6 1
10 Gävleborg Ockelbo M 46.5 1
11 Gävleborg Ockelbo F 34 1
12 Dalarna Malung-Sälen M 47 6
13 Dalarna Ludvika M 28 0
14 Dalarna Ludvika F 36 4
15 Dalarna Vansbro M 38 2
16 Dalarna Ludvika M —
a
8
17 Dalarna Ludvika F 36 1
18 Gävleborg Ljusdal M 49 6
19 Västra Götaland Mellerud M 49.5 3
20 Värmland Munkfors F 40 1
21 Värmland Sunne M 32
0
22 Värmland Eda M 28 0
23 Örebro Örebro F 37.5 1
24 Värmland Arvika F 38 3
25 Örebro Lindesberg F 29 0
26 Värmland Torsby M 48 1
27 Värmland Sunne M 40 5
28 Västra Götaland Dals-Ed F 38 5
Source: Table 1 of Ref. [II.2].
a
—:data not available.
211
RADIOCAESIUM,
210
Po AND
210
Pb IN WOLVES
II.3. RESULTS AND DISCUSSION
The activity concentrations of
137
Cs and
210
Po in different tissues from the
wolves are Table
II.2. The information comprises summaries from Refs [II.2–II.4].
After uptake, polonium mainly accumulates in the liver and kidneys,
while caesium is more homogenously distributed in the body (but is lower in
bone). Food is the major intake pathway for both caesium and polonium. For
210
Po, there is also in vivo buildup from
210
Pb. Reindeer have enhanced levels
of both
137
Cs and
210
Po, especially after winter due to the consumption of lichen.
However, during the summer, 74% of the wolf diet consists of moose. The
aggregated TF for
210
Po shows large variations (0.000 5–0.27) depending on the
organ (see Table II.2). Gjelsvik et al. [II.2] report that:
“The bio-kinetics of polonium in the body is rather complicated. The
fractional uptake by man has been reported from 0.1 to 0.8. The biological
residence time has been reported to be between 20 and 80
days [Ref. [II.5]].
Thomas et al. [Ref. [II.3]] have studied the transfer of
210
Po and
210
Pb
through the lichen–caribou–wolf food chain of northern Canada where
caribou was the main food item for the wolves. Their results are in good
agreement with our data. Thomas et al.
[Ref. [II.3]] reported
210
Po/
210
Pb
activity ratios of 30–56 for liver and kidney. Fuller
[Ref. [II.6]] estimated
that the average daily intake was 2.7 kg per day when eating caribou and
5.4
kg per day when eating moose.”
The fresh weight activity concentrations are: 7.5–13
Bq/kg in muscle;
145–369
Bq/kg in the liver; 142–343 Bq/kg in the kidneys; and 20–29 Bq/kg
in bone. The lower values are from a region with less
210
Po in lichen and is thus
reflected in the food chain. Gjelsvik et al. [II.2] continue that:
“Howard et al. [Ref. [II.7]] estimated the transfer coefficient F
f
(d kg
−1
)
of
210
Po from caribou to wolves as the ratio of activity concentration in
the muscle of wolf (Bq kg
−1
w.w.) divided by the daily amount intake of
caribou meat (kg w.w. d
−1
) with the activity concentration of
210
Po in the
quantity Bq kg
−1
w.w. which gave a value of 0.089 d kg
−1
w.w.”
Using the same coefficient for the Swedish wolves, the food
would contain 8–170
Bq/kg (DW). The caribou from Canada contained
30–50
Bq/kg (DW) in muscle, 500–1300 Bq/kg (DW) in the kidneys and
550–1400
Bq/kg (DW) in the liver, depending on the region [II.3]. In the muscle
of moose from Finland, sampled in March, the concentrations were 25
Bq/kg
and in reindeer, 45
Bq/kg [II.4]. Persson [II.8] reported in 1972 concentrations

212
APPENDIX II
TABLE II.2. SUMMARY OF DATA ON
137
Cs,
210
Po AND
210
Pb IN WOLVES
Matrix
137
Cs
(Bq/kg)
210
Po
(Bq/kg)
210
Po:
210
Pb Remarks Ref.
Blood
a
—
b
17 19 Canada, Snowdrift, 1993 [II.3]
4–959 2.2–54 2.3–54 Sweden, 2010–2011 [II.2]
Bone
c
—
b
4.2–25 0.29–0.7 Canada, Baker Lake, 1993 [II.3]
—
b
20 0.6 Canada, Snowdrift, 1993 [II.3]
—
b
4.8 0.26 E. Finland, 1968 [II.4]
—
b
11–67 0.29–0.7 N. Finland, 1968 [II.4]
Liver
d
—
b
1 400 58 Canada, Baker Lake, 1993 [II.3]
—
b
551 30 Canada, Snowdrift, 1993 [II.3]
—
b
6.8 22 E. Finland, 1968 [II.4]
—
b
49–310 26–115 N. Finland, 1968 [II.4]
36–4 050 20–523 9.4–56 Sweden, 2010–2011 [II.2]
Kidney
d
—
b
1 300 58 Canada, Baker Lake, 1993 [II.3]
—
b
539 28 Canada, Snowdrift, 1993 [II.3]
31–3 453 24–942 1.8–7.9 Sweden, 2010–2011 [II.2]
Muscle
d
—
b
49 —
b
Canada, Baker Lake, 1993 [II.3]
—
b
29 —
b
Canada, Snowdrift, 1993 [II.3]
350 1.2 57 E. Finland, 1968 [II.4]
270–7 000 0.3–1.0 30–80 N. Finland, 1968 [II.4]
70–8 410 1–43 2–77 Sweden, 2010–2011 [II.2]
Aggregated transfer factor (Po·kg
−1
·m
−1
) Ref.
Liver 0.012–0.23
[II.2]
Kidney 0.001–0.27
Muscle
0.000 5–0.021
0.01–0.26
Source: Adapted from Ref. [II.2].
a
Bq/L.
b
—: data not available.
c
Bq/kg, fresh weight.
d
Bq/kg,
dry weight.
213
RADIOCAESIUM,
210
Po AND
210
Pb IN WOLVES
in the muscle of Swedish reindeer as 16–48 Bq/kg, 28–220 Bq/kg in the kidneys
and 88–148
Bq/kg in the liver. Kauranen et al. [II.4] observed in 1971 lower
concentrations in wolves, lynx and wolverine in southern Finland compared to
northern Finland
[II.2].
The gastrointestinal uptake of
210
Po is higher than
210
Pb. Consequently, the
210
Po:
210
Pb activity ratio increases with level in the food chain. In lichen, the ratio
is slightly less than 1, and in reindeer about 5 in the liver and 20 in muscle
[II.8].
Thomas et al.
[II.3] find
210
Po:
210
Pb activity ratios of 28–58 for the liver and
kidneys of wolves, which is in fair agreement with the the data in Ref. [II.2].
In the south of Sweden, moose is the major food item for wolves. The
activity concentration is generally higher in reindeer than in moose but in the
north the concentrations do not differ much. However, the data do not support the
tendency for higher concentrations in northern Sweden in contrast to the results
in Ref.
[II.4] for Finland.
Rather high concentrations of
137
Cs are found in areas with high deposition
from the Chernobyl accident, such as in Gävleborg county. However, wolves
move long distances to find their prey.
Gjelsvik et al. [II.2] report that the activity ratio of
210
Po:
137
Cs in liver is
in the range of 0.011–4.0, in muscle 0.002–0.05 and in kidneys 0.012–2.1. This
differential bio-distribution shows the much larger accumulation of
210
Po in
the liver and kidneys relative to
137
Cs. The large spread in the data is due to the
variability in
137
Cs deposition in different areas and also reflects the range over
which wolves travel to obtain prey.
The dose conversion factors in humans for
137
Cs and
210
Po are
1.2
× 10
−8
Sv/Bq and 7 × 10
−7
Sv/Bq, respectively. This is for a person with
a body weight of 70 kg and using a quality factor of 20 for
210
Po. The dose is
not applicable to animals, given the lack of specific tissue weighting factors for
non-human biota, and only the absorbed dose in grays can be calculated. The
absorbed dose depends on the biological residence time, organ distribution and,
for
137
Cs, the body weight as well (a substantial fraction of the gamma energy
from 662 keV photons escapes from the body depending on body mass). Gjelsvik
et al. [II.2] report that:
“The biological half-time of caesium in the human body is about 70 days
but is supposed to be shorter for wolves both for caesium and polonium.
Under an equilibrium situation doses can be calculated even if the biological
half-time is not known but this is not possible under a dynamic situation.
We suppose a stable condition and 35
days half-life. The maximal dose
from
137
Cs to a wolf would be 3000 μGy per year…. The maximal dose
from
210
Po to liver would be 2600 μGy per year.”
214
APPENDIX II
For estimating the aggregated TFs for
137
Cs, charts of known deposition in
Sweden from the Chernobyl accident should be used. Estimates of the aggregated
TF can be as high as 0.1 but with large variations.
II.4. SUMMARY
There are significantly high activity concentrations of caesium and
polonium in wolves, and the concentrations are higher in areas where reindeer
are a significant part of the diet. Wolves are believed to prefer organs such as
the liver and kidneys, which contain higher quantities of polonium. However,
the proportion of different organs eaten is less important for
137
Cs, since it is
more homogenously distributed in the body. A high Po:Cs ratio might indicate
that moose is a major food source. Particularly high activity concentrations of
137
Cs are found in tissues from wolves from areas with high fallout from the
Chernobyl accident. Further studies of wolves from the summer season could
yield important information on food habits.
REFERENCES TO APPENDIX II
[II.1] PERSSON, R.B.R.,
55
Fe,
90
Sr,
134
Cs,
137
Cs, and
210
Po in the Biosphere: Radiological
Health Aspects of the Environmental Contamination from Radioactive Materials in
Northern Sweden, PhD Thesis, Lund Univ.
(1970).
[II.2] GJELSVIK, R., HOLM, E., KÅLÅS, J.A., PERSSON, B., ÅSBRINK, J.,
Polonium-210 and Caesium-137 in lynx (Lynx lynx), wolverine (Gulo gulo) and wolves
(Canis lupus), J. Environ. Radioact.
138 (2014) 402–409.
[II.3] THOMAS, P.A., SHEARD, J.W., SWANSON, S., Transfer of
210
Po and
210
Pb through
the lichen–caribou–wolf food chain for northern Canada, Health Phys.
66
(1994)
666–677.
[II.4] KAURANEN, P., MIETTINEN, J.K., PULLIANINEN, E., Polonium-210 and
lead-210 in some terrestrial animals in Finland, Ann. Zool. Fenn. 8 (1971) 318–323.
[II.5] HENRICSSON, C.F., RANEBO, Y., HANSSON, M., RÄÄF, C.L., HOLM, E., A
biokinetic study of
209
Po in man, Sci. Total Environ. 437 (2012) 384–389.
[II.6] FULLER, T.K., Population dynamics of wolves in north-central Minnesota, Wildl.
Monogr. 105 (1989) 1–41.
[II.7] HOWARD, B.J., BERESFORD, N.A., HOVE, K., Transfer of radiocesium to
ruminants in natural and semi-natural ecosystems and appropriate countermeasures,
Health Phys. 61 (1991) 715–725.
[II.8] PERSSON, B.R., “Lead-210, polonium-210 and stable lead in the food-chain lichen,
reindeer and man”, The Natural Radiation Environment
II (Proc. 2nd Int. Symp.
Houston, 1972) (ADAMS, J.A.S., LOWDER, W.M., GESELL, T.F., Eds), United
States Department of Commerce, Springfield, VA (1972)
347–367.
215
Appendix III
210
Po IN MARINE MAMMALS
III.1. INTRODUCTION
Marine mammals encompass 125
species in 20 families that merge in
5
different mammalian groups [III.1]:
(a) Cetaceans (whales, dolphins and porpoises);
(b) Sirenians (manatees and dugongs);
(c) Pinnipeds (sea lions, walruses and seals);
(d) Marine and sea otters;
(e) Polar bears.
These marine mammalian groups are more closely related to one or another
terrestrial mammalian group (e.g. horses, bears or elephants) than they are to
each other. However, all marine mammals have one thing in common: they derive
virtually all of their food from the marine environment
[III.1–III.3]. Jefferson et
al. [III.1] report that:
“Marine mammals are not randomly distributed in the world’s oceans.
It has long been known, for example, that certain species are found
exclusively or primarily in waters of a particular depth, temperature range,
or oceanographic regime, and not in areas lacking one or all of these
characteristics. For most species, however, little is known of the particular
factors that cause them to be found in one area and not in another that
appears, qualitatively at least, the same.
“One major factor affecting productivity, and thus indirectly influencing the
distribution of marine mammals, is the pattern of major ocean currents.
…….
“Wherever oceanic conditions promote high nutrient content, it is likely that
some species of marine mammal will be present to exploit that richness.
Thus, the presence of marine mammals and other high order predators and
consumers in an area is related primarily to prey, and secondarily to the
water conditions supporting that productivity.”
216
APPENDIX III
Mammals do not substantially absorb chemical elements, including radionuclides,
directly from sea water, as do fish through the gill epithelium. The mammalian
diet is exclusively based on common marine species, and the large majority of
radionuclide intake takes place through ingestion and inhalation.
III.2. MARINE MAMMAL FOOD CHAINS
In general, marine mammals are at the top of marine food chains, for
example: the blue whale, the largest animal, feeds on zooplankton (krill); the
sperm whale feeds on large size pelagic cephalopods and deep-sea sharks; the
dolphins of coastal seas (Delphinus delphis and other Delphinidae) feed on
common coastal fish species such as Clupeidae and Carangidae fish; sea otters
and seals feed on crustaceans, bivalves and gastropods; and the killer whale
feeds on sea otters and penguins. In their environments, marine mammals occupy
different trophic levels (planktivorous, piscivores and carnivores) and often
occupy the top position in their food webs, but marine mammals may, in turn,
have humans, sharks and bears, as well as larger cetaceans and pinnipeds as
predators
[III.3, III.4].
Owing to their position in marine food webs and following international
conventions on enhancing the protection and conservation of marine
mammals, studies have been conducted on several species to investigate
contamination by pesticide residues, polychlorinated biphenyls, heavy metals
and other toxicants, as well as on reproductive success and health impairment
(e.g. Refs [III.5, III.6]). Several reports have also covered the accumulation of
radionuclides in marine mammals. The radiosensitivity of marine mammals is
often comparable to that of terrestrial mammals. Thus knowledge on food chain
transfer and radionuclide absorption into internal organs can be used as a proxy
for radionuclide accumulation and radiation effects in other mammals, including
humans [III.7–III.9].
III.3. BIOACCUMULATION AND BIODISTRIBUTION
Although there have been limited studies on radioactivity in marine
mammals, current knowledge includes data on
210
Po activity concentrations
for some species as well as computed whole body radiation doses from from
210
Po and several other natural and artificial radionuclides [III.10–III.13]. Most
210
Po analyses on marine mammals have been performed on samples from beach
stranded animals or on samples from by-catch netted in tuna fishing gear, which
explains the scattered nature of data, both in species and geography.
217
210
Po IN MARINE MAMMALS
In 1974, Cherry and Shannon [III.14] published a review of the few results
for
210
Po reported. Since then, more data have been published ,but they do not yet
encompass most marine mammal families and, furthermore, many reports focus
on determination of artificial radionuclides. This is the case, for example, for
reports on:
137
Cs and
239+240
Pu in harbour porpoises and seals with regard to local
radioactive waste discharges from Sellafield, the United Kingdom; temporal
trends of global radioactive fallout from nuclear weapon tests in seals from Lake
Baikal, the Russian Federation; and
137
Cs geographical distribution in oceans and
specific coastal areas (such as Antarctic seas) determined in dolphins, porpoises
and seals (see Refs [III.10, III.12, III.15–III.20]).
Less attention has been given to measuring naturally occurring radionuclides
in marine mammals, with the exception of
40
K, which has often been used to
account for the natural radiation background and for comparison with internal
radiation dose received from artificial radionuclides [III.10, III.13, III.18].
However, current concerns regarding radiation protection of non-human biota and
the definition of reference species for environmental radiological risk assessment
has tilted radioecologists’ research interests to increasing knowledge about the
actual radiation dose received by biota [III.7–III.9, III.21–III.23]. Furthermore,
it has been recognized that, in marine organisms at least, the radiation dose
from internally deposited alpha emitters, such as
210
Po, is much larger than that
from internally deposited gamma emitters, such as
137
Cs and other artificial
radionuclides [III.24, III.25].
The results on
210
Po in internal tissues of marine mammals show large
inter-individual variation. In the same species, and for individuals collected in
the same season and same area,
210
Po concentrations in muscle tissues vary over
relatively wide ranges. For example, in the common dolphin of the north-eastern
Atlantic Ocean,
210
Po concentrations (FW) are in the range of 9–87 Bq/kg,
averaging 56 Bq/kg [III.13]. A wide activity concentration (FW) range of
48–102 Bq/kg is also found in the South Atlantic Franciscana dolphin, averaging
66.1 Bq/kg [III.26]. Of all marine mammals analysed so far,
210
Po concentrations
in muscle fall in the range of 1–102 Bq/kg (FW) (see Table III.1).
Polonium-210 concentrations (FW) in other organs are higher than in
muscle, averaging 123
Bq/kg in the liver and 110 Bq/kg in the kidneys of the
north-eastern Atlantic common dolphin
[III.13], and are even higher in the
liver of the South Atlantic Franciscana dolphin, averaging 315
Bq/kg [III.26]
(see Table III.1).
Analysis of the data indicates that in each marine mammal species, the
highest
210
Po concentrations are consistently in the liver and kidneys, followed
by muscle. Lower concentrations are measured in mammary glands, gonads,
bone and fat tissue (see Table
III.1). In each species, the liver to muscle
210
Po
concentration ratio is always much higher than unity and among individual

218
APPENDIX III
TABLE III.1. ACTIVITY CONCENTRATIONS OF
210
Po IN MARINE
MAMMALS
Region Species
210
Po
(Bq/kg, FW)
Ref.
NE Atlantic
Common dolphin
Muscle
Liver
Kidney
Gonad
Fat tissue
Bone
Mammary gland
56 ± 32
123 ± 42
110 ± 49
11 ± 2
11 ± 11
4.63 ± 0.12
29.5 ± 0.9
[III.13]
Striped dolphin
Muscle
Mammary gland
42.0 ± 0.8
8.2 ± 0.2
[III.13]
Pilot whale
Muscle
Fat tissue
13.7 ± 0.3
0.70 ± 0.06
[III.13]
North Atlantic
Sperm whale
Muscle 5.0 ± 0.2
[III.25]
South Atlantic
Franciscana dolphin
Muscle
Liver
66.1 ± 18.9
315 ± 168
[III.26]
Guiana dolphin
Muscle
Liver
24.6 ± 17.2
243 ± 213
[III.26]
Clymene dolphin
Muscle 15.7 ± 1.1
[III.26]
Atlantic spotted dolphin
Muscle
Liver
37.1 ± 36.4
125.7 ± 6.6
[III.26]
Bottlenose dolphin
Muscle
Liver
5.8 ± 6.3
180.5 ± 244.0
[III.26]

219
210
Po IN MARINE MAMMALS
TABLE III.1. ACTIVITY CONCENTRATIONS OF
210
Po IN MARINE
MAMMALS (cont.)
Region Species
210
Po
(Bq/kg, FW)
Ref.
South Atlantic
Common dolphin
Muscle
Liver
9.34 ± 0.7
54.3 ± 3.2
[III.26]
Common dolphin
a
Muscle
Liver
Kidney
Gonad
Fat tissue
Bone
86 ± 2
122 ± 0.4
180 ± 71
3.6 ± 0.5
6.4 ± 0.6
26 ± 0.5
[III.27]
Whale
a
Muscle
Liver
Kidney
Gonad
Bone
1.1 ± 0.03
18 ± 0.3
43.1 ± 0.9
1.8 ± 0.1
2.5 ± 0.2
[III.27]
Pacific
Common dolphin
Muscle 4.5
[III.28]
Sperm whale
Muscle 23.3 ± 0.4
[III.29]
Atlantic
Fin whale
Muscle
Kidney
Fat tissue
1.9 ± 0.1
9.07 ± 0.30
1.3 ± 0.1
[III.29]
Arctic
Ringed seal
Muscle
Liver
Kidney
20.9
98.7
82.7
[III.11]
Bering Sea
Pacific walrus
Muscle
Liver
Kidney
28.7 ± 17
189 ± 157
174 ± 85
[III.30]

220
APPENDIX III
dolphins, this ratio varies from 2 to 39 [III.26]. High
210
Po concentrations in the
liver are thought to reflect
210
Po absorption from recently ingested food (within
a few hours), as in terrestrial mammals. From human and animal data, it can
be inferred that one week after intake the liver retains, on average, 30% of the
210
Po absorbed into the blood, while 8–10% is retained in the kidneys, 9–10%
TABLE III.1. ACTIVITY CONCENTRATIONS OF
210
Po IN MARINE
MAMMALS (cont.)
Region Species
210
Po
(Bq/kg, FW)
Ref.
Bering Sea
Bearded seal
Muscle
Liver
Kidney
27.1 ± 2.3
207 ± 30.4
128 ± 20.5
[III.30]
Swedish coast
Grey and harbour seals
Muscle
Liver
Kidney
Gonad
2.5–20
6–63
5–65
0.5–1.8
[III.19]
Greenland, Bylot
Sound
Seal
Muscle
Liver
8.1 ± 0.8
43 ± 4
[III.31]
Antarctica
Leopard seal
Muscle
Liver
Kidney
1.6 ± 0.1
11.5 ± 0.3
15.8 ± 0.4
[III.15]
Crabeater seal
Muscle
Liver
Kidney
1.3 ± 0.2
22.5 ± 3.3
11.4 ± 2.4
[III.15]
Weddell seal
Muscle
Liver
Kidney
5.0 ± 2.5
48.8 ± 22.5
33.0 ± 11.7
[III.15]
Note: The arithmetic mean and standard deviation are recalculated from the original data.
a
Converted to fresh weight using dry weight to fresh weight conversion factors determined
in dolphins, as follows: muscle: 0.31; liver: 0.25; kidney: 0.22; gonad: 0.22; fat tissue: 0.64;
bone: 0.47; mammary gland: 0.24 [III.13].
221
210
Po IN MARINE MAMMALS
in blood and 5–6% in the skeleton [III.32]. Polonium-210 in the muscle and in
systemic circulation is mostly derived from ingestion as well. Bone is the long
term site for deposition of
210
Pb, which decays into
210
Po. Concentrations of
210
Pb
and
210
Po in dolphin bone were measured in near radioactive equilibrium [III.13].
It is interesting to note that radionuclide distribution modelling in the ringed seal
concluded that probably not more than 1% of the
210
Po in the ringed seal soft
tissue would be from ingrown
210
Po derived from decay of
210
Pb deposit in the
bone
[III.11].
Polonium-210 activity concentration determined in the dorsal muscle of
dolphins is always sampled in the same muscle and body zone, and is therefore
comparable. It shows an increase with dolphin body length and thus with
age
[III.13], which suggests a steady increase of
210
Po body burden, probably due
to the higher ingestion rate by larger individuals and increasing contribution of
210
Po from
210
Pb in the bone as the animal ages, such as observed in humans (see
Chapter
9). In dolphins and whales, other authors have reported an allometric
variation in
210
Po concentration, with lower
210
Po in larger animals [III.27].
However, body size is not the only parameter which influences
210
Po
concentration in marine organisms. A relationship was also identified between
high
210
Po concentrations in muscle and muscle tissue myoglobin content,
ranging from low in white muscle to high in red muscle
[III.27]. This is likely
due to a strong association between
210
Po and metal binding proteins (such as
found with ferritin in fish), contributing to enhancing
210
Po accumulation in red
muscle tissue compared to white muscle tissue
[III.33].
Among individuals of the same species, such as observed in the common
dolphin, the variable
210
Po concentrations in muscle and other internal organs
might be dependent on diet composition and
210
Po assimilation efficiency from
recently ingested food. This receives strong support from previous studies
in other marine species, such as fish and shrimp
[III.34, III.35], as well as in
humans
[III.36].
The diet of marine mammals is not constant every day and changes over
the lifespan of the species and to vary from one feeding ground to another. In
spite of this,
210
Po ingestion is still the main source of
210
Po accumulated in
internal organs. Therefore, the differences in average
210
Po concentrations
between species might reflect the different diets of marine mammals. With
this assumption in mind,
210
Po concentrations (FW) in muscle tissue are:
10–100
Bq/kg in dolphins; 2.5–20 Bq/kg in seals off the Swedish coast;
5
Bq/kg in sperm whales; and as low as 1.1 Bq/kg in South Atlantic whales
(see Table III.1). All of these mammals have different food chains which could
assist data interpretation. However, data interpretation is difficult because
samples have origins in different oceanic regions and ecosystems, and were
obtained at different times. This is important because whales, for example, starve
222
APPENDIX III
during migration. Furthermore, in most cases the exact muscle sampled was not
recorded or described, and
210
Po results for each species have different statistical
weight, in many cases being represented by only a single sample.
III.4. REGIONAL DIFFERENCES
Consistent datasets for the same ecosystem are even more scant. For
the Antarctic Ocean food chain, however, Roos et al.
[III.15] report
210
Po
concentrations in muscle tissue for single individuals of the Weddell seal, the
crabeater seal and the leopard seal of 16, 4.1 and 5.0
Bq/kg (FW), respectively. In
the Antarctic food web, the crabeater seal specializes in krill and other crustaceans;
the leopard seal eats krill, fish and, on occasion, crabeater seals; and the Weddell
seal feeds on fish, squid, krill and, infrequently, penguins
[III.3]. While krill
and other crustaceans generally display relatively higher
210
Po concentrations,
penguins were reported to display relatively low
210
Po concentration in their
flesh, 1.5–2.2
Bq/kg (FW) [III.15]. Therefore, the different
210
Po levels in these
three mammal species seem to reasonably mirror their feeding habits.
Conversely,
210
Po concentrations could be expected to be similar in the
same or related species from different oceanic regions when they occupy the same
ecological niche and trophic level. This was the case for dolphins in the tropical
South Atlantic and temperate north-eastern Atlantic Ocean
[III.13, III.26].
Globally,
210
Po transfer in marine food chains seems to be associated with
protein transfer and
210
Po concentration in consumer tissues mostly reflects
210
Po
levels in the prey and thus the trophic level occupied by marine species [III.37].
Although
210
Po concentrations in mammals are also expected to reflect
210
Po food
chain transfer and their trophic level, the low number of marine mammal species
and specimens analysed so far is not sufficient to draw clear conclusions.
While polonium concentrations in marine mammal tissues are highly
variable and depend on absorption from food,
40
K concentrations determined
in tissues of porpoises, bottlenose dolphins and seals do not show wide
inter-individual variation within the same species
[III.10, III.13, III.20]. This
is consistent with the homeostatic control of
40
K concentration in the internal
organs of mammals.
The generally higher
210
Po concentrations in marine mammal tissue
compared to terrestrial mammals, such as muscle tissue from cows, lambs
and rabbits (see Chapter
6), is a direct consequence of the
210
Po concentration
in the diet. While the diet of marine mammals is exclusively based on marine
prey that consistently have
210
Po:
210
Pb activity ratios well above unity, the
diet of terrestrial mammals (which can be herbivorous, omnivorous or strictly
carnivorous) is generally lower in
210
Po than in
210
Pb, at least for herbivorous
223
210
Po IN MARINE MAMMALS
and omnivorous species. Similarly, some human populations (e.g. in Japan
and Portugal) have a diet rich in marine food (
210
Po:
210
Pb > 1 in the diet), with
average
210
Po intake substantially higher than for populations with a continental
diet (
210
Po:
210
Pb < 1) [III.38, III.39]. These divergent radionuclide intakes are
likely to be reflected in
210
Po concentrations in human tissues and whole body
burdens; however, this has not yet been evaluated (see Chapter
9).
Radiation doses due to natural (
210
Pb,
210
Po and
40
K) and artificial
(
137
Cs) radionuclides accumulated in internal organs have been computed for
some marine mammals. Due to its alpha radiation emission,
210
Po is the main
contributor to the absorbed radiation dose and is estimated to account for more
than 97% of the dose in dolphins [III.13, III.26,
III.40].
In 1982,
210
Po activity concentrations in dolphin muscle tissue mostly
fell in the range of 10–100
Bq/kg (FW), and are two to three orders of
magnitude higher than the average activity concentration in tissues of the
human body, 0.2
Bq/kg (FW) [III.41]. From internally accumulated
210
Po, the
whole body radiation dose for dolphins is around 1.5
μGy/h and 1.9 μGy/h
for Delphinus delphis and Pontoporia blainvillei, respectively. Such natural
radiation doses received by these marine mammals (~15
mGy/a) are unusually
high compared to humans who receive an average dose from internal
210
Po of
about 6.2
× 10
−4
μGy/h [III.26, III.37]. Nevertheless, such dose rates in marine
mammals are still below the threshold radiation dose rate of 100 μGy/h, at which
biological effects have been observed in other mammals [III.9, III.42].
Further study of
210
Po in marine mammals would be of great value, in
particular to understand how they cope with relatively high radiation doses and
to identify detoxification and repair mechanisms for genetic damage caused by
radiation in internal tissues.
REFERENCES TO APPENDIX III
[III.1] JEFFERSON, T.A., LEATHERWOOD, S., WEBBER, M.A., Marine Mammals of
the World, Food and Agriculture Organization of the United Nations, Rome
(1993).
[III.2] CULIK, B.M., Review of Small Cetaceans: Distribution, Behaviour, Migration and
Threats, Marine Mammal Action Plan/Regional Seas Reports and Studies No.
177,
United Nations Environment Programme/Secretariat of the Convention on the
Conservation of Migratory Species of Wild Animals, Bonn
(2004).
[III.3] NATIONAL OCEANIC AND ATOMOSPHERIC ADMINISTRATION, Marine
Mammals,
www.nmfs.noaa.gov/pr/species/mammals
[III.4] PERRIN, W.F., WÜRSIG, B., THEWISSEN, J.G.M. (Eds), Encyclopaedia of Marine
Mammals, Academic Press, San Diego, CA
(2009).
224
APPENDIX III
[III.5] DUIGNAN, P.J., Disease Investigations in Stranded Marine Mammals, 1999–2002,
DOC Science Internal Series
104, New Zealand Department of Conservation,
Wellington (2003).
[III.6] BOSSART, G.D., Marine mammals as sentinel species for oceans and human health,
Oceanogr. 19 (2006) 134–137.
[III.7] PRÖHL, G. (Ed.), Dosimetric Models and Data for Assessing Radiation Exposures to
Biota, FASSET Deliverable 3, Norwegian Radiation Protection Authority,
Østerås (2003).
[III.8] BROWN, J.E., STRAND, P., HOSSEINI, A., BØRRETZEN, P. (Eds), Handbook for
Assessment of the Exposure of Biota to Ionising Radiation from Radionuclides in the
Environment, FASSET Deliverable 5 (2003).
[III.9] BROWN, J.E., BØRRETZEN, P., HOSSEINI, A., Biological transfer of radionuclides
in marine environments: Identifying and filling knowledge gaps for environmental
impact assessments, Radioprot. 40 Suppl. 1 (2005) S533–S539.
[III.10] CALMET, D., WOODHEAD, D., ANDRÉ, J.M.,
210
Pb,
137
Cs and
40
K in three species
of porpoises caught in the Eastern Tropical Pacific Ocean, J. Environ. Radioact.
15
(1992)
153–169.
[III.11] GWYNN, J.P., BROWN, J.E., KOVACS, K.M., LYDERSEN, C., The derivation of
radionuclide transfer parameters for and dose-rates to adult ringed seal (Phoca
hispida) in an Arctic environment, J. Environ. Radioact.
90 (2006) 197–209.
[III.12] UDAKA, M., et al., Radionuclide (
137
Cs and
40
K) concentrations in the muscle of
Baikal seal (Pusa sibirica) from Lake Baikal, Mar. Pollut. Bull.
58 (2009) 290–294.
[III.13] MALTA, M., CARVALHO, F.P., Radionuclides in marine mammals off the
Portuguese coast, J. Environ. Radioact.
102 (2011) 473–478.
[III.14] CHERRY, R.D., SHANNON, L.V., The alpha radioactivity of marine organisms,
Atom. Energ. Rev.
12 (1974) 3–45.
[III.15] ROOS, P., HOLM, E., PERSSON, R.B.R., AARKROG, A., NIELSEN, S.P., “Flux of
210
Pb,
137
Cs,
239+240
Pu and
241
Am in the Antarctic Peninsula area”, Det Sjette Nordiske
Radioøkologi Seminar (6th Nordic Sem. Radioecology, Torshavn, Faroe Islands,
1992), Forskningscenter Risø, Roskilde (1992)
1–12.
[III.16] BERROW, S.D., et al., Radionuclides (
137
Cs and
40
K) in harbour porpoises Phocoena
phocoena from British and Irish coastal waters, Mar. Pollut. Bull.
36 (1998) 569–576.
[III.17] WATSON, W.S., et al., Radionuclides in seals and porpoises in the coastal waters
around the UK, Sci. Total Environ.
234 (1999) 1–13.
[III.18] YOSHITOME, R., et al., Global distribution of radionuclides (
137
Cs and
40
K) in
marine mammals, Environ. Sci. Technol.
37 (2003) 4597–4602.
[III.19] HOLM, E., et al., “Radioactivity in seals from the Swedish coast”, Scientific Trends
in Radiological Protection of the Environment: ECORAD 2004 (BRÉCHIGNAC, F.,
HOWARD, B.J., Eds), Institut de radioprotection et de sûreté nucléaire, Fontenay-
aux-Roses (2005) 85–95.
[III.20] NIELSEN, S.P., JOENSEN, H.P., Recent trends of environmental radioactivity in
Greenland and the Faroe Islands, Radioprot. 44 (2009) 843–848.
225
210
Po IN MARINE MAMMALS
[III.21] PENTREATH, R.J., WOODHEAD, D.S., A system for protecting the environment
from ionizing radiation: Selecting reference fauna and flora, and possible dose
models and environmental geometrics that could be applied to them, Sci. Total
Environ.
277 (2001) 33–43.
[III.22] BRÉCHIGNAC, F., Protection of the environment: How to position radioprotection
in an ecological risk assessment perspective, Sci. Total Environ.
307 (2003) 35–54.
[III.23] INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION,
Environmental Protection: The Concept and Use of Reference Animals and Plants,
Publication
108, Elsevier (2008).
[III.24] AARKROG, A., et al., A comparison of doses from
137
Cs and
210
Po in marine food: A
major international study, J. Environ. Radioact.
34 (1997) 69–90.
[III.25] CARVALHO, F.P., OLIVEIRA, J.M., “Radioactivity in marine organisms from
Northeast Atlantic Ocean”, The Natural Radiation Environment (NRE VIII) (Proc.
8th Int. Symp. Rio de Janeiro, 2007) (PASCHOA, A.S., STEINHAUSER, F., Eds),
American Institute of Physics, Melville, NY (2008)
387–392.
[III.26] GODOY, J.M., SICILIANO, S., DE CARVALHO, Z.L., DE MOURA, J.F., GODOY,
M.L.D.P.,
210
Polonium content of small cetaceans from Southeastern Brazil, J.
Environ. Radioact.
106 (2012) 35–39.
[III.27] CHERRY, R.D., HEYRAUD, M., RINDFUSS, R., Polonium-210 in teleost fish and
in marine mammals: Interfamily differences and a possible association between
polonium-210 and red muscle content, J. Environ. Radioact.
24 (1994) 273–291.
[III.28] FOLSOM, T.R., WONG, K.M., HODGE, V.F., “Some extreme accumulations of
natural polonium radioactivity observed in marine organisms”, The Natural Radiation
Environment (Proc. 2nd Int. Symp. Houston, 1972) (ADAMS, J.A.S., LOWDER,
W.M., GESELL, T.F., Eds), United States Department of Commerce, Springfield, VA
(1972) 863–882.
[III.29] HOLTZMAN, R.B., Natural levels of lead-210, polonium-210 and radium-226 in
humans and biota of the Arctic, Nature
210 (1966) 1094–1097.
[III.30] HAMILTON, T., SEAGARS, D., JOKELA, T., LAYTON, D.,
137
Cs and
210
Po in
Pacific walrus and bearded seal from St.
Lawrence Island, Alaska, Mar. Pollut.
Bull.
56 (2008) 1158–1167.
[III.31] NIELSEN, S.P., ROOS, P., Radionuclides in Seal Flesh and Liver, Summary Sem.
within the NKS B Programme, 2002–2005 (2006).
[III.32] HARRISON, J., LEGGER, R., LLOYD, D., PHIPPS, A., SCOTT, B., Polonium-210
as a poison, J. Radiol. Prot. 27 (2007) 17–40.
[III.33] DURAND, J.P., et al.,
210
Po binding to metallothioneins and ferritin in the liver of
teleost marine fish, Mar. Ecol. Prog. Ser.
177 (1999) 189–196.
[III.34] CARVALHO, F.P., FOWLER, S.W., An experimental study on the bioaccumulation
and turnover of polonium-210 and lead-210 in marine shrimp, Mar. Ecol. Prog.
Ser.
102 (1993) 125–133.
[III.35] CARVALHO, F.P., FOWLER, S.W., A double tracer technique to determine the
relative importance of water and food as sources of polonium-210 to marine prawns
and fish, Mar. Ecol. Prog. Ser.
103 (1994) 251–264.
226
APPENDIX III
[III.36] HUNT, G.J., ALLINGTON, D.J., Absorption of environmental polonium-210 by the
human gut, J. Radiol. Prot.
13 (1993) 119–126.
[III.37] CARVALHO, F.P., Polonium (
210
Po) and lead (
210
Pb) in marine organisms and their
transfer in marine food chains, J. Environ. Radioact.
102 (2011) 462–72.
[III.38] CARVALHO, F.P.,
210
Po and
210
Pb intake by the Portuguese population: The
contribution of seafood in the dietary intake of
210
Po and
210
Pb, Health Phys. 69
(1995)
469–480.
[III.39] YAMAMOTO, M., SAKAGUCHI, A., TOMITA, J., IMANKA, T., SIRAICHI, K.,
Measurements of
210
Po and
210
Pb in total diet samples: Estimate of dietary intakes of
210
Po and
210
Pb for Japanese, J. Radioanal. Nucl. Chem. 279 (2009) 93–103.
[III.40] BROWN, J.E., JONES, S.R., SAXÉN, R., THØRRING, H., VIVES i BATLLE, J.,
Radiation doses to aquatic organisms from natural radionuclides, J. Radiol. Prot.
24
(2004)
A63–A77.
[III.41] UNITED NATIONS SCIENTIFIC COMMITTEE ON THE EFFECTS OF ATOMIC
RADIATION, Ionizing Radiation: Sources and Biological Effects, UNSCEAR 1982
Report to the General Assembly, with Annexes, United Nations, New
York (1982).
[III.42] REAL, A., SUNDELL-BERGMAN, S., KNOWLES, J.F., WOODHEAD, D.S.,
ZINGER, I., Effects of ionising radiation exposure on plants, fish and animals:
Relevant data for environmental radiation protection, J. Radiol. Prot.
24
(2004)
A123–A138.
227
Appendix IV
POLONIUM RELEASES FROM
VEGETATION AND FOREST FIRES
IV.1. INTRODUCTION
Humans absorb
210
Po much more than other heavy radioactive elements,
such as thorium and uranium [IV.1]. In the human body, the main origin of
210
Po
is in food and water; however, it can also be accumulated from the air through
inhalation and lung absorption [IV.2, IV.3] (see also Chapter 9). Early studies
from the 1970s on
210
Pb and
210
Po activity concentrations in atmospheric aerosols
reported the occurrence of higher than expected
210
Po activities and abnormally
high
210
Po:
210
Pb activity ratios (i.e. >1) [IV.4, IV.5]. Those reports often attributed
these exceptional ratios to volcanic emissions (see Ref. [IV.5]). Enhanced
210
Po
concentrations and
210
Po:
210
Pb activity ratios were also attributed to releases from
industrial sources and vegetation fires, but these were usually reported with scarce
experimental evidence in support of that attribution
[IV.4, IV.6]. Later work
measuring
210
Pb and
210
Po in smoke from savannah fires in Africa showed that
smoke particles can contain high
210
Pb and
210
Po levels and increase the usually
low activity concentration ratios in surface air aerosols in the region
[IV.7, IV.8].
The determination of radionuclides in effluent releases to the atmosphere
(gases and particulate matter) from non-nuclear industries also reveal enhanced
concentrations of
210
Po and occasionally high
210
Po:
210
Pb ratios. These are
reported, for example, for industries such as coal fired power plants, metal
smelters and elemental phosphorus producing plants
[IV.9]. High
210
Po
concentrations and other naturally occurring radioactive material (NORM)
in effluents from these industries are generally associated with relatively high
temperatures used in their core processes.
In 1982, the United Nations Scientific Committee on the Effects of
Atomic Radiation compiled what was known about radioactive emissions from
NORM industries, pointing out that substantial enrichment of natural radioactive
elements with low boiling point could be observed within the wastes generated
in some of those industries
[IV.2]. Later, reports were provided of the existence
of high activity deposits and scales of certain volatile radioisotopes, particularly
210
Po, in filters, ash and on the surfaces of the furnaces and subsequent devices
where the processes take place
[IV.10–IV.13]. Mora et al. [IV.13] attempt to
model the behaviour of
210
Po in these industries was made based on its physical
properties, particularly its low melting point.
228
APPENDIX IV
Morawska [IV.14] investigates burning wood in stoves for house heating
as a source of contaminants to indoor air. The
210
Po concentrations are found to
be enhanced in smoke from domestic wood combustion and to contribute to poor
indoor air quality; although in modern houses the smoke is efficiently extracted
to the atmosphere. Nonetheless, measurable concentrations of
210
Po are reported
in association with soot and organic compounds in the extracted smoke
[IV.15].
Enhanced
210
Po levels and elevated
210
Po:
210
Pb ratios in aerosols in the atmosphere
of Seoul, Republic of Korea, are associated with coal burning
[IV.10].
High concentrations of
210
Po in cigarette smoke and enhanced radiation
doses to the smokers’ lung have been reported. Although tobacco leaves have
moderate
210
Po concentrations,
210
Po volatilization and re-concentration
in smoke particles are associated with the high temperature of tobacco
combustion
[IV.16–IV.19].
Altogether, evidence from investigations of NORM industries, wood
combustion and cigarette smoke case studies has sufficiently underpinned
the case for enhanced
210
Po activity concentrations occurring in particulates
formed during the transformation of common raw materials that undergo high
temperature processes. Despite this knowledge, assessment of
210
Po and other
natural radionuclides released by forest and bush fires has been essentially
under-investigated in atmospheric radioactivity research.
IV.2. VEGETATION AND FOREST FIRES
Wildfires burn many thousands of hectares of forest and bush around the
world every year. Although global statistics of vegetation fires are far from
complete, analysis of satellite data for 2000 revealed that 350
million ha were
affected by vegetation fires
[IV.20]. The plant biomass burned annually can
vary according to the ecosystem affected, but reaches many millions of tonnes.
This combustion releases considerable amounts of carbon, particles and gases
(including toxic organic substances) to the atmosphere
[IV.21–IV.23]. More
than 200 volatile substances have already been identified in forest fire smoke,
including toxic gases, such as carbon monoxide, formaldehyde, acrolein,
hydrocarbon compounds and dioxins
[IV.15, IV.23, IV.24]. Smoke particles also
adversely affect the respiratory tract and their inhalation in areas impacted by
forest fires have been connected to a higher frequency of asthma and other lung
diseases
[IV.23]. Furthermore, smoke rapidly travels around the globe and raises
concerns about air quality in areas far from forest fires
[IV.25].
Compared with what is known about the environmental stressors described
in Section
IV.1, the release of radionuclides by vegetation fires has been less
investigated and attention has been focused almost exclusively on artificial
229
VEGETATION AND FOREST FIRES
radionuclides. One example is the work undertaken to evaluate resuspension
of artificial radioactivity, such as
137
Cs, released by the Chernobyl accident, in
1986, and deposited onto soils and forests in Eastern Europe
[IV.26–IV.31]. This
resuspension was observed recently, following summer forest fires in Belarus
and Ukraine. These forest fires reinitiated atmospheric transport processes to
carry
137
Cs to central, northern and western European countries, although on a
much smaller scale than in 1986
[IV.1, IV.32, IV.33].
However, besides radioactivity of artificial origin, vegetation fires are
also likely to release naturally occurring radionuclides, such as
14
C and
40
K, and
members of the uranium, thorium and actinium decay series
[IV.1]. Plants absorb
naturally occurring radionuclides through root and foliar uptake, and accumulate
them throughout their tissues (see Chapter
6). So far, the radiological impact of
smoke from forest or grass fires has scarcely been investigated
[IV.32, IV.34].
Nevertheless, work done on
137
Cs resuspension by forest fires in Eastern Europe
allows for comparison of measurements of naturally occurring radionuclides
in surface air in Finland. The air activity concentrations of resuspended
137
Cs
are of little radiological significance and are comparable to, or lower than,
those of naturally occurring radionuclides in aerosol particles
[IV.32]. Artificial
transuranic radionuclides and total alpha radioactivity associated with smoke
from prescribed fires have also been measured at the Savannah River Site and
other woodland areas in the southern United States of America
[IV.34].
With fire, natural radionuclides concentrated in plants are released to
the atmosphere as smoke components. It is known that concentrations of
naturally occurring radionuclides are generally low in vegetation. Atmospheric
radionuclides, such as the long lived
222
Rn progeny,
210
Pb,
210
Bi and
210
Po, deposit
onto the surface of leaves and bark, where the highest
210
Po concentrations in
plants are usually measured. In the tree trunk and branch wood,
210
Po content is
usually lower than in leaves
[IV.35]. The burning of this wood still releases some
210
Po along with other compounds [IV.15]. More significant than combustion
of tree wood are the
210
Po releases by grass and bush vegetation fires to the
atmosphere (see Table
IV.1).
Carvalho et al. [IV.1] hypothesize that the release of radionuclides from
plant biomass can occur through two mechanisms:
“One consists in the volatilization of elements [such as polonium] when the
flame temperature exceeds the volatilization point of those elements. The
other is the enrichment through enhancement of element concentrations in
ash/smoke particles (refractory fraction) due to reduction of plant material
mass with the volatilization of water, organic compounds, and elements of
low volatilization point.”

230
APPENDIX IV
In contrast to the more refractory elements, such as uranium and thorium,
Carvalho et al. [IV.1] report that polonium is volatile at lower temperatures and
can be released to the atmosphere as gaseous compounds.
More research has recently been conducted on
210
Po and other natural
radionuclides released from vegetation by wildfires [IV.24, IV.35]. Carvalho et
al. [IV.1] report that in aerosols sampled in the vicinity of fires, “the radionuclide
activities per unit of air volume was systematically higher with higher particle
load in the air, suggesting that smoke particles collected on filters were the
vehicle for transport of radioactivity in the atmosphere” (see Fig. IV.1). The
210
Po
activity concentrations in smoke particles were enriched by a factor of 100–1000
over those in the vegetation on a mass basis [IV.24]. Furthermore, Carvalho et
al. [IV.1] find (see also Refs [IV.35, IV.36]):
“The proportions of radionuclides in the smoke were modified in comparison
with background aerosols and with unburned plants. For example, the
concentration ratios
210
Po/
210
Pb in vegetation before the fire were often
about 0.1, while in the smoke from vegetation fires this ratio increased up
to 12, revealing an extraordinary enrichment of smoke particles in
210
Po.”
Paatero et al.
[IV.32] report that the
210
Po:
210
Pb activity ratio in air increased
by almost an order of magnitude when smoke particulates were present. Carvalho
et al. [IV.36] determine
210
Po in smoke from vegetation fires was determined
in several particle size classes of smoke aerosols sampled with a cascade
impactor
[IV.36]. This reveals an enrichment of the activity concentration of
TABLE IV.1. RADIONUCLIDE ACTIVITY CONCENTRATIONS IN PLANTS
AND IN SMOKE FROM PLANT COMBUSTION (Bq/kg, DW)
238
U
234
U
226
Ra
210
Pb
210
Po
232
Th
Leaves —
a
—
a
—
a
9.4 1.05 —
a
Trunk —
a
—
a
—
a
4.3 0.14 —
a
Smoke of:
Eucalyptus wood
Acacia wood
Bush wildfire
Pinewood wildfire
65 ± 5
11 ± 1
169 ± 7
204 ± 11
88 ± 7
36 ± 4
167 ± 7
217 ± 12
318 ± 65
503 ± 165
2492 ± 639
3547 ± 1678
52.2 ± 4
440 ± 19
369 ± 36
—
a
125 ± 10
611 ± 28
4422 ± 186
3972 ± 157
35 ± 3
<29
—
a
117 ± 8
Source: Table 1 of Ref. [IV.1] (see also Refs [IV.24, IV.35]).
a
—: data not available.
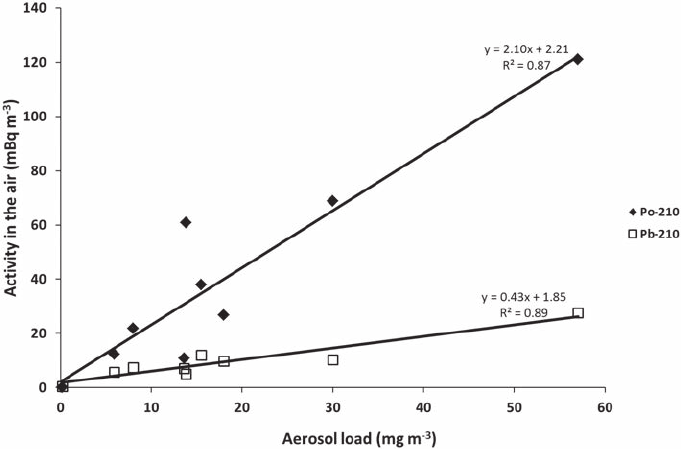
231
VEGETATION AND FOREST FIRES
radionuclides in aerosol particles collected near the vegetation fire compared to
reference aerosol samples collected in other areas in the absence of vegetation
fires
[IV.1]. In the case of
210
Po, this enrichment is observed mostly in the
smallest particles, with aerodynamic diameters less than 0.5
μm. In contrast,
the distribution of refractory elements, such as
232
Th and
238
U, in smoke particle
aerosols across the range of size classes show reasonable homogeneity
[IV.1].
Owing to the much higher number of smaller particles (<0.5
μm) per unit
volume, these are the main contributors for inhaled activity, particularly for
210
Po
(see Fig. IV.2.)
Polonium atoms in the gas phase are positive ions and can easily be
recaptured by electrostatic charges onto the aerosol particles, and smaller
particles (coagulation nuclei) can gradually enter accumulation mode and later
settle as coarse aerosol particles
[IV.14]. Considering the
210
Po radionuclide
distribution in aerosol fractions, inhalation of smoke in the vicinity of a
vegetation fire can lead to inhalation of significant activity. Inhalation of up to
70
mBq/m
3
, mostly due to the
210
Po fraction associated with very fine particles,
has been estimated
[IV.35]. Prolonged exposure to smoke from vegetation fires
Source: Figure 1 of Ref. [IV.24].
Note: Correlations are statistically significant at p < 0.01.
FIG.
IV.1. Aerosol load in surface air with smoke from vegetation fires, and
210
Pb and
210
Po
radioactivity in the air (reproduced with permission courtesy of EIT Press).

232
APPENDIX IV
might lead to enhanced radiation doses to the lung, especially from
210
Po, that
could be comparable or even higher than the radiation dose received by heavy
cigarette smokers. This exposure to ionizing radiation is sufficient justification
for recommending respiratory protection, in particular for firefighters and
members of the public in areas heavily affected by smoke from vegetation and
forest fires
[IV.35, IV.36]. Increasing worldwide consumption of coal is also
likely to enhance
210
Po levels in the atmosphere and its impact on public health
should be carefully monitored
[IV.10].
REFERENCES TO APPENDIX IV
[IV.1] CARVALHO, F.P., OLIVEIRA, J.M., MALTA, M., “Exposure to forest fires,
radioactivity and health risks”, Occupational Safety and Hygiene (SHO 2012) (Int.
Symp. Guimarães, 2012) (AREZES, P., Eds.), Portuguese Society of Occupational
Safety and Hygiene, Guimarães (2012) 126–130.
[IV.2] UNITED NATIONS SCIENTIFIC COMMITTEE ON THE EFFECTS OF ATOMIC
RADIATION, Ionizing Radiation: Sources and Biological Effects, UNSCEAR 1982
Report to the General Assembly, with Annexes, United Nations, New York (1982).
Source: Figure 2 of Ref. [IV.36].
FIG.
IV.2. Radionuclide concentrations in aerosol particles in the smoke from a forest fire
sorted by aerodynamic size classes (AS) (reproduced with permission courtesy of Elsevier).
233
VEGETATION AND FOREST FIRES
[IV.3] CARVALHO, F.P.,
210
Po and
210
Pb intake by the Portuguese population: The
contribution of seafood in the dietary intake of
210
Po and
210
Pb, Health Phys. 69
(1995) 469–480.
[IV.4] POET, S.E., MOORE, H.E., MARTELL, E.A., Lead 210, bismuth 210, and polonium
210 in the atmosphere: Accurate ratio measurement and application to aerosol
residence time determination, J. Geophys. Res.
77 (1972) 6515–6527.
[IV.5] LAMBERT, G., BUISSON, A., SANAK, J., ARDOUIN, B., Modification of the
atmospheric polonium 210 to lead 210 ratio by volcanic emissions, J. Geophys.
Res.
84 (1979) 6980–6986.
[IV.6] CARVALHO, F.P., Origins and concentrations of
222
Rn,
210
Pb,
210
Bi and
210
Po in the
surface air at Lisbon, Portugal, at the Atlantic edge of the European continental
landmass, Atmos. Environ.
29 (1995) 1809–1819.
[IV.7] NHO, E.-Y., ARDOUIN, B., LE CLOAREC, M.-F., RAMONET, M., Origins of
210
Po in the atmosphere at Lamto, Ivory Coast: Biomass burning and Saharan dusts,
Atmos. Environ.
30 (1996) 3705–3714.
[IV.8] NHO, E.-Y., LE CLOAREC, M.-F., ARDOUIN, B., RAMONET, M.,
210
Po, an
atmospheric tracer of long-range transport of volcanic plumes, Tellus
B 49
(1997)
429–438.
[IV.9] KOSTER, H.W., et al., Po-210, Pb-210, Ra-226 in Dutch aquatic ecosystems and
polders: Anthropogenic sources, distribution and radiation doses, Radiat. Prot.
Dosim. 45 (1992) 715–719.
[IV.10] YAN, G., CHO, H.-M., LEE, I., KIM, G., Significant emissions of
210
Po by coal
burning into the urban atmosphere of Seoul, Korea, Atmos. Environ.
54 (2012) 80–85.
[IV.11] KHATER, A.E.M., BAKR, W.F., Technologically enhanced
210
Pb and
210
Po in iron
and steel industry, J. Environ. Radioact.
102 (2011) 527–530.
[IV.12] TROTTI, F., et al., “A study concerning NORM in integrated steelworks”, Naturally
Occurring Radioactive Material (NORM
V) (Proc. Int. Symp. Seville, 2007), IAEA,
Vienna (2008)
351–354.
[IV.13] MORA, J.C., ROBLES, B., CORBACHO, J.A., GASCÓ, C., GÁSQUEZ, M.J.,
Modelling the behaviour of
210
Po in high temperature processes, J. Environ.
Radioact.
102 (2011) 520–526.
[IV.14] MORAWSKA, L., “Indoor particles, combustion products and fibres”, The Handbook
of Environmental Chemistry, Vol.
4, Part F: Indoor Air Pollution (PLUSCHKE, P.,
Ed.) Springer, Heidelberg (2004)
117–147.
[IV.15] GONÇALVES, C., et al., Characterisation of PM10 emissions from woodstove
combustion of common woods grown in Portugal, Atmos. Environ.
44
(2010)
4474–4480.
[IV.16] KILTHAU, G.F., Cancer risk in relation to radioactivity in tobacco, Radiol.
Technol.
67 (1996) 217–222.
[IV.17] PAPASTEFANOU, C., Radioactivity in tobacco leaves, J. Environ. Radioact. 53
(2001)
67–73.
[IV.18] CARVALHO, F.P., OLIVEIRA, J.M., Polonium in cigarette smoke and radiation
exposure of lungs, Czechoslov. J. Phys.
56 (2006) 697–703.
234
APPENDIX IV
[IV.19] DESIDERI, D., MELI, M.A., FEDUZI, L., ROSELLI, C.,
210
Po and
210
Pb inhalation
by cigarette smoking in Italy, Health Phys.
92 (2007) 58–63.
[IV.20] FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS,
Fire Management Global Assessment
2006, FAO Forestry Paper No. 151, FAO,
Rome
(2007).
[IV.21] SAARIKOSKI, S., HILLAMO, R., “Wildfires as a source of aerosol particles
transported to the Northern European regions”, The Handbook of Environmental
Chemistry, Vol. 26: Urban Air Quality in Europe (VIANA, M., Ed.), Springer,
Heidelberg (2012)
101–121.
[IV.22] LAZARIDIS, M., et al., Contribution of forest fire emissions to atmospheric pollution
in Greece, Air Qual. Atmos. Health
1 (2008) 143–158.
[IV.23] DE VOS, A.J.M., REISEN, F., COOK, A., DEVINE, B., WEINSTEIN, P.,
Respiratory irritants in Australian bushfire smoke: Air toxics sampling in a smoke
chamber and during prescribed burns, Arch. Environ. Contam. Toxicol.
56
(2009)
380–388.
[IV.24] CARVALHO, F.P., OLIVEIRA, J.M., MALTA, M., “Vegetation fires and release of
radioactivity into the air”, Environmental Health and Biomedicine (BREBBIA, C.A.,
et al., Eds), WIT Press, Southhampton (2011) 3–9.
[IV.25] DAMOAH, R., et al., Around the world in 17 days: Hemispheric-scale transport of
forest fire smoke from Russia in May
2003, Atmos. Chem. Phys. 4 (2004) 1311–1321.
[IV.26] PALIOURIS, G., TAYLOR, H.W., WEIN, R.W., SVOBODA, J., MIERZYNSKI, B.,
Fire as an agent in redistributing fallout
137
Cs in the Canadian boreal forest, Sci. Total
Environ.
160/161 (1995) 153–166.
[IV.27] AMIRO, B.D., SHEPPARD, S.C., JOHNSTON, F.L., EVENDEN, W.G., HARRIS,
D.R., Burning radionuclide question: What happens to iodine, cesium and chlorine in
biomass fires? Sci. Total Environ.
187 (1996) 93–103.
[IV.28] KASHPAROV, V.A., et al., Forest fires in the territory contaminated as a result of the
Chernobyl accident: Radioactive aerosol resuspension and exposure of fire-fighters,
J. Environ. Radioact.
51 (2000) 281–298.
[IV.29] YOSCHENKO, V.I., et al., Resuspension and redistribution of radionuclides during
grassland and forest fires in the Chernobyl exclusion zone — Part
II: Modeling the
transport process, J. Environ. Radioact.
87 (2006) 260–278.
[IV.30] YOSCHENKO, V.I., et al., Resuspension and redistribution of radionuclides during
grassland and forest fires in the Chernobyl exclusion zone — Part
I: Fire experiments,
J. Environ. Radioact.
86 (2006) 143–163.
[IV.31] MIN HAO, W., BONDARENKO, O.O., ZIBTSEV, S., HUTTON, D., “Vegetation
fires, smoke emissions, and dispersion of radionuclides in the Chernobyl exclusion
zone”, Developments in Environmental Science, Vol.
8 (BYTNEROWICZ, A.,
ARBAUGH, M., RIEBAU, A., ANDERSEN, C., Eds), Elsevier (2009)
265–275.
[IV.32] PAATERO, J., et al., Resuspension of radionuclides into the atmosphere due to forest
fires, J. Radioanal. Nucl. Chem.
282 (2009) 473–476.
[IV.33] JARGIN, S.V., Forest fires in the former Soviet Union: No reasons for radiophobia, J.
Environ. Radioact.
102 (2011) 218–219.
235
VEGETATION AND FOREST FIRES
[IV.34] COMMODORE, A.A., et al., Radioactivity in smoke particulates from prescribed
burns at the Savannah River Site and at selected southeastern United States forests,
Atmos. Environ.
54 (2012) 643–656.
[IV.35] CARVALHO, F.P., OLIVEIRA, J.M., MALTA, M., Forest fires and resuspension of
radionuclides into the atmosphere, Am. J. Environ. Sci.
8 (2012) 1–4.
[IV.36] CARVALHO, F.P., OLIVEIRA, J.M, MALTA, M., Exposure to radionuclides in
smoke from vegetation fires, Sci. Total Environ.
472 (2012) 421–424.
237
Appendix V
POLONIUM IN TOBACCO AND DOSES TO HUMANS
V.1. INTRODUCTION
Tobacco (Nicotina rustica) is in the Solonaceae family, together with bell
pepper (capsicum), tomato and potato, and there are many species in the tobacco
genus, Nicotiana. It was originally grown as a crop in Central America and is
named after the Caribbean island of Tobago. Most tobacco is smoked, but other
forms exist, such as snuff and chewing tobacco. Nicotine, the active substance in
tobacco, is present in lower doses in potato plants, tomato plants and, together
with cocaine, in coca plants. It is named after a French ambassador to Portugal,
Jean Nicot, who introduced snuff, taken through the nose, to the French court
in 1560.
V.2.
210
Pb AND
210
Po IN TOBACCO
Inhalation of tobacco smoke is ranked second to food as a source of
210
Pb
and
210
Po in humans (see Ref. [V.1] for a review), and there is a clear correlation
between lung cancer and smoking, and evidence of a synergistic effect when
smoking and elevated radon levels are combined
[V.2–V.4]. There are several
publications which deal with
210
Pb and
20
Po in tobacco and the transfer to humans
(see Table
V.1). Activity concentrations are in the range of 2.8–37 mBq/g. There
are few data on activity concentrations in pipe tobacco, cigar tobacco and snuff.
A cigarette contains about 1
g of tobacco.
When
210
Pb is also analysed, it is generally in radioactive equilibrium to
210
Po. In addition, while all results are of the same order of magnitude, the activity
concentrations of
210
Pb and
210
Po in tobacco vary depending on the geographical
region in which the tobacco is grown and harvested. If foliar deposition is the
main source for the radionuclides, the
210
Po:
210
Pb activity ratio should be lower
than one at harvest. However, owing to the time elapsed from harvest to purchase,
equilibrium has usually been attained in tobacco products.

238
APPENDIX V
V.3. TRANSFER TO HUMANS AND RADIOLOGICAL DOSES
The effective dose conversion factors for
210
Pb and
210
Po for inhalation
by adults are 1.1
× 10
−6
Sv/Bq and 3.3 × 10
−6
Sv/Bq, respectively [V.12]. The
dose conversion factors by ingestion are slightly lower, at 6.7 × 10
−7
Sv/Bq and
1.2 × 10
−6
Sv/Bq, respectively.
Additional studies from the 1960s include information on the radionuclide
contents in the wrapping paper, filters, cigarette ash, post-smoking filters and
cigarette ends, as well as in the mainstream and sidestream
smoke, and that
sometimes a smoking machine was used for the radionuclide budget in the
different matrices
[V.10, V.11] (see Table V.2).
There are large variations in the data. Apart from the variation of
radionuclide concentrations in tobacco, the authors often have different estimates
of the amount of smoke inhaled and the fraction of
210
Po retained in the lungs.
Different dose conversion factors have been used where recommendations
have changed over time. Sometimes, calculations are based on assumed lung
TABLE V.1. EXAMPLES OF
210
Po ACTIVITY CONCENTRATIONS
IN TOBACCO
Country
Activity per gram
(mBq/g)
Activity per cigarette
(mBq)
Ref.
Various 6.6–24 7.4–23 [V.4]
Egypt 12.6–24 9.7–21.8 [V.5]
Greece (tobacco leaves) 6–18 5–15 [V.6]
Poland 5.7–33 5.7–33.2 [V.7]
Portugal 2.8–37 2.6–27.7 [V.8]
Sweden (snuff) 6–60 —
a
[V.9]
USA
Cigarettes
Pipe tobacco
Cigar tobacco
13.3–20.7
10.5–22
7.6–13.9
10.5–30
12.6–17.8 [V.10]
—
a
USA
Pipe tobacco
Cigar tobacco
7–13
17
12–18
—
a
—
a
[V.11]
a
—: data not available.

239
POLONIUM IN TOBACCO
concentrations. The quality factor used to estimate dose from alpha radiation
(now 20) is not always given in these studies. As for less common exposure
routes, using tobacco as snuff gives a lower dose than smoking. It would be of
interest to study the impact of
210
Po derived from using water pipes (hookahs).
A fraction of the radionuclides will transfer to the smoker and will also
expose persons in the vicinity. Smokers have higher concentrations of these
radionuclides in their lungs but also in other organs, such as the liver and
kidneys (see Chapter
9). The dose to the lungs is much higher from smoking
TABLE V.2. ACTIVITY CONCENTRATIONS IN DIFFERENT
SMOKING MATRICES AND ANNUAL DOSES (SMOKING
20 CIGARETTES PER DAY) FROM
210
Po
Study
Activity per gram
(mBq/g)
Dose
(μSv/a)
Skwarzek et al. [V.7]
Filter
Paper
Filter after smoking
Ash only
0.1–4.3
1.5
0.3–9.1
0.43–14.7
—
a
Ferri and Baratta [V.11]
Filter after smoking
Inhaled
End+ash
1.1–2.0
1.7–4.0
4.3–7.1
165–460 (lungs)
Khater [V.5]
Filter
Paper
Filter after smoking
Ash only
0.9–9.7
0.3–8.1
1.5–12.6
2.2–35
193 (effective dose)
Carvalho and Oliveira [V.8]
Inhaled
End+ash
0.97–1.52
0.3–4.9
420 (lungs)
Black and Bretthauer [V.10]
Inhaled
End only
2.6
7.0
—
a
Watson [V.4] —
a
5–220 (lung)
Papastefanou [V.6] —
a
47–135
(effective dose)
a
—: data not available.
240
APPENDIX V
than from breathing outdoor ambient air, and a correlation exists between the
prevalence of lung cancer in smokers with those living in houses with high radon
concentrations.
REFERENCES TO APPENDIX V
[V.1] FINKELSTEIN, M.M., Clinical measures, smoking, radon exposure, and risk of lung
cancer in uranium miners, Occup. Environ. Med.
53 (1996) 697–702.
[V.2] STEENLAND, K., Age specific interactions between smoking and radon among
United States uranium miners, Occup. Environ. Med.
51 (1994) 192–194.
[V.3] HARLEY, N.H., HARLEY, J.H., Potential lung cancer risk from indoor radon
exposure, CA: Cancer J. Clin.
40 (1990) 265–275.
[V.4] WATSON, A.P., Polonium-210 and Lead-210 in Food and Tobacco Products: A Review
of Parameters and an Estimate of Potential Exposure and Dose, ORNL/TM-8831, Oak
Ridge Natl Lab., TN
(1983).
[V.5] KHATER, A.E.M., Polonium-210 budget in cigarettes, J. Environ. Radioact. 71
(2004)
33–41.
[V.6] PAPASTEFANOU, C., Radioactivity of tobacco leaves and radiation doses induced
from smoking, Int. J. Environ. Res. Public Health
6 (2009) 558–567.
[V.7] SKWARZEK, B., STRUMIŃSKA, D.I., BORYŁO, A., ULATOWSKI, J., Polonium
210
Po in cigarettes produced in Poland, J. Environ. Sci. Health A 36 (2001) 465–474.
[V.8] CARVALHO, F.P., OLIVEIRA, J.M., Polonium in cigarette smoke and radiation
exposure of lungs, Czechoslov. J. Phys.
56 (2006) 697–703.
[V.9] SAMUELSSON, C., Medicinska konsekvenser av polonium i snus, Läkartidningen 86
(1989) 2290–2292.
[V.10] BLACK, S.C., BRETTHAUER, E.W., Polonium-210 in tobacco, Radiol. Health Data
Rep.
9 (1968) 145–152.
[V.11] FERRI, E.S., BARATTA, E.J., Polonium-210 in tobacco, cigarette smoke, and selected
human organs, Public Health Rep.
81 (1966) 121–127.
[V.12] INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION,
Age-dependent Doses to the Members of the Public from Intake of Radionuclides —
Part 5: Compilation of Ingestion and Inhalation Coefficients, Publication 10, Pergamon
Press, Oxford and New York (1995).
241
Appendix VI
ESTIMATION OF AEROSOL RESIDENCE TIMES
IN THE ATMOSPHERE
VI.1. AEROSOL RESIDENCE TIME
The residence time (τ) for a radionuclide in a parcel of air (or any other
environmental compartment) represents the average time an atom of that
radionuclide is present in the air parcel before it is removed
[VI.1]. If N is the total
number of atoms of the radionuclide in the air parcel, I is the rate of introduction
from sources (e.g. by ingrowth from a parent or transfer from another air parcel)
and R is the rate of removal (e.g. by radioactive decay, or wet or dry deposition),
then:
d
d
N
IR
t
=-
(VI.1)
If N is a constant, then steady state conditions hold, and I = R. In this case,
the residence time is given by:
NN
RI
t
==
(VI.2)
In the case of the total amount of
222
Rn in the atmosphere, the only
significant removal process is radioactive decay. Under the assumption that a
steady state condition applies, then:
1
5.5 days
N
N
t
ll
= ==
(VI.3)
However, for a portion of the whole atmosphere, such as the planetary
boundary layer (PBL), the average residence time for
222
Rn will be less than this
due to mixing, which results in additional net removal from the PBL to the free
troposphere. These residence times are not long in comparison with characteristic
mixing times in the troposphere; as a result,
222
Rn is not well mixed either
horizontally or vertically.
The
222
Rn progeny,
210
Pb,
210
Bi and
210
Po, are attached to aerosols in the
atmosphere, and their behaviour reflects that of those aerosols. In the troposphere,
242
APPENDIX VI
aerosol residence times range from days to weeks, depending on the size of the
aerosol, height above ground level and the prevailing meteorological conditions.
In contrast, residence times in the stratosphere are of the order of one year
[VI.1].
The degrees of disequilibrium between
222
Rn and
210
Pb, and between
210
Pb and its radioactive progeny,
210
Bi and
210
Po, have sometimes been used
to calculate apparent atmospheric aerosol residence times
[VI.2–VI.4]. For this
purpose, concentrations of the pair (
222
Rn,
210
Pb) in air can be used [VI.5]. For
the pairs (
210
Pb,
210
Bi) and (
210
Pb,
210
Po), concentrations in air [VI.6–VI.8], in
precipitation
[VI.9–VI.11] and in dry fallout [VI.11] have been used.
In the case that
210
Pb,
210
Bi and
210
Po are produced solely by decay of
222
Rn
in the atmosphere, that their production and removal are in steady state at the
time and place of measurement, and that the removal term follows first order
kinetics, then:
( )
2
11 2 2 R
d
0
d
N
NN
t
l ll
= - +=
(VI.4)
where 1 is a parent radionuclide, 2 is its immediate daughter, λ
1
and λ
2
are their radioactive decay constants, and λ
R
is the first order rate constant for
removal of aerosols by all processes. The mean aerosol residence time is:
R
R
1
t
l
=
(VI.5)
For the individual parent–progeny pairs, the apparent aerosol residence
time is given by
[VI.2, VI.5]:
( )
( )
( )
Pb
R
Pb Rn
Bi
R
Bi Pb Bi
2
R
1
Rn, Pb
1
Pb, Bi
4
Pb, Po
2
A
A
A
AA
b b ac
a
t
l
t
l
t
æö
÷
ç
÷
ç
=
÷
ç
÷
÷
ç
èø
æö
÷
ç
÷
ç
=
÷
ç
÷
÷
ç
-
èø
-+ -
=
(VI.6)
(VI.7)
(VI.8)

243
AEROSOL RESIDENCE TIMES
where
Pb Po
Po
Bi Po
Po
Bi Po
;
;
11
;
and .
X XX
AN
aA A
bA
A
c
l
ll
ll
=
=-
æö
÷
ç
÷
ç
=- +
÷
ç
÷
÷
ç
èø
-
=
In each case, these should represent the residence time of the aerosols to
which
210
Pb is attached. In practice, the apparent residence times calculated from
these three radionuclide pairs often do not agree (e.g. see Refs
[VI.2, VI.5]).
Table
VI.1 shows a summary of apparent residence times calculated for
tropospheric aerosols from a number of studies
[VI.6, VI.12]. The residence
times are highly variable. In general, those obtained from the (
210
Pb,
210
Po) pair
are higher than for the other two pairs. However, relatively high residence times
are also sometimes obtained for the (
210
Pb,
210
Bi) pair.
Resuspension of dust can be an interfering factor in these calculations, since
210
Pb and its progeny will be at, or close to, secular equilibrium on this dust. This
factor can be greater for the larger aerosol size fractions
[VI.14, VI.23, VI.24].
It can also be greater for dry fallout than for precipitation
[VI.11]. One approach
to this problem is to use the determination of a member of the uranium decay
chain above
210
Pb to apply a correction to the measured
210
Pb,
210
Bi and
210
Po
concentrations to allow calculation of corrected residence times
[VI.6, VI.10].
In the case of the (
210
Pb,
210
Po) pair, another factor is the influence of
significant sources of
210
Po from the Earth’s surface due to the volatility of
polonium, such as volcanic emissions, biomass burning and coal combustion (see
Chapter
5). The influence of such sources is expected to be much lower in the
case of (
210
Pb,
210
Bi). In addition, the mean residence time of
210
Bi (τ
Bi
= 7.2 d)
is more comparable to aerosol residence times in the troposphere than that of
210
Po. Consequently, a residence time calculation based on (
210
Pb,
210
Bi) is more
reliable than one based on (
210
Pb,
210
Po).
The apparent residence times obtained represent an average for the activity
in the air or precipitation sample. They can therefore represent an average of
residence times for several air masses which have mixed, such as a mixture
of upper tropospheric and lower tropospheric air with different residence
times
[VI.5, VI.24]. For precipitation samples, they can represent an average of
residence times for in-cloud rainout and below-cloud washout, and mixing of air
masses during the storm
[VI.9, VI.10].

244
APPENDIX VI
The use of the (
210
Pb,
210
Bi) and (
210
Pb,
210
Po) pairs relies on determination
of these radionuclides in air filter or precipitation samples. The
210
Bi and
210
Po
determinations should be carried out as soon as practicable after collection of
the sample in order to minimize uncertainties resulting from decay and ingrowth
corrections
[VI.25]. When determining the sample
210
Po activity, ingrowth from
TABLE VI.1. APPARENT AEROSOL RESIDENCE TIMES OBTAINED FOR
ATMOSPHERIC OR PRECIPITATION SAMPLES
Location
τ
R
(Rn, Pb)
(d)
τ
R
(Pb, Bi)
(d)
τ
R
(Pb, Po)
(d)
Ref.
Portugal
Savacem —
a
0.5–82 (5.7) 7–757 (33) [VI.6]
USA
Oak Ridge, TN
Boulder, CO
Argonne, IL
Madison, WI
Fayetteville, AR
Fayetteville, AR
Poker Flat, AK
Eagle, AK
—
a
2.2–3.4
—
a
—
a
—
a
—
a
—
a
—
a
4.8–15.3 (8.2)
1.59–13.0 (5.4)
6–67
—
2.4–25.6 (8.5)
3–240 (20)
—
a
—
a
—
a
11–77
33–66
9.6
—
a
2–320 (40)
11.9–32
0–38.9
[VI.12]
[VI.2, VI.5, VI.13]
[VI.14]
[VI.15]
[VI.16]
[VI.9]
[VI.7]
[VI.7]
Switzerland
Jungfraujoch —
a
—
a
1–12 (6) [VI.17]
Germany
Freiburg —
a
—
a
20 [VI.18]
France
Gif-sur-Yvette 6.5
6.77
8.8–10.5
7–9
—
a
[VI.19]
United Kingdom
Milford Haven 6.77 —
a
40 [VI.20]
India
Mumbai —
a
8 —
a
[VI.21]
Egypt
El-Minia —
a
4.3–12.86 (9.83) —
a
[VI.22]
Source: Table 3 of Ref. [V.12].
Note: Averages in parentheses.
a
—: data not available.
245
AEROSOL RESIDENCE TIMES
both
210
Pb and
210
Bi between sample collection and analysis needs to be allowed
for. In this case, a separate
210
Bi determination can be used to avoid unquantifiable
errors in the ingrowth and decay correction
[VI.8, VI.26].
REFERENCES TO APPENDIX VI
[VI.1] SEINFELD, J.H., PANDIS, S.N., Atmospheric Chemistry and Physics: From Air
Pollution to Climate Change, 3rd edn, John Wiley and Sons, Hoboken, NJ (2006).
[VI.2] POET, S.E., MOORE, H.E., MARTELL, E.A., Lead 210, bismuth 210 and polonium
210 in the atmosphere: Accurate ratio measurement and application to aerosol
residence time determination, J. Geophys. Res.
77 (1972) 6515–6527.
[VI.3] TUREKIAN, K.K., NOZAKI, Y., BENNINGER, L.K., Geochemistry of atmospheric
radon and radon products, Annu. Rev. Earth Planet Sci.
5 (1977) 227–255.
[VI.4] RANGARAJAN, C., EAPEN, C.D., The use of natural radioactive tracers in a study
of atmospheric residence times, Tellus
B 42 (1990) 142–147.
[VI.5] MOORE, H.E., POET, S.E., MARTELL, E.A.,
222
Rn,
210
Pb,
210
Bi and
210
Po profiles
and aerosol residence times versus altitude, J. Geophys. Res.
78 (1973) 7065–7075.
[VI.6] CARVALHO, F.P., Origins and concentrations of
222
Rn,
210
Pb,
210
Bi and
210
Po in the
surface air at Lisbon, Portugal, at the Atlantic edge of the European continental
landmass, Atmos. Environ.
29 (1995) 1809–1819.
[VI.7] BASKARAN, M., SHAW, G.E., Residence time of arctic haze aerosols using the
concentrations and activity ratios of
210
Po,
210
Pb and
7
Be, J. Aerosol Sci. 32
(2001)
443–452.
[VI.8] DŁUGOSZ-LISIECKA, M., BEM, H., Determination of the mean aerosol residence
times in the atmosphere and additional
210
Po input on the base of simultaneous
determination of
7
Be,
22
Na,
210
Pb,
210
Bi and
210
Po in urban air, J. Radioanal. Nucl.
Chem.
293 (2012) 135–140.
[VI.9] GAVINI, M.B., BECK, J.N., KURODA, P.K., Mean residence times of the long-lived
radon daughters in the atmosphere, J. Geophys. Res.
79 (1974) 4447–4452.
[VI.10] MARTIN, P., Uranium and thorium series radionuclides in rainwater over several
tropical storms, J. Environ. Radioact.
65 (2003) 1–18.
[VI.11] McNEARY, D., BASKARAN, M., Residence times and temporal variations of
210
Po
in aerosols and precipitation from southeastern Michigan, United States, J. Geophys.
Res.
112 D4 (2007).
[VI.12] PAPASTEFANOU, C., Residence time of tropospheric aerosols in association with
radioactive nuclides, Appl. Radiat. Isot.
64 (2006) 93–100.
[VI.13] MOORE, H.E., POET, S.E., MARTELL, E.A., “Tropospheric aerosol residence
times indicated by radon and radon-daughter concentrations”, Natural Radiation
Environment
II (Proc. 2nd Int. Symp. Houston, 1972) (ADAMS, J.A.S., LOWDER,
W.M., GESELL, T.F., Eds), United States Department of Commerce, Springfield, VA
(1972)
775–786.
246
APPENDIX VI
[VI.14] MARLEY, N.A., et al., Measurement of
210
Pb,
210
Po and
210
Bi in size-fractionated
atmospheric aerosols: An estimate of fine-aerosol residence times, Aerosol Sci.
Technol.
32 (2000) 569–583.
[VI.15] FRANCIS, C.W., CHESTERS, G., HASKIN, L.A., Determination of
210
Pb mean
residence time in the atmosphere, Environ. Sci. Technol.
4 (1970) 586–589.
[VI.16] FRY, L.M., MENON, K.K., Determination of the tropospheric residence time of
lead-210, Science
137 (1962) 994–995.
[VI.17] GÄGGELER, H.W., JOST, D.T., BALTENSPERGER, U., SCHWIKOWSKI, M.,
Radon and thoron decay product and
210
Pb measurements at Jungfraujoch,
Switzerland, Atmos. Environ.
29 (1995) 607–616.
[VI.18] LEHMANN, L., SITTKUS, A., Bestimmung von Aerosolverweilzeiten aus den RaD
und RaF-Gehalt der atmosphärischen Luft und des Niederschlages,
Naturwissenschaft
46 (1959) 9–10.
[VI.19] LAMBERT, G., et al., Cycle du radon et de ses descendants: Application à 1’étude
des échanges troposphère-stratosphère, Ann. Geophys.
38 (1982) 497–531.
[VI.20] PEIRSON, D.H., CAMBRAY, R.S., SPICER, G.S., Lead-210 and polonium-210 in
the atmosphere, Tellus
18 (1966) 427–433.
[VI.21] RANGARAJAN, C., A study of the mean residence time of the natural radioactive
aerosols in the planetary boundary layer, J. Environ. Radioact.
15 (1992) 193–206.
[VI.22] AHMED, A.A., MOHAMMED, A., ALI, A.E., EL-HUSSEIN, A., BARAKAT, A., A
study on aerosol residence time in El-Minia, Egypt, J. Aerosol Sci.
31 (2000) 470–471.
[VI.23] GAFFNEY, J.S., MARLEY, N.A., CUNNINGHAM, M.M., Natural radionuclides in
fine aerosols in the Pittsburgh area, Atmos. Environ.
38 (2004) 3191–3200.
[VI.24] BASKARAN, M., Po-210 and Pb-210 as atmospheric tracers and global atmospheric
Pb-210 fallout: A review, J. Environ. Radioact.
102 (2011) 500–513.
[VI.25] KIM, C.-K., MARTIN, P., FAJGELJ, A., Quantification of measurement uncertainty
in the sequential determination of
210
Pb and
210
Po by liquid scintillation counting and
alpha-particle spectrometry, Accredit. Qual. Assur.
13 (2008) 691–702.
[VI.26] LOZANO, R.L., SAN MIGUEL, E.G., BOLIVAR, J.P., Assessment of the influence
of in
situ
210
Bi in the calculation of in situ
210
Po in air aerosols: Implications on
residence time calculations using the
210
Po/
210
Pb activity ratios, J. Geophys.
Res.
116 (2011).
247
Appendix VII
USE OF
210
Po AS A RADIOTRACER IN BIOGENIC
PARTICULATE FLUX STUDIES IN THE OCEANS
VII.1. INTRODUCTION
Polonium-210 is present in the world’s oceans, and
210
Po sources and its
distribution and dynamics in the water column are described in more detail in
Chapter 8. The distribution of
210
Po in the water column of the open ocean is
generally similar in various oceanic regions. In the upper layer of the ocean,
210
Po activity concentration in water is lower than that of
210
Pb due to the
overriding importance of atmospheric deposition where
210
Po:
210
Pb is roughly
around 0.1. In the mesopelagic zone, under the euphotic/epipelagic layer,
210
Po
is in excess relative to
210
Pb, probably due to release of
210
Po soluble forms from
sinking faecal pellets and biogenic debris originating from the upper layer. In the
bathypelagic and abyssopelagic zones,
210
Po concentrations are lower than
210
Pb,
and although continuously produced in the water from
226
Ra–
210
Pb radioactive
decay,
210
Po is also continuously removed by sinking particulate matter [VII.1].
The mean residence time of
210
Pb and
210
Po radionuclides can be determined
in the water column in several oceanic regions using the disequilibria between
226
Ra,
210
Pb and
210
Po. In general,
210
Po residence time in the upper layer of the
ocean is short, around 0.6–0.7 a
–1
[VII.1–VII.4]. This short residence time is
due to rapid
210
Po adsorption onto particles such as viruses, bacteria, plankton,
organic debris and inorganic particles that make the gravitational flux of settling
particles. Furthermore,
210
Po is taken up into the cells of phytoplankton and
becomes associated with soluble proteins in the cytosol [VII.5]. Zooplankton,
as phytoplankton grazers, easily absorb
210
Po with cytosolic proteins of ingested
phytoplankton, while metals, including
210
Pb, are segregated and remain mostly
associated with the ejected cell debris.
The grazing activity of zooplankton in the epipelagic zone processes and
removes particulate matter from the ocean surface layer, mostly packing it into
faecal pellets that rapidly sink throughout the water column reach the abyssal
sea-floor in a relatively short time [VII.6, VII.7]. The role of zooplankton in the
removal of
210
Po and other elements from the epipelagic layer of the ocean has
long been known and the associated transport of contaminants (e.g. plutonium
from fallout) in the oceans has been investigated [VII.8–VII.11].
248
APPENDIX VII
Besides phytoplankton and zooplankton, all organisms of marine food
chains in the upper layer of the ocean contribute through similar processes,
although not quantified yet, to concentrate
210
Po, starting from ionic
210
Po in the
soluble phase and gradually incorporating it into large particles and biota tissues.
With the seasons, and in line with the highs and lows of primary productivity in
the epipelagic layer,
210
Po removal rates and flux to the deep-sea with the sinking
particles vary throughout the year [VII.3, VII.12].
VII.2. USE OF
210
Po AS A RADIOTRACER
Studies on the biogenic particle flux to the deep ocean started in the 1960s
with the supply of organic carbon from the euphotic zone to the permanently dark
deep-sea layers and the sustainability of deep-sea food chains. In the 1970s, the
existence of hydrothermal vents and deep-sea food chains based on the synthesis
of organic matter by sulphur reducing bacteria were discovered, which sustain
localized deep-sea food chains that do not depend on sunlight to synthesize
organic compounds. For most of the ocean sea-floor, however, deep-sea life
depends on the organic matter synthesized in the euphotic zone and exported to
the deep sea [VII.13].
Investigations on the carbon cycle have highlighted the importance of the
oceans as a sink for atmospheric carbon. Phytoplankton in the oceans, similar to
forests on land, is a major sink for atmospheric CO
2
[VII.14, VII.15]. Dissolution
of atmospheric CO
2
in the ocean is followed by incorporation of CO
2
into organic
molecules through photosynthesis by phytoplankton, and this incorporation is
followed by organic carbon recycling in, and export from, the euphotic zone with
the sinking particle flux. This association of oceanic organic matter production
and particulate matter export, conceptualized as the ‘biological carbon pump’,
contributes to controlling CO
2
levels in the atmosphere and buffering the
global climate system [VII.14, VII.16]. Therefore, the downward transfer and
sequestration of carbon in the oceans is a central issue in global climate change.
Studies on particulate organic carbon, particulate inorganic carbon and
biomineral (e.g. calcium and silicon) transfers in the ocean looked into natural
tracers to help quantify transfer fluxes [VII.3, VII.7]. The behaviour of several
radionuclides from the uranium radioactive series in the oceans has been assessed,
especially
210
Pb,
210
Po and
234
Th, and their potential as natural radiotracers for
particle flux [VII.17].
Phytoplankton productivity is connected to enhanced
210
Po absorption from
sea water, and association of this naturally occurring radionuclide with the organic
matter cycle in the surface ocean layer has been established [VII.15, VII.18].
249
BIOGENIC PARTICULATE FLUX STUDIES
Progress has been made in the direct measurement of the biogenic particle
flux in the ocean, especially due to the use of drifting or moored particle
interceptor devices or sediment traps
[VII.6]. Determination of radionuclide
concentrations in samples of particulate material indicate a high association of
several particle reactive radionuclides, such as
210
Pb,
210
Po,
239+240
Pu and
234
Th,
with the total flux of particulate and detrital material
[VII.18–VII.20]. However,
recent work has shown a better correlation between
210
Po and particulate organic
carbon fluxes
[VII.3]. Owing to
210
Po binding to amino acids and incorporation
into proteins in living organisms, the use of
210
Po as a tracer for particulate
organic carbon flux in the ocean water column seems to have a scientific basis
and has recently been explored. Polonium-210 seems to offer a good indirect
means of computing the flux of organic particles and thus particulate organic
carbon in the ocean
[VII.3, VII.18–VII.20].
Although
210
Pb is also a particle reactive radionuclide, as is
234
Th,
210
Pb is adsorbed onto organic and inorganic surfaces but is not significantly
absorbed into marine organisms to the extent that
210
Po is (see Chapter 8). For
suspended particulate matter in coastal environments throughout the year, both
210
Pb and
210
Po total concentrations in water are positively correlated with the
load of suspended particulate matter (p
< 0.01). However,
210
Po concentration
in particulate matter is better correlated with chlorophyll-a (p
< 0.10), while
210
Pb was correlated with the inorganic particle residue (p < 0.01) but not with
chlorophyll-a
[VII.21]. All evidence suggests that
210
Po is bound, and mostly
incorporated in, living matter, while
210
Pb seems to undergo adsorption onto the
mineral and detrital particle fraction. Other naturally occurring radionuclides,
such as
234
Th, have been used in combination with
210
Po to determine the mean
residence time of particles in the surface ocean layer [VII.3]. It seems that
210
Po
is a suitable tracer in oligotrophic conditions (non-bloom of phytoplankton),
while
210
Pb and
234
Th display a better correlation and are thus better tracers of the
inorganic fraction of particulate flux. Different natural radiotracers can provide
different information and, actually, the measurement of
210
Po and
234
Th might
be complementary to better understand the particle flux
[VII.3, VII.7, VII.19,
VII.20,
VII.22].
Further research could be on the relationship of radionuclides and
particulate organic carbon in particles of different size classes, and on the binding
and partitioning (K
d
) of radiotracers and organic particles. Further studies on
210
Po binding adsorption and absorption by microscopic life forms in the ocean
are still needed, as are studies on the release rates of
210
Po from sinking particles
(carcasses and faecal pellets).
Determination of
210
Po in association with other parameters is a tool to
assess the organic particle flux in various regions of the ocean, and it is expected
250
APPENDIX VII
that by using these natural radiotracers a global picture of the biominerals and
particulate organic carbon fluxes in the world’s oceans will emerge.
REFERENCES TO APPENDIX VII
[VII.1] BACON, M.P., SPENCER, D.W., BREWER, P.G.,
210
Pb/
226
Ra and
210
Po/
210
Pb
disequilibria in seawater and suspended particulate matter, Earth Planet. Sci. Lett.
32
(1976)
277–296.
[VII.2] MASQUÉ, P., SANCHEZ-CABEZA, J.A., BRUACH, J.M., PALACIOS, E.,
CANALS, M., Balance and residence times of
210
Pb and
210
Po in surface waters of
the northwestern Mediterranean Sea, Cont. Shelf Res.
22 (2002) 2127–2146.
[VII.3] LE MOIGNE, F.A.C., et al., Export of organic carbon and biominerals derived from
234
Th and
210
Po at the Porcupine Abyssal Plain, Deep Sea Res. Part I 72
(2013)
88–101.
[VII.4] CARVALHO, F.P., “Uranium series radionuclides in the water column of the
Northeast Atlantic Ocean”, Isotopes in Hydrology, Marine Ecosystems and Climate
Change Studies (Proc. Int. Symp. Monaco, 2011), Vol.
2, IAEA, Vienna
(2013)
427–441.
[VII.5] REINFELDER, J.R., FISHER, N.S., The assimilation of elements ingested by
marine plankton, Science
251 (1991) 794–796.
[VII.6] FOWLER, S.W., KNAUER, G.A., Role of large particles in the transport of elements
and organic compounds through the oceanic water column, Prog. Oceanogr.
16
(1986)
147–194.
[VII.7] VERDENY, E., et al., POC export from ocean surface waters by means of
234
Th/
238
U
and
210
Po/
210
Pb disequilibria: A review of the use of two radiotracer pairs, Deep Sea
Res. Part
II 56 (2009) 1502–1518.
[VII.8] CHERRY, R.D., FOWLER, S.W., BEASLEY, T.M., HEYRAUD, M., Polonium-210:
Its vertical oceanic transport by zooplankton metabolic activity, Mar. Chem.
3
(1975)
105–110.
[VII.9] BEASLEY, T.M., HEYRAUD, M., HIGGO, J.J.W., CHERRY, R.D., FOWLER,
S.W.,
210
Po and
210
Pb in zooplankton fecal pellets, Mar. Biol. 44 (1978) 325–328.
[VII.10] HIGGO, J.J.W., CHERRY, R.D., HEYRAUD, M., FOWLER, S.W., BEASLEY,
T.M., “Vertical oceanic transport of alpha-radioactive nuclides by zooplankton fecal
pellets”, Natural Radiation Environment
III (Proc. Symp. Houston, 1978), Vol. 1
(GESELL, T.F., LOWDER, W.M., Eds), Department of Energy, Oak Ridge, TN
(1980)
502–513.
[VII.11] LINDAHL, P., LEE, S.-H., WORSFOLD, P., KEITH-ROACH, M., Plutonium
isotopes as tracers for ocean processes: A review, Mar. Environ. Res.
69
(2010)
73–84.
[VII.12] MARTIN, P., et al., Export and mesopelagic particle flux during a North Atlantic
spring diatom bloom, Deep Sea Res. Part
I 58 (2011) 338–349.
251
BIOGENIC PARTICULATE FLUX STUDIES
[VII.13] MILLER, C.B., WHEELER, P.A., Biological Oceanography, Wiley and Blackwell,
New York
(2012).
[VII.14] EPPLEY, R.W., PETERSON, B.J., Particulate organic matter flux and planktonic
new production in the deep ocean, Nature
282 (1979) 677–680.
[VII.15] RILEY, J.S, et al., The relative contribution of fast and slow sinking particles to
ocean carbon export, Global Biogeochem. Cycles
26 (2012).
[VII.16] HENSON, S., et al., A reduced estimate of the strength of the ocean’s biological
carbon pump, Geophys. Res. Lett.
38 (2011).
[VII.17] COCHRAN, J.K., MASQUÉ, P., Short-lived U/Th series radionuclides in the ocean:
Tracers for scavenging rates, export fluxes and particle dynamics, Rev. Mineral.
Geochem.
52 (2003) 461–492.
[VII.18] FRIEDRICH, J., RUTGERS VAN DER LOEFF, M.M., A two-tracer (
210
Po–
234
Th)
approach to distinguish organic carbon and biogenic silica export flux in the
Antarctic Circumpolar Current, Deep Sea Res. Part
I 49 (2002) 101–120.
[VII.19] STEWART, G.M., et al., Comparing POC export from
234
Th/
238
U and
210
Po/
210
Pb
disequilibria with estimates from sediment traps in the northwest Mediterranean,
Deep Sea Res. Part
I 54 (2007) 1549–1570.
[VII.20] STEWART, G.M., et al., Exploring the connection between
210
Po and organic matter
in the northwestern Mediterranean, Deep Sea Res. Part I 54 (2007) 415–427.
[VII.21] CARVALHO, F.P., OLIVEIRA, J.M., MALTA, M., “Vegetation fires and release of
radioactivity into the air”, Environmental Health and Biomedicine (BREBBIA,
C.A., et al., Eds), WIT Press, Southhampton (2011)
3–9.
[VII.22] STEWART, G.M., MORAN, S.B., LOMAS, M.W., Seasonal POC fluxes at BATS
estimated from
210
Po deficits, Deep Sea Res. Part I 57 (2010) 113–124.
253
ABBREVIATIONS
APDC ammonium pyrrolidine dithiocarbamate
CR concentration ratio
DCC dose conversion coefficient
DW dry weight
FW fresh weight
ICRP International Commission on Radiological Protection
K
d
distribution coefficient
NORM naturally occurring radioactive material
PBL planetary boundary layer
TF transfer factor
255
CONTRIBUTORS TO DRAFTING AND REVIEW
Carvalho, F. University of Lisbon, Portugal
Chambers, D. SENES Consultants Ltd, Canada
Fernandes, S. SENES Consultants Ltd, Canada
Fesenko, S. International Atomic Energy Agency
Holm, E. University of Gothenburg, Sweden
Howard, B. Centre for Ecology and Hydrology, United Kingdom
Martin, P. International Atomic Energy Agency
Phaneuf, M. International Atomic Energy Agency
Porcelli, D. University of Oxford, United Kingdom
Pröhl, G. International Atomic Energy Agency
Twining, J. Consultant
Vandenhove, H. SCK•CEN, Belgian Nuclear Research Centre, Belgium
Consultants Meetings
Vienna, Austria: 22–26 November 2010, 16–20 May 2011, 20–24 February 2012
@
No. 25
ORDERING LOCALLY
In the following countries, IAEA priced publications may be purchased from the sources listed below or
from major local booksellers.
Orders for unpriced publications should be made directly to the IAEA. The contact details are given at
the end of this list.
CANADA
Renouf Publishing Co. Ltd
22-1010 Polytek Street, Ottawa, ON K1J 9J1, CANADA
Telephone: +1 613 745 2665 Fax: +1 643 745 7660
Email: [email protected] Web site: www.renoufbooks.com
Bernan / Rowman & Littlefield
15200 NBN Way, Blue Ridge Summit, PA 17214, USA
Tel: +1 800 462 6420 • Fax: +1 800 338 4550
Email: [email protected] Web site: www.rowman.com/bernan
CZECH REPUBLIC
Suweco CZ, s.r.o.
Sestupná 153/11, 162 00 Prague 6, CZECH REPUBLIC
Telephone: +420 242 459 205 Fax: +420 284 821 646
Email: [email protected] Web site: www.suweco.cz
FRANCE
Form-Edit
5 rue Janssen, PO Box 25, 75921 Paris CEDEX, FRANCE
Telephone: +33 1 42 01 49 49 Fax: +33 1 42 01 90 90
Email: [email protected] Web site: www.form-edit.com
GERMANY
Goethe Buchhandlung Teubig GmbH
Schweitzer Fachinformationen
Willstätterstrasse 15, 40549 Düsseldorf, GERMANY
Telephone: +49 (0) 211 49 874 015 Fax: +49 (0) 211 49 874 28
Email: [email protected] Web site: www.goethebuch.de
INDIA
Allied Publishers
1st Floor, Dubash House, 15, J.N. Heredi Marg, Ballard Estate, Mumbai 400001, INDIA
Telephone: +91 22 4212 6930/31/69 Fax: +91 22 2261 7928
Email: [email protected] Web site: www.alliedpublishers.com
Bookwell
3/79 Nirankari, Delhi 110009, INDIA
Telephone: +91 11 2760 1283/4536
Email: [email protected] Web site: www.bookwellindia.com
ITALY
Libreria Scientifica “AEIOU”
Via Vincenzo Maria Coronelli 6, 20146 Milan, ITALY
Telephone: +39 02 48 95 45 52 Fax: +39 02 48 95 45 48
Email: [email protected] Web site: www.libreriaaeiou.eu
JAPAN
Maruzen-Yushodo Co., Ltd
10-10 Yotsuyasakamachi, Shinjuku-ku, Tokyo 160-0002, JAPAN
Telephone: +81 3 4335 9312 Fax: +81 3 4335 9364
Email: [email protected] Web site: www.maruzen.co.jp
RUSSIAN FEDERATION
Scientific and Engineering Centre for Nuclear and Radiation Safety
107140, Moscow, Malaya Krasnoselskaya st. 2/8, bld. 5, RUSSIAN FEDERATION
Telephone: +7 499 264 00 03 Fax: +7 499 264 28 59
Email: [email protected] Web site: www.secnrs.ru
UNITED STATES OF AMERICA
Bernan / Rowman & Littlefield
15200 NBN Way, Blue Ridge Summit, PA 17214, USA
Tel: +1 800 462 6420 • Fax: +1 800 338 4550
Email: [email protected] Web site: www.rowman.com/bernan
Renouf Publishing Co. Ltd
812 Proctor Avenue, Ogdensburg, NY 13669-2205, USA
Telephone: +1 888 551 7470 Fax: +1 888 551 7471
Email: [email protected] Web site: www.renoufbooks.com
Orders for both priced and unpriced publications may be addressed directly to:
Marketing and Sales Unit
International Atomic Energy Agency
Vienna International Centre, PO Box 100, 1400 Vienna, Austria
Telephone: +43 1 2600 22529 or 22530 • Fax: +43 1 2600 29302 or +43 1 26007 22529
Email: [email protected] • Web site: www.iaea.org/books
16-49041

@
RELATED PUBLICATIONS
www.iaea.org/books
HANDBOOK OF PARAMETER VALUES FOR THE
PREDICTION OF RADIONUCLIDE TRANSFER IN
TERRESTRIAL AND FRESHWATER ENVIRONMENTS
Technical Reports Series No. 472
STI/DOC/010/472 (194 pp.; 2010)
ISBN 978–92–0–113009–9 Price: €45.00
THE ENVIRONMENTAL BEHAVIOUR OF RADIUM:
REVISED EDITION
Technical Reports Series No. 476
STI/DOC/010/476 (267 pp.; 2014)
ISBN 978–92–0–143310–7 Price: €52.00
HANDBOOK OF PARAMETER VALUES FOR THE
PREDICTION OF RADIONUCLIDE TRANSFER
TO WILDLIFE
Technical Reports Series No. 479
STI/DOC/010/479 (211 pp.; 2014)
ISBN 978–92–0–100714–8 Price: €55.00
Atoms for Peace
Atoms for Peace

INTERNATIONAL ATOMIC ENERGY AGENCY
VIENNA
ISBN 978–92–0–112116–5
ISSN 0074–1914
Polonium-210 is an alpha emitting radionuclide
with no radioactive progeny and produces
only very-low-intensity gamma rays at very low
abundance. This means doses largely arise
from internal exposure. In addition to the
relatively high ingestion dose coefficient of
210
Po, radionuclide transfer in the environment
results in high activity concentrations in certain
foods. This publication focuses on radionuclide
transfers in terrestrial, freshwater and marine
environments, and provides information on key
transfer processes, concepts and models.
The Environmental Behaviour of Poloniumtechnical reportS series no. 484
