
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT® Copyright 2004-2020 American Medical Association.
1
Multiple Sclerosis
Quality Measurement Set
2020 Update
Approved by the Multiple Sclerosis Quality Measure Development Work Group on September 1, 2020.
Approved by the AANI Quality Measure Subcommittee on September 10, 2020. Approved by AANI
Quality Committee on October 13, 2020. Approved by AANI Board of Directors on November 5, 2020.

©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT® Copyright 2004-2020 American Medical Association.
2
Disclaimer
Quality Measures published by the American Academy of Neurology Institute and its affiliates are
assessments of current scientific and clinical information provided as an educational service. The
information: 1) should not be considered inclusive of all proper treatments, methods of care, or as a
statement of the standard of care; 2) is not continually updated and may not reflect the most recent
evidence (new evidence may emerge between the time information is developed and when it is published
or read); 3) addresses only the question(s) or topic(s) specifically identified; 4) does not mandate any
particular course of medical care; and 5) is not intended to substitute for the independent professional
judgment of the treating provider, as the information does not account for individual variation among
patients. In all cases, the selected course of action should be considered by the treating provider in the
context of treating the individual patient. Use of the information is voluntary. AANI provides this
information on an “as is” basis, and makes no warranty, expressed or implied, regarding the information.
AANI specifically disclaims any warranties of merchantability or fitness for a particular use or purpose.
AANI assumes no responsibility for any injury or damage to persons or property arising out of or related
to any use of this information or for any errors or omissions.
©2020 American Academy of Neurology Institute. All rights reserved.
Limited proprietary coding is contained in the measure specifications for convenience. Users of the
proprietary coding sets should obtain all necessary licenses from the owners of these code sets. The AANI
and its members disclaim all liability for use or accuracy of any Current Procedural Terminology (CPT®)
or other coding contained in the specifications. ICD-10 copyright 2012 International Health Terminology
Standards Development Organization
CPT ® is a registered trademark of the American Medical Association and is copyright 2020. CPT®
codes contained in the Measure specifications are copyright 2004-2020 American Medical Association.

©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT® Copyright 2004-2020 American Medical Association.
3
Contents
Work Group Members .................................................................................................................................. 4
Importance and Prevalence ........................................................................................................................... 5
Defining Multiple Sclerosis Quality Measures ......................................................................................... 5
Prevalence and Impact .......................................................................................................................... 5
Measure Development Process ............................................................................................................. 5
2020 Multiple Sclerosis (MS) Quality Measurement Set ............................................................................. 6
2014 Multiple Sclerosis Quality Measures Retired .................................................................................. 6
Other Potential Measures .......................................................................................................................... 8
Measure Harmonization ............................................................................................................................ 8
Introductory References ............................................................................................................................ 8
2020 Multiple Sclerosis (MS) Quality Measure Specifications .................................................................... 9
Magnetic Resonance Imaging (MRI) Monitoring for Patients with Multiple Sclerosis (MS) .................. 9
Disease Modifying Therapies (DMT) Monitoring for Patients with Multiple Sclerosis (MS) ............... 15
Bladder, Bowel, and Sexual Dysfunction Screening and Follow-Up for Patients with Multiple Sclerosis
(MS) ........................................................................................................................................................ 21
Cognitive Impairment Screening and Follow-Up for Patients with Multiple Sclerosis (MS) ................ 30
Fatigue Screening and Follow-Up for Patients with Multiple Sclerosis (MS) ....................................... 39
Exercise and Appropriate Physical Activity Counseling for Patients with Multiple Sclerosis (MS) ..... 47
Contact Information .................................................................................................................................... 52
Appendix A: Disclosures ............................................................................................................................ 53

4
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Work Group Members
American Academy of Neurology
Alex Rae-Grant, MD, FRCPC, FAAN
Neeta Garg, MD
Barbara Giesser, MD, FAAN
Iris Vanessa Marin Collazo, MD
Michael Olek, DO
Consortium of Multiple Sclerosis Centers
Jeffrey English, MD
Multiple Sclerosis Association of America
Amanda Montague, EdM
National Multiple Sclerosis Society
Lilyana Amezcua, MD, MS, FAAN
Elizabeth Page
American Academy of Neurology Facilitators - non-voting work group members
Tracie Caller, MD
Adam G. Kelly, MD, FAAN
American Academy of Neurology Staff
Amy Bennett, JD
Molly Byrne, MPH
Erin Lee
Karen Lundgren, MBA
Brandon Magliocco, MS
Piper Ranallo, PhD
Becky Schierman, MPH

5
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Importance and Prevalence
Defining Multiple Sclerosis Quality Measures
The American Academy of Neurology Institute (AANI) has developed quality measures since 2008 based on the belief
that specialists should play a major role in selecting and creating measures that will drive performance improvement and
possibly be used in accountability programs in the future. In 2014, the AANI developed a set of multiple sclerosis quality
measures, which was released in 2015 and reaffirmed on July 29, 2017. In 2019, the AANI formed a standing Multiple
Sclerosis (MS) Quality Measurement Set Work Group (work group). The AANI charged this work group with updating
existing and developing new quality measures for patients diagnosed with multiple sclerosis.
Additionally, the Work Group is charged with the surveillance of evolving evidence base to determine if future updates
are needed, as well as development and release of quality improvement tools to assist in the implementation of the
measures in practice. The work group will meet twice yearly to review any new guideline or evidence developments and
testing data. This will allow for more timely updates and maintenance of measures for use in public reporting and
accountability programs.
Prevalence and Impact
The estimated cumulated prevalence of MS among adults in the United States in 2010 was approximately 727,000. In
2017, that number was substantially higher, approximately 914,000.
1,2
The prevalence is higher among women with a
female: male ratio of 2.8.
1
A recent study found that multiple sclerosis health care spending in the United States was $13.9 billion in 2016.
3
Multiple
sclerosis was one of the health conditions with the highest annual spending growth paid by both public and private
insurance for the year, which may be correlated with the introduction of specialty drug treatment.
3
Measure Development Process
The AANI identified non-voting facilitators from the Quality Measure Subcommittee and Quality Committee to provide
methodological support and guide the work group to consensus decisions. A call for work group volunteers was made and
a subject matter expert Chair was identified. Work group members were selected based on review of disclosure
statements, subject matter expertise, and measure development experience. All work group members are required to
disclose relationships with industry and other entities to avoid actual, potential, or perceived conflicts of interest. Seated
work group members were instructed to abstain from voting on individual measure concepts if a conflict was present. See
Appendix A.
The AANI measure development process involves a modified Delphi review by the work group to reach consensus on
measures to be developed prior to a 21-day public comment and following the public comment for further refinement.
4
The measures in this set are being made available without any prior testing. The AAN encourages testing of this
measurement set for feasibility and reliability by organizations or individuals positioned to do so. Select measures will be
beta tested once the set has been released, prior to submission to the Centers for Medicare & Medicaid Services (CMS)
for consideration in Quality Payment Program’s (QPP) Merit-based Incentive Payment System (MIPS) and the National
Quality Forum for possible endorsement. The measurement set will be reviewed for updates at least every six months by
the standing multiple sclerosis measure development work group.
Below is an illustration of the measure development process from proposals, discussion, research, evaluation, to approval.

6
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
2020 Multiple Sclerosis (MS) Quality Measurement Set
The work group approved six measures for the 2020 update.
Magnetic Resonance Imaging (MRI) Monitoring for Patients with MS
Disease Modifying Therapies (DMT) Monitoring for Patients with MS
Bladder, Bowel, and Sexual Dysfunction Screening and Follow-up for Patients with MS
Cognitive Impairment Screening and Follow-up for Patients with MS
Fatigue Screening and Follow-up for Patients with MS
Exercise and Appropriate Physical Activity Counseling for Patients with MS
There is no requirement that all the measures in the measurement set be used. Providers and treatment teams are
encouraged to identify the one or two measures that would be most meaningful to their patient population and implement
those measures to drive performance improvement in practice. Data should be collected for an initial benchmark period,
and results used to drive meaningful changes to improve performance and overall care.
2014 Multiple Sclerosis Quality Measures Retired
Seven of the original multiple sclerosis quality measures were retired. Measures may be retired for multiple reasons, and
retirement does not reflect a lack of value in quantifying a concept. The work group strongly believes these concepts
remain of value, but measures were retired due to feasibility concerns or existence of cross-cutting measures that include
patients with MS in the denominator. The AANI is encouraging quality measurement development work groups to reduce
the number of measures available for an individual disease topic to reduce clinician burden, focus on fewer meaningful
measures for quality improvement, and allow for testing of measures developed. Rationale for individual measure
retirement is detailed in the following section:
11 Existing Measures Due
for Triennial Review
3 new concepts advanced
6 measures approved
Data Review via Concept
Rankings
Public comment and
Refinement
Group Discussions
5 new measures advanced
1 measure reaffirmed
2 additional measures
retired
Medical librarian search
Existing measure decisions
• 1 measure
reaffirmed
• 5 measures retired
• 4 measures
identified for
updates
• 1 measure held for
further discussion
25 new concepts identified

7
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Multiple Sclerosis (MS) Diagnosis – Retired in 2020
Current MS Disability Scale Score – Retired in 2020
Fall Risk Screening for Patients with MS – Retired in 2020
Fatigue Outcome for Patients with MS - Retired in 2020
Clinical Depression Screening for Patients with MS – Retired in 2020
Depression Outcome for Patients with MS – Retired in 2020
Maintained or Improved Baseline Quality of Life for Patients with MS – Retired in 2020
• Multiple Sclerosis (MS) Diagnosis – The work group retired the MS diagnosis measure because the data
collection for these measures placed large burden on physicians and care provider teams including potentially
modifying their documentation practices.
• Current MS Disability Scale Score – This measure was previously incorporated in the AANI’s Axon Registry.
Implementation concerns were identified as the data were being collected in the registry. It was noted that some of
the disability scales approved for use in the measure would not be collected on the date of the patient visit and
collected at a later follow-up visit. CMS had approved the measure for use by a Qualified Clinical Data Registry
through 2019 following which, CMS added a modification to include a follow-up component to the collection of
the scale score. Adding a follow-up component would add another layer of complexity reducing feasibility
further. The work group noted that an outcome measure would be difficult to develop on the topic given the
varied scale use by neurologists and by practice settings. Given these concerns, the measure was retired and
discussion held on development of a relapse or disability related measure.
• Fall Risk Screening for Patients with MS – The work group retired this measure given the existence of cross-
cutting falls measures. The work group encourages providers to utilize one of the below measures to monitor and
track falls and fall outcomes in practice. MIPS Quality measure specifications are available at qpp.cms.gov.
o For patients 65 and older
▪ MIPS Quality ID #318 Falls: Screening for Future Fall Risk
▪ MIPS Quality ID #154 Falls: Risk Assessment
▪ MIPS Quality ID #155 Falls: Plan of Care
o For patients 64 and younger
▪ Axon Registry #45 Falls Outcome
▪ Axon Registry #53 Falls Plan of Care
• Fatigue Outcome for Patients with MS – The fatigue outcome measure was retired due to concerns that a
physician or MS treatment team has little control over changes in fatigue screening scores that are likely impacted
by multiple causes including other co-morbid conditions treated by other specialists. As a result, the measure was
changed to a screening and follow-up measure.
• Clinical Depression Screening for Patients with MS & Depression Outcome for Patients with MS – The work
group retired the prior depression assessment and outcome measures given the existence of cross-cutting
depression measures. The work group encourages providers to utilize one of the below measures to monitor and
track depression outcomes in practice:
o MIPS Quality ID #134 Preventive Care and Screening: Screening for Depression and Follow Up Plan
o MIPS Quality ID#370 Depression Remission at Twelve Months. Outcome measures at twelve months for
patients age 18 years and older diagnosed with major depression or dysthymia utilizing PHQ-9 scores
• Maintained or Improved Baseline Quality of Life for Patients with MS – The work group retired the prior quality
of life for patients with MS measure due to the existence of cross-cutting measures addressing quality of life for
patients with MS. The work group encourages providers to utilize Axon Registry #54 Quality of Life for Patients
with Neurologic Conditions.

8
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Other Potential Measures
The work group proposed 25 measure concepts based on extensive literature search/review. The AANI encourages work
groups to focus development of measure concepts that are feasible to collect, do not pose an excessive burden on
providers to collect data, meaningful to quality improvement efforts, and address a known treatment or care gap. It is
important to recognize the fact that it is not feasible for the work group to develop all the appropriate concepts due to
resource limitations and consideration for minimizing provider reporting burden.
Through one round of ranking, work group members prioritized three newer concepts for discussion. Two concepts on
disease modifying therapy and fatigue screening were developed for public comment. One concept, relapse or disability
monitoring for patients with MS was not. This concept of relapse and disability monitoring is of utmost importance. The
determination to not make a measure at this time is detailed below. The work group plans to monitor this space to
determine if a measure can be developed during a future update of the measurement set.
Three relapse or disability options were discussed:
• a process measure assessing disability,
• an intermediate patient reported outcome focused on missed work or school days, and
• an intermediate patient reported outcome focused on healthcare utilization for relapses.
The above three concepts were not advanced to public comment due to feasibility concerns. The work group noted the
existing disability scale score measure was difficult to collect in practice and a new process measure would have similar
issues/limitations. Any intermediate patient reported outcome measure would require substantial practice and
documentation changes to implement. These concerns prevent any such measure from being developed at this time. The
work group will continue to revisit this concept during biannual reviews.
Measure Harmonization
The AANI encourages work groups to avoid duplication of measures that already exist in the field. Further details on
measure harmonization is included in individual measure specifications below.
The measurement set includes measures that require the use of validated screening tools. The work group discussed and
determined that multiple tools should be offered to allow providers to determine which tool best meets their individual
practice needs. Tools may be subject to copyright and require licensing fees. The work group notes that effective
September 2020 that Montreal Cognitive Assessment use requires completion of a proprietary examination and fee.
The AANI has developed additional measures that may be of interest to clinicians and teams treating patients with
neurologic conditions. All AANI measures are available for free at: https://www.aan.com/policy-and-
guidelines/quality/quality-measures2/quality-measures/
Introductory References
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States. Neurology.
2019;92(10):e1029-e1040.
2. Nelson LM, Wallin MT, Marrie RA, et al. A new way to estimate neurologic disease prevalence in the United
States. Neurology. 2019; 92(10): 469-480.
3. Dieleman JL, Cao J, Chapin A, et al. US Health Care Spending by Payer and Health Condition, 1996-2016.
JAMA. 2020; 323(9):863-884.
4. Quality Measure Subcommittee. American Academy of Neurology Quality Measurement Manual 2019 Update.
24p. January 2020. Available at: https://www.aan.com/policy-and-guidelines/quality/quality-measures2/how-
measures-are-developed/ Accessed on November 13, 2020.

9
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
2020 Multiple Sclerosis (MS) Quality Measure Specifications
Magnetic Resonance Imaging (MRI) Monitoring for Patients with Multiple Sclerosis (MS)
Measure Title
MRI Monitoring for Patients with MS
Description
Percentage of patients who had a brain MRI scan in the last 24 months and care management
decisions updated.
Measurement
Period
January 1, 20xx to December 31, 20xx
Eligible
Population
Eligible Providers
Medical Doctor (MD), Doctor of Osteopathy (DO), Pharmacist (PharmD),
Physician Assistant (PA), Advanced Practice Registered Nurse (APRN)
Care Setting(s)
Outpatient Care
Ages
Any
Event
Office or telehealth encounter
Diagnosis
Multiple Sclerosis
Denominator
Patients with a diagnosis of MS
Numerator
Patients who had a brain MRI scan in the last 24 months and care management decisions
updated*.
Definition
*Care management decisions updated defined as reaffirmation or adjustment to the
treatment plan, adjustment or initiation of appropriate medication, or further testing.
Required
Exclusions
• Active diagnosis of Radiologically Isolated Syndrome (RIS) or Clinically Isolated
Syndrome (CIS) on date of encounter
Allowable
Exclusions
• Patient declines referral to MRI in the last 24 months
• MRI not clinically indicated given patient circumstances on date of encounter
• Patient unable to have an MRI and this reason documented during measurement period
Allowable
Exclusion
Inclusion Logic
Allowable exclusions can only help measure performance. If a patient has an allowable
exclusion but is found to meet the numerator that patient is included in the count to meet the
measure.
Exclusion
Rationale
Patients with RIS and CIS are not included in the eligible population given the lack of guidelines
on timing of periodic surveillance imaging, as well as diagnostic variability for these conditions.
A required exclusion is appropriate as a result.
Allowable exclusions are needed for the following reasons:
• Patients must agree to undergo an MRI, and it is not appropriate to force a patient to
undergo testing they are opposed to having done. There are multiple reasons a patient
may decline MRI including claustrophobia, unable to access MRI due to limited MRI
availability, religious preference, and cost.
• MRI may not be clinically indicated for some patients and physician and treatment team
judgement should allow for these patients being excluded. Some examples of where
MRI is not clinically indicated include patients who have a 20-year history of MS or
current diagnosis of a progressive form of MS.
• Patient may meet an exclusion for MRI given a history of trauma or surgery which may
have left ferromagnetic material in the body, ferromagnetic implants or pacemakers, and
inability to lie still for 1 hour or more. These patients are appropriate to exclude due to
the potential harm that may result from undergoing an MRI. Also, if a patient is actively
having a clinical relapse, MRI may not add to clinical decision making about changing
the treatment regimen.
Measure Scoring
Percentage

10
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Interpretation of
Score
Higher Score Indicates Better Quality
Measure Type
Process
Level of
Measurement
Provider
Risk Adjustment
Not Applicable
Opportunity to
Improve Gap in
Care
This measure is not intended to monitor baseline or re-baseline activity specifically. The
measure is intended to address a gap in care for patients and prompt providers to consider
modifying management decisions based on imaging results. The MRI changes may not always
prompt treatment modifications, but providers consider the MRI data to assess if they are
indicated in an individual patient. There are patients with MS who may require more frequent
monitoring such as those prescribed natalizumab to monitor for Progressive Multifocal
Leukoencephalopathy (PML) or those switching disease-modifying therapies; these patients
should be treated in accordance with current guideline statements.
1
The work group notes this measure may have a positive, unintended consequence of addressing
rural disparities by ensuring all patients with MS are provided routine MRI monitoring.
Monitoring of measure performance will occur to address any negative, unintended
consequences.
For Process
Measures
Relationship to
Desired Outcome
The following evidence statements are quoted verbatim from the referenced clinical guidelines:
• “Timing of brain MRI protocol for patients with an established diagnosis of MS:
…Every 1-2 years while on disease-modifying therapy to assess for subclinical disease
activity (i.e. new T2 lesions or gadolinium enhancing lesions). Less frequent MRI scans
required in clinically stable patients after 2-3 years of stable treatment (gadolinium-
based contrast optional)”
1
Harmonization
with Existing
Measures
Other draft measures impacting MRI use for patients with multiple sclerosis were reviewed; the
draft measures utilize different denominator based on subtypes of multiple sclerosis. The work
group developed this measure with a denominator of all patients with MS to address feasibility
of data collection in practice, as MS subtypes are not easily identified due to lack of consistent
coding practices.
References
1. Consortium of Multiple Sclerosis Centers. Consortium of MS Centers MRI Protocol for the
Diagnosis and Follow-up of MS 2018 Revised Guidelines. Available at:
https://www.mscare.org/page/MRI_protocol Accessed on November 13, 2020.
Process
Comparison MRI scan
collected
Treatment team
intervention for
identified patients
Intermediate
Outcome
Disease activity &
progression identified
Treatment
modification/optimized
to reduce disease
activity
Outcome
Reduced disease
activity

11
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Code System
Code
Code Description
Initial Population
CPT
99201-99205
Office or other outpatient visit, new patient
CPT
99211-99215
Office or other outpatient visit, established patient
CPT
99241-99245
Office or other outpatient consultation, new or established patient
CPT
Telehealth TBD
Denominator
ICD-10
G35
Multiple Sclerosis
SNOMEDCT
24700007
Multiple sclerosis (disorder)
SNOMEDCT
192929006
Exacerbation of multiple sclerosis (disorder)
SNOMEDCT
230372003
Acute relapsing multiple sclerosis (disorder)
SNOMEDCT
425500002
Secondary progressive multiple sclerosis (disorder)
SNOMEDCT
426373005
Relapsing remitting multiple sclerosis (disorder)
SNOMEDCT
428700003
Primary progressive multiple sclerosis (disorder)
SNOMEDCT
438511000
Benign multiple sclerosis (disorder)
SNOMEDCT
92926004
Multiple sclerosis of the brainstem (disorder)
SNOMEDCT
192927008
Multiple sclerosis of the spinal cord (disorder)
SNOMEDCT
439567002
Malignant multiple sclerosis (disorder)
SNOMEDCT
724778008
Progressive relapsing multiple sclerosis (disorder)
SNOMEDCT
733028000
Multiple sclerosis, ichthyosis, factor VIII deficiency syndrome (disorder)
SNOMEDCT
766246000
Marburg acute multiple sclerosis (disorder)
SNOMEDCT
816984002
Progressive multiple sclerosis (disorder)
Numerator – MRI component
CPT
70551
Magnetic resonance imaging, brain without contrast material
CPT
70553
Magnetic resonance imaging, brain without contrast material, followed by
contrast material and further sequences
SNOMEDCT
241601008
MRI of head
SNOMEDCT
702724004
MRI of head and neck with contrast
SNOMEDCT
29567006
MRI of brain and brain stem (procedure)
SNOMEDCT
395611000119106
MRI of brain and brain stem with contrast
SNOMEDCT
443603002
MRI of brain with contrast using isotropic resolution
SNOMEDCT
698355003
Magnetic resonance imaging for measurement of brain volume with
contrast
SNOMEDCT
3313508016
Magnetic resonance imaging of brain and brain stem with contrast
(procedure)
SNOMEDCT
3320261014
MRI of brain and brain stem with contrast
SNOMEDCT
3313509012
Magnetic resonance imaging of brain and brain stem with contrast
Numerator – Care management decisions updated component
SNOMEDCT
1779009018
Development of care plan
SNOMEDCT
1767604017
Development of care plan (procedure)
SNOMEDCT
1196083017
Development of individualized plan of care (procedure)
SNOMEDCT
1209518012
Development of individualized plan of care
SNOMEDCT
1228792012
Develops individualized plan of care
SNOMEDCT
566252018
Change of medication (procedure)
SNOMEDCT
282660014
Change of medication
SNOMEDCT
282659016
Medication changed
SNOMEDCT
750861000124112
Recommendation to change medication to lower cost therapeutic
equivalent (procedure)
SNOMEDCT
750871000124117
Recommendation to change medication to lower cost therapeutic
equivalent
SNOMEDCT
616161000124116
Recommendation to change medication dose form (procedure)

12
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
SNOMEDCT
616171000124111
Recommendation to change medication dose form
SNOMEDCT
616181000124114
Advice to change medication dose form
SNOMEDCT
616281000124118
Recommendation to change medication dose (procedure)
SNOMEDCT
616291000124115
Recommendation to change medication dose
SNOMEDCT
616301000124119
Advice to change medication dose
SNOMEDCT
616161000124116
Recommendation to change medication dose form (procedure)
SNOMEDCT
566927011
Referral for further care (procedure)
SNOMEDCT
283512014
Referral for further care
SNOMEDCT
183444007
Referral for further care (procedure)
SNOMEDCT
709318013
Provision of specialist further education (procedure)
SNOMEDCT
456380014
Provision of specialist further education
SNOMEDCT
706904013
Further opinion sought (finding)
SNOMEDCT
453917017
Further opinion sought
Presence of key phrases in clinical note may meet numerator component for Axon Registry.
Suggested key phrases to locate numerator component via Axon Registry
®
are included below. This list is not
exhaustive and will be updated annually if adopted into the Axon Registry:
• “Treatment plan remains appropriate”
• “No treatment plan changes needed”
• “Treatment plan updated”
• “Treatment plan changed”
• “Further testing conducted”
• “Additional tests ordered”
• “Pharmacological updates made”
• “Initiated Disease Modifying Therapy”
• “Initiated DMT”
• “Adjusted DMT”
• “Medication adjusted”
• “Continue same DMT”
Required Exclusions
ICD-10
G36.9
Acute disseminated demyelination, unspecified
ICD-10
G37.8
Other specified demyelinating diseases of the central nervous system
ICD-10
G37.9
Demyelinating disease of the central nervous system, unspecified
SNOMEDCT
445967004
Clinically isolated syndrome
SNOMEDCT
2880226016
Clinically isolated syndrome (disorder)
SNOMEDCT
2883049010
Clinically isolated syndrome
SNOMEDCT
633651000124112
Clinically isolated syndrome of brainstem (disorder)
SNOMEDCT
633661000124114
Clinically isolated syndrome of brainstem
SNOMEDCT
3009685011
Monofocal clinically isolated syndrome (disorder)
SNOMEDCT
3009542010
Monofocal clinically isolated syndrome
SNOMEDCT
703622004
Monofocal clinically isolated syndrome
SNOMEDCT
703621006
Multifocal clinically isolated syndrome
SNOMEDCT
3009533015
Multifocal clinically isolated syndrome (disorder)
SNOMEDCT
3009313015
Multifocal clinically isolated syndrome
SNOMEDCT
3009649011
Polysymptomatic clinically isolated syndrome
SNOMEDCT
16415361000119105
Radiologically isolated syndrome
SNOMEDCT
3774704015
Radiologically isolated syndrome (disorder)
SNOMEDCT
3774703014
Radiologically isolated syndrome
Allowable Exclusions
SNOMEDCT
183932001
Procedure contraindicated (situation)

13
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
SNOMEDCT
397745006
Medical contraindication (finding)
SNOMEDCT
407563006
Treatment not tolerated (situation)
SNOMEDCT
428119001
Procedure not indicated (situation)
SNOMEDCT
408548005
Magnetic resonance imaging scan declined
SNOMEDCT
2612982013
Magnetic resonance imaging scan declined (situation)
SNOMEDCT
2160098019
Magnetic resonance imaging scan declined
SNOMEDCT
746791000124111
Recommendation refused by patient (situation)
SNOMEDCT
746801000124112
Recommendation refused by patient
SNOMEDCT
2608177018
Refused procedure - after thought (situation)
SNOMEDCT
284171012
Refused procedure - after thought
SNOMEDCT
183947005
Refused procedure - after thought (situation)
SNOMEDCT
2606319010
Refusal of treatment by patient (situation)
SNOMEDCT
169559019
Refusal of treatment by patient
SNOMEDCT
105480006
Refusal of treatment by patient (situation)
SNOMEDCT
2612741019
Refusal of treatment by parents (situation)
SNOMEDCT
1209841012
Refusal of treatment by parents
SNOMEDCT
2608092019
Refused procedure - parent's wish (situation)
SNOMEDCT
284172017
Refused procedure - parent's wish
SNOMEDCT
183948000
Refused procedure - parent's wish (situation)
SNOMEDCT
183944003
Procedure refused (situation)
SNOMEDCT
183945002
Procedure refused for religious reason (situation)
SNOMEDCT
413310006
Patient non-compliant - refused access to services (situation)
SNOMEDCT
413311005
Patient non-compliant - refused intervention / support (situation)
SNOMEDCT
413312003
Patient non-compliant - refused service (situation)
SNOMEDCT
183948000
Refused procedure - parent's wish (situation)
SNOMEDCT
416432009
Procedure not wanted (situation)
SNOMEDCT
443390004
Refused (qualifier value)
Presence of key phrases in clinical note may meet allowable exclusion component for Axon Registry.
Suggested key phrases to locate exclusions via Axon Registry
®
are included below. This list is not exhaustive and will
be updated annually if adopted into the Axon Registry:
• “Patient has clinically evident disease activity.”
• “Patient declines referral to MRI.”
• “Patient refuses referral to MRI.”
• “Patient refuses MRI.”
• “Patient declines MRI.”
• “MRI not clinically indicated.”
• “Patient history prevents MRI.”
• “Patient unable to have MRI.”
• “MRI contraindicated.”
• “Patient meets MRI exclusion.”
• “Patient has new Dx of MS; MRI not indicated.”
• “MRI not ordered due to patient cost concerns”

14
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Yes
No
No
Yes
No
Yes
No
Yes
No
No
No
Yes
Yes
Yes
No
No
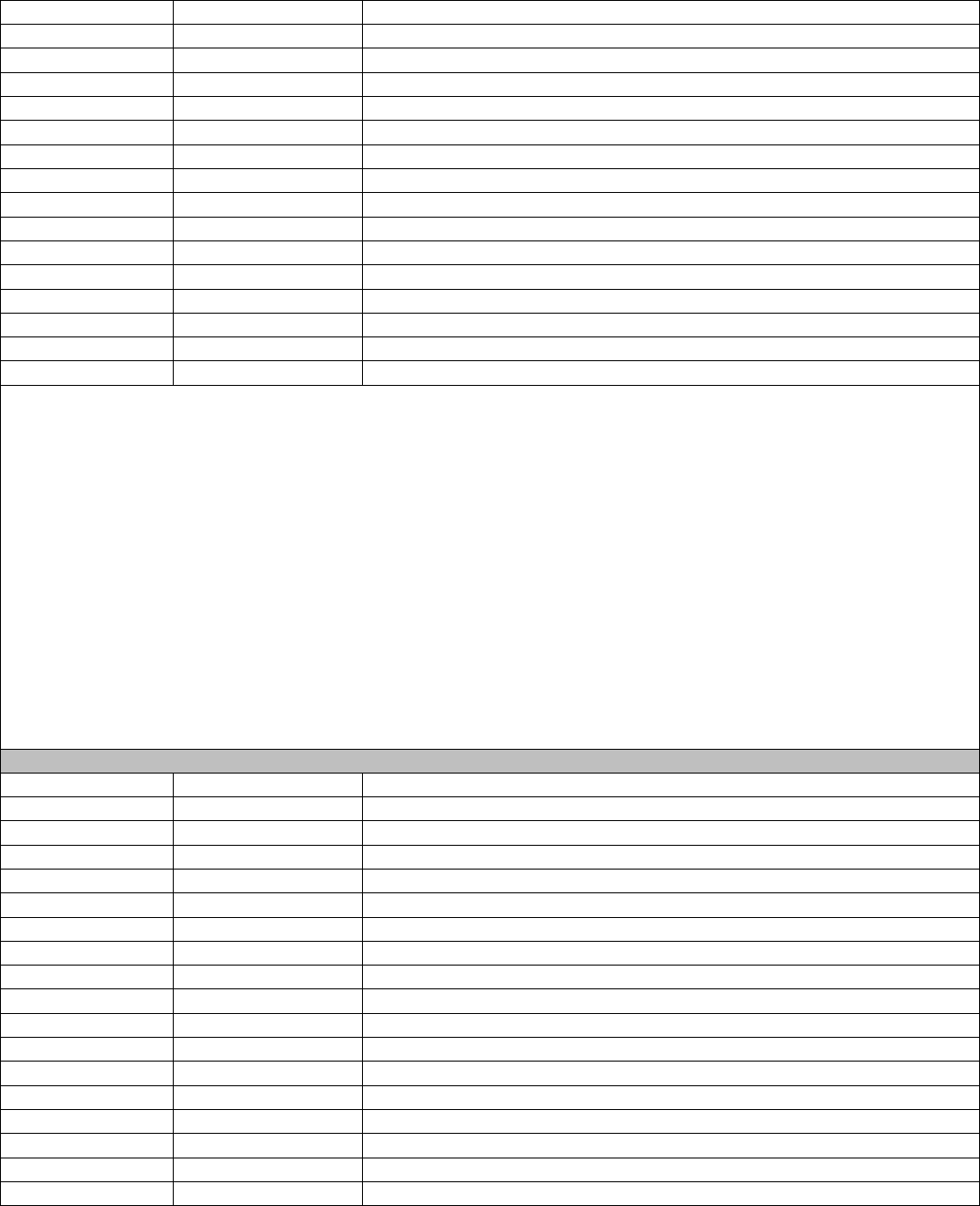
Flow Chart Diagram: MRI Surveillance for Patients with MS
Patient
INCLUDED in
Eligible
Population
Patient NOT
Included in
Eligible
Population
Patient met
numerator
criteria
Patient did
NOT meet
numerator
criteria
In the last 24 months, did patient have a brain
MRI scan and care management decisions
updated?
Did the patient have at least one new or
established visit with an eligible provider
during the measurement period?
Did patient have an active diagnosis of RIS
or CIS on the date of the visit?
Patient
INCLUDED in
Denominator
On date of encounter, did patient decline
MRI?
Remove from
denominator*
*Do not remove/exclude
if patient meets the
numerator
Did patient have a MS diagnosis on the
date of the visit?
On date of encounter, was MRI not
clinically indicated?
During the measurement period, was
there a notation patient meets MRI
exclusion?

15
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Disease Modifying Therapies (DMT) Monitoring for Patients with Multiple Sclerosis (MS)
Measure Title
DMT Monitoring for Patients with MS
Description
Percentage of patients with MS prescribed a DMT who were screened for side effects and
compliance/adherence.
Measurement
Period
January 1, 20xx to December 31, 20xx
Eligible
Population
Eligible Providers
Medical Doctor (MD), Doctor of Osteopathy (DO), Pharmacist (PharmD),
Physician Assistant (PA), Advanced Practice Registered Nurse (APRN)
Care Setting(s)
Outpatient Care
Ages
Any
Event
Office or telehealth encounter for patients with a DMT prescription
Diagnosis
Multiple Sclerosis
Denominator
Patients with a diagnosis of MS who were prescribed a new DMT during the measurement
period.
Numerator
Patients who were screened on date of encounter for:
• DMT side effects and
• compliance/adherence with DMT
Required
Exclusions
• Patient had a new DMT initiated on date of encounter
Allowable
Exclusions
• Patient declines a discussion on date of encounter
Allowable
Exclusion
Inclusion Logic
Allowable exclusions can only help measure performance. If a patient has an allowable
exclusion but is found to meet the numerator that patient is included in the count to meet the
measure.
Exclusion
Rationale
• Patients with a new DMT initiated on date of encounter should be excluded due to
insufficient exposure to drug to necessitate monitoring and may have been switched to a
new DMT on the date in which case no need for a full monitoring assessment of the
prior DMT.
• DMT monitoring requires assessment of subjective symptom that requires patient
cooperation to assess.
Measure Scoring
Percentage
Interpretation of
Score
Higher Score Indicates Better Quality
Measure Type
Process
Level of
Measurement
Provider
Risk Adjustment
Not Applicable
Opportunity to
Improve Gap in
Care
The work group notes there is limited evidence about gaps in care surrounding DMT monitoring
and documentation, but anecdotally believes gaps exist for both. Evidence indicates that patients
will self-report adherence and compliance concerns when using DMT for MS.
1
This current measure is focused on monitoring following a new DMT initiation. Future
iterations of the measure may evolve over time to include all DMT monitoring. The work group
will assess feasibility, documentation burden and unintended consequences during future
reviews.
For Process
Measures
Relationship to
Desired Outcome
By screening and monitoring patients with MS prescribed DMTs, clinicians will be able to
identify patients with side effects and patients who are not adhering to treatment. Once these
issues are identified, clinicians will be able to modify/alter treatment plans or propose measures
to address side effects and compliance/adherence issues to help improve outcomes and quality of
life.

16
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Following evidence statements are quoted verbatim from the referenced clinical guidelines:
• “Level B Clinicians should monitor for medication adherence, AEs, tolerability, safety,
and effectiveness of the therapy in people with MS on DMTs.”
2
• “Level B Clinicians should follow up either annually or according to medication-
specific REMs in people with MS on DMTs.”
2
• “Level B Clinicians should discuss a change to noninjectable or less frequently
injectable DMTs in people with MS who report intolerable discomfort with the
injections or in those who report injection fatigue on injectable DMTs.”
2
Harmonization
with Existing
Measures
Other draft measures for patients with multiple sclerosis utilizing DMTs were reviewed; the
draft measures utilize different denominator based on subtypes of multiple sclerosis. The work
group developed this measure with a denominator of all patients with MS to address feasibility
of data collection in practice, as MS subtypes are not easily identified due to lack of consistent
coding practices.
References
1. McKay KA, Evans A, Fisk JD, et al. Disease-Modifying Therapies and Adherence in
Multiple Sclerosis: Comparing Patient Self-Report with Pharmacy Records.
Neuroepidemiology. 2017; 48:124-130.
2. Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary:
Disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018;
90(17):777-788.
Process
DMT initiated
DMT monitoring
Intermediate
Outcome
DMT adherence
DMT tolerability and
safety assessed
Outcome
DMT use and
adherence resulting in
decreased MS-disease
activity

17
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Initial Population
CPT
99201-99205
Office or other outpatient visit, new patient
CPT
99211-99215
Office or other outpatient visit, established patient
CPT
99241-99245
Office or other outpatient consultation, new or established patient
CPT
Telehealth codes TBD
Denominator
ICD-10
G35
Multiple Sclerosis
SNOMEDCT
24700007
Multiple sclerosis (disorder)
SNOMEDCT
192929006
Exacerbation of multiple sclerosis (disorder)
SNOMEDCT
230372003
Acute relapsing multiple sclerosis (disorder)
SNOMEDCT
425500002
Secondary progressive multiple sclerosis (disorder)
SNOMEDCT
426373005
Relapsing remitting multiple sclerosis (disorder)
SNOMEDCT
428700003
Primary progressive multiple sclerosis (disorder)
SNOMEDCT
438511000
Benign multiple sclerosis (disorder)
SNOMEDCT
92926004
Multiple sclerosis of the brainstem (disorder)
SNOMEDCT
192927008
Multiple sclerosis of the spinal cord (disorder)
SNOMEDCT
439567002
Malignant multiple sclerosis (disorder)
SNOMEDCT
724778008
Progressive relapsing multiple sclerosis (disorder)
SNOMEDCT
733028000
Multiple sclerosis, ichthyosis, factor VIII deficiency syndrome (disorder)
SNOMEDCT
766246000
Marburg acute multiple sclerosis (disorder)
SNOMEDCT
816984002
Progressive multiple sclerosis (disorder)
AND presence of one of the below RxNorm codes for the first time in the patient record for the first time in the 12
months prior to the date of the encounter.
RxNorm
Reviewed annually
Glatiramer acetate
RxNorm
Reviewed annually
Interferon b-1a
RxNorm
Reviewed annually
Interferon b-1b
RxNorm
Reviewed annually
Pegylated interferon b-1a
RxNorm
Reviewed annually
Dimethyl fumarate
RxNorm
Reviewed annually
Fingolimod
RxNorm
Reviewed annually
Teriflunomide
RxNorm
Reviewed annually
Siponimod
RxNorm
Reviewed annually
Cladribine
RxNorm
Reviewed annually
Diroximel fumarate
RxNorm
Reviewed annually
Alemtuzumab
RxNorm
Reviewed annually
Natalizumab
RxNorm
Reviewed annually
Ocrelizumab
RxNorm
Reviewed annually
Ofatumumab
Numerator – Side effect component
SNOMED
2612797016
No drug side effect reported (situation)
SNOMED
1488760019
No drug side effect reported
SNOMED
395061001
No drug side effect reported
SNOMED
1769129019
Medication side effects present (finding)
SNOMED
1780402015
Medication side effects present
SNOMED
1787478017
Has shown side effects from medication
SNOMED
3013779011
Medication side-effect
SNOMED
401207004
Medication side effects present (finding)
SNOMED
2612971017
Medication stopped - side effect (situation)
SNOMED
1488709015
Medication stopped - side effect
SNOMED
395009001
Medication stopped – side effect (finding)
SNOMED
704417003
At risk of medication side effect (finding)
18
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
SNOMED
3013270011
At risk of medication side effect (finding)
SNOMED
3013275018
At risk of medication side effect
SNOMED
129850005
At risk for negative response to medication
SNOMED
2608043013
Doctor stopped drugs - side effect (situation)
SNOMED
282667012
Doctor stopped drugs - side effect
SNOMED
282668019
Dr stopped drugs - side effect
SNOMED
182842009
Doctor stopped drugs – side effect (situation)
SNOMED
552913018
High risk drug side effect (finding)
SNOMED
264902014
High risk drug side effect
SNOMED
170909007
High risk drug side effect (finding)
SNOMED
2719553017
Repeat prescription drug side effect (finding)
SNOMED
2770170014
Repeat prescription drug side effect
SNOMED
170926001
Repeat prescription drug side effect (finding)
SNOMED
2152188017
Drug side effect - acceptable to patient (finding)
SNOMED
2159921014
Drug side effect - acceptable to patient
SNOMED
408357000
Drug side effect - acceptable to patient (finding)
Presence of key phrases in clinical note may meet numerator component for Axon Registry.
Suggested key phrases to locate numerator components via Axon Registry
®
are included below. This list is not
exhaustive and will be updated annually if adopted into the Axon Registry:
• “Patient reports no side effects”
• “Patient reports side effects”
• “DMT side effects discussed”
• “DMT side effects are severe”
• “DMT side effects are moderate”
• “DMT side effects are mild”
• “DMT side effects are minimal”
• “DMT side effects reported”
• “DMT side effects not reported”
• “DMT side effects are tolerable”
• “DMT side effects are improving”
Numerator - Medication adherence/compliance component:
SNOMED
2788859011
Compliance behavior to prescribed medication (observable entity)
SNOMED
2792744019
Compliance behavior to prescribed medication
SNOMED
2793641011
Compliance behaviour to prescribed medication
SNOMED
439914008
Compliance behavior to prescribed medication (observable entity)
SNOMED
3036826013
Compliance behavior to therapeutic regimen (observable entity)
SNOMED
3036723011
Compliance behavior to therapeutic regimen
SNOMED
3037872015
Compliance behaviour to therapeutic regimen
SNOMED
709007004
Compliance behavior to therapeutic regimen (observable entity)
SNOMED
1768899016
Drug compliance poor (finding)
SNOMED
1780186012
Drug compliance poor
SNOMED
400978007
Drug compliance poor (finding)
SNOMED
2152204014
Drug compliance checked (finding)
SNOMED
2159937015
Drug compliance checked
SNOMED
408373006
Drug compliance checked (finding)
SNOMED
2573535016
Verbalizes medication compliance (finding)
SNOMED
2576832015
Verbalizes medication compliance
SNOMED
2579791015
Verbalizes medication adherence
SNOMED
4190110006
Verbalizes medication compliance (finding)

19
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
SNOMED
566303015
Drug compliance good (finding)
SNOMED
282724013
Drug compliance good
SNOMED
182884001
Drug compliance good (finding)
SNOMED
1491000124115
Prescription compliance status (finding)
SNOMED
1501000124111
Prescription compliance status
SNOMED
671000124101
Prescription compliance status (finding)
SNOMED
5631000175104
Patient sequesters unused medication
SNOMED
2834718017
Assessment of compliance with medication regimen (procedure)
SNOMED
2471844010
Assessment of compliance with medication regimen
SNOMED
2477776019
Assess compliance with medication regimen
SNOMED
740391000124114
Assessment of adherence to medication regimen
SNOMED
410122002
Assessment of compliance with medication regimen (procedure)
SNOMED
2529544010
Drug therapy compliance observations (finding)
SNOMED
2576445011
Drug therapy compliance finding
SNOMED
2532949019
Drug therapy compliance observations
SNOMED
414059009
Drug therapy compliance observations (finding)
SNOMED
182884001
Drug compliance good
SNOMED
400978007
Drug compliance poor
SNOMED
408373006
Drug compliance checked
SNOMED
668770011
Drugs - total non-compliance (finding)
SNOMED
411900018
Drugs - total non-compliance
SNOMED
275927006
Drugs - total non-compliance (finding)
SNOMED
668771010
Drugs - partial non-compliance (finding)
SNOMED
411901019
Drugs - partial non-compliance
SNOMED
275928001
Drugs - partial non-compliance (finding)
SNOMED
709008009
Complies with therapeutic regimen
SNOMED
7058009
Noncompliance with treatment
SNOMED
2638881015
Noncompliance with treatment (finding)
SNOMED
2647221012
Noncompliance with treatment
SNOMED
2536432016
Does not comply with treatment
SNOMED
740101000124117
Nonadherence with treatment
SNOMED
734021017
Noncompliance with medication regimen (finding)
SNOMED
208675012
Noncompliance with medication regimen
SNOMED
208676013
Noncompliance: medication regimen
SNOMED
129834002
Noncompliance with medication regimen (finding)
SNOMED
3004395012
Non-compliance of drug therapy (finding)
SNOMED
3004295010
Non-compliance of drug therapy
SNOMED
726441000124114
Drug therapy non adherence
SNOMED
726431000124116
Medication therapy non-adherence
SNOMED
702565001
Non-compliance of drug therapy (finding)
SNOMED
778331000124111
Medication non-adherence due to intolerance (finding)
SNOMED
778341000124118
Medication non-adherence due to intolerance
SNOMED
778321000124113
Medication non-compliance due to intolerance
SNOMED
457621000124107
Medication non-adherence due to intolerance (finding)
SNOMED
3004326019
Suspected non-compliance of drug therapy (situation)
SNOMED
3449739016
Suspected non-adherence of medication therapy
SNOMED
3004393017
Suspected non-compliance of drug therapy
SNOMED
702566000
Suspected non-compliance of drug therapy (situation)
SNOMED
778351000124116
Medication non-adherence due to language barrier (finding)
SNOMED
778361000124119
Medication non-adherence due to language barrier

20
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
SNOMED
778371000124114
Medication non-compliance due to language barrier
SNOMED
457631000124105
Medication non-adherence due to language barrier (finding)
SNOMED
778381000124112
Medication non-adherence due to psychosocial issues (finding)
SNOMED
778391000124110
Medication non-adherence due to psychosocial issues
SNOMED
778401000124112
Medication non-compliance due to psychosocial issues
SNOMED
457641000124100
Medication non-adherence due to psychosocial issues (finding)
SNOMED
751831000124111
Medication non-compliance due to excessive pill burden (finding)
SNOMED
751841000124118
Medication non-compliance due to excessive pill burden
SNOMED
751851000124116
Medication non-adherence due to excessive pill burden
SNOMED
454171000124105
Medication non-compliance due to excessive pill burden (finding)
Presence of key phrases in clinical note may meet numerator component for Axon Registry.
Suggested key phrases to locate numerator components via Axon Registry
®
are included below. This list is not
exhaustive and will be updated annually if adopted into the Axon Registry:
• “Patient taking DMT consistently”
• “Patient not taking DMT consistently”
• “Patient inconsistently taking DMT”
• “Patient not adherent with DMT”
• “Patient adherent with DMT”
• “Patient unable to afford DMT”
Required Exclusions
Presence of one of the above RxNorm codes for the first time in the patient record on the date of the encounter.
Allowable Exclusions
SNOMEDCT
2606319010
Refusal of treatment by patient (situation)
SNOMEDCT
169559019
Refusal of treatment by patient
SNOMEDCT
105480006
Refusal of treatment by patient (situation)
SNOMEDCT
2612741019
Refusal of treatment by parents (situation)
SNOMEDCT
1209841012
Refusal of treatment by parents
SNOMEDCT
183945002
Procedure refused for religious reason (situation)
SNOMEDCT
413310006
Patient non-compliant - refused access to services (situation)
SNOMEDCT
413311005
Patient non-compliant - refused intervention / support (situation)
SNOMEDCT
413312003
Patient non-compliant - refused service (situation)
SNOMEDCT
443390004
Refused (qualifier value)
Presence of key phrases in clinical note may meet allowable exclusion component for Axon Registry.
Suggested key phrases to locate exclusions via Axon Registry
®
are included below. This list is not exhaustive and will
be updated annually if adopted into the Axon Registry:
• “Patient declines to discuss DMT use”
• “Patient refuses to discuss DMT use”

21
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
No
Yes
Yes
No
No
Yes
No
Yes
No
No
Yes
No
No
Yes
No
No
Flow Chart Diagram: DMT Monitoring for Patients with MS
Patient
INCLUDED in
Eligible
Population
Patient met
numerator
criteria
Patient did
NOT meet
numerator
criteria
On date of encounter, was patient
prescribed a new DMT?
Remove from
denominator*
*Do not remove/exclude
if patient meets the
numerator
Did patient have a MS diagnosis on the date
of the visit?
On date of encounter, did patient decline or
refuse discussion on DMT use?
?
Was patient screened for DMT side effects, AND
medication adherence/non-compliance?
Patient
INCLUDED in
Denominator
Patient NOT
Included in
Eligible
Population
Did the patient have at least one new or
established visit with an eligible provider
during the measurement period?
Did patient have a new DMT prescribed
during the measurement period?

22
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Bladder, Bowel, and Sexual Dysfunction Screening and Follow-Up for Patients with Multiple Sclerosis (MS)
Measure Title
Bladder, Bowel, and Sexual Dysfunction Screening and Follow-Up for Patients with MS
Description
Percentage of patients with MS who were screened for at least one of three symptoms: bladder,
bowel, or sexual dysfunction in the past 12 months, and if screening positive for any one of
these symptoms had appropriate follow-up care.
Measurement
Period
January 1, 20xx to December 31, 20xx
Eligible
Population
Eligible Providers
Medical Doctor (MD), Doctor of Osteopathy (DO), Pharmacist (PharmD),
Physician Assistant (PA), Advanced Practice Registered Nurse (APRN),
Physical Therapy (PT), Occupational Therapy (OT)
Care Setting(s)
Outpatient Care
Ages
Any
Event
Office or telehealth encounter
Diagnosis
Multiple Sclerosis
Denominator
Patients with a diagnosis of MS.
Numerator
Patients with MS who were screened* for at least one of three symptoms: bladder, bowel, or
sexual dysfunction in the past 12 months, and if screening positive had appropriate follow-up**
care.
Definitions:
* Screened is defined as an assessment of symptoms.
**Appropriate follow-up is defined as adjustment to the treatment plan, adjustment or
initiation of appropriate medication, further testing, counseling on lifestyle changes, or
referral to an appropriate healthcare provider.
Required
Exclusions
None
Allowable
Exclusions
• Patient refuses or patient declines on date of encounter
Allowable
Exclusion
Inclusion Logic
Allowable exclusions can only help measure performance. If a patient has an allowable
exclusion but is found to meet the numerator that patient is included in the count to meet the
measure.
Exclusion
Rationale
• Patients need to be willing to complete the screening for the screening to be valid.
Measure Scoring
Percentage
Interpretation of
Score
Higher Score Indicates Better Quality
Measure Type
Process
Level of
Measurement
Provider
Risk Adjustment
Not Applicable
Opportunity to
Improve Gap in
Care
2010 North American Research Committee on Multiple Sclerosis (NARCOMS) Registry data
indicated that 91% of 9,341 patients with MS responding were mildly, moderately, or severely
bothered by bladder, bowel, or sexual symptoms.
1
Between 50 to 90% of men with MS and 40
to 80% of women with MS experience sexual dysfunction which is significantly more than in
general population
2
. Sexual dysfunction symptoms are often overlooked in clinical evaluations
of patients with MS.
2
Schairer, et al., found that sexual dysfunction has a larger detrimental
impact on the mental health of health-related quality of life for patients with MS than physical
disability.
3

23
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
For this first iteration of the measure a broad definition of screening was provided; clinicians
may use a validated instrument. The work group notes these instruments are not widely used in
practice. The measure may be updated in future reviews, to detail specific instruments as they
become more widely used in practice.
For Process
Measures
Relationship to
Desired Outcome
By screening annually for bladder, bowel, and sexual dysfunction, clinicians will be able to
identify patients needing appropriate treatment to address these issues, leading to improved
outcomes and better quality of life.
Following evidence statements are quoted verbatim from the referenced clinical guidelines or :
• “Ensure all people with MS have a comprehensive review of all aspects of their care at
least once a year.”
4
• “Tailor the comprehensive review to the needs of the person with MS assessing:
o …bladder, bowel, and sexual function…”
4
• “Refer any issues identified during the comprehensive review of the person with MS to
members of the MS multidisciplinary team and other appropriate teams so that they can
be managed.”
4
• “When assessing lower urinary tract dysfunction in a person with neurological disease,
take a clinical history, including information about:
o urinary tract symptoms
o neurological symptoms and diagnosis (if known)
o clinical course of the neurological disease
o bowel symptoms
o sexual function
o comorbidities
o use of prescription and other medication and therapies.”
5
• “Refer people for urgent investigation if they have any of the following ‘red flag’ signs
and symptoms:
o haematuria
o recurrent urinary tract infections (for example, three or more infections in the
last 6 months)
o loin pain
o recurrent catheter blockages (for example, catheters blocking within 6 weeks of
being changed)
o hydronephrosis or kidney stones on imaging
o biochemical evidence of renal deterioration.”
5
o “Be aware that unexplained changes in neurological symptoms (for example,
confusion or worsening spasticity) can be caused by urinary tract disease, and
consider further urinary tract investigation and treatment if this is suspected.”
4
• “Consider pelvic floor muscle training for people with: lower urinary tract dysfunction
due to multiple sclerosis….”
5
Fletcher, et al., state, “All MS patients should be specifically queried about sexual function.”
6
Further, they noted, “A variety of factors, including MS related disease activity, MS symptoms,
depression & effects of pharmacologic therapy can contribute to sexual dysfunction in patients
with MS.”
6

24
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Harmonization
with Existing
Measures
No known similar measures
References
1. Wang G, Marrie RA, Fox RJ, et al. Treatment satisfaction and bothersome bladder, bowel, sexual
symptoms in multiple sclerosis. Multiple Sclerosis and Related Disorders. 2018; 20: 16-21.
2. Pöttgen J, Rose A, van de Vis W, et al. Sexual dysfunctions in MS in relation to
neuropsychiatric aspects and its psychological treatment: A scoping review. PLoS One.
2018;13(2):e0193381.
3. Schairer LC, Foley FW, Zemon V, et al. The impact of sexual dysfunction on health-
related quality of life in people with multiple sclerosis. Multiple Sclerosis. 2014;
20(5):610-616.
4. National Clinical Guideline Centre (NICE) (UK). Multiple Sclerosis: Management of Multiple
Sclerosis in Primary and Secondary Care. London: National Institute for Health and Care
Excellence; 2014 Oct. 2019 update available at: https://www.nice.org.uk/guidance/cg186
Accessed on November 13, 2020.
5. National Clinical Guideline Centre (NICE) (UK). Urinary incontinence in neurological
disease: assessment and management. London: National Institute for Health and Care
Excellence; 2012 Aug. Available at: https:/www.nice.org.uk/guidance/cg148 Accessed
on March 5, 2020.
6. Fletcher SG, Castro-Borrero W, Remington G, et al. Sexual dysfunction in patients with
multiple sclerosis: a multidisciplinary approach to evaluation and management. Nature
Clinical Practice Urology. 2009; 6: 96-107.
Process
Screening completed
Intermediate
Outcome
Treatment of bladder,
bowel, or sexual
dysfunction symptoms
Reduction of secondary
complications
Outcome
Improved quality of life
Reduction or
elimination of bladder,
bowel, and sexual
dysfunction symptoms

25
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Code System
Code
Code Description
Initial Population
CPT
99201-99205
Office or other outpatient visit, new patient
CPT
99211-99215
Office or other outpatient visit, established patient
CPT
99241-99245
Office or other outpatient consultation, new or established patient
CPT
97003, 97004
Occupational therapy, evaluation and re-evaluation
CPT
97161-97164
Physical therapy, evaluation and re-evaluation
CPT
Telehealth codes TBD
Denominator
ICD-10
G35
Multiple Sclerosis
SNOMEDCT
24700007
Multiple sclerosis (disorder)
SNOMEDCT
192929006
Exacerbation of multiple sclerosis (disorder)
SNOMEDCT
230372003
Acute relapsing multiple sclerosis (disorder)
SNOMEDCT
425500002
Secondary progressive multiple sclerosis (disorder)
SNOMEDCT
426373005
Relapsing remitting multiple sclerosis (disorder)
SNOMEDCT
428700003
Primary progressive multiple sclerosis (disorder)
SNOMEDCT
438511000
Benign multiple sclerosis (disorder)
SNOMEDCT
92926004
Multiple sclerosis of the brainstem (disorder)
SNOMEDCT
192927008
Multiple sclerosis of the spinal cord (disorder)
SNOMEDCT
439567002
Malignant multiple sclerosis (disorder)
SNOMEDCT
724778008
Progressive relapsing multiple sclerosis (disorder)
SNOMEDCT
733028000
Multiple sclerosis, ichthyosis, factor VIII deficiency syndrome (disorder)
SNOMEDCT
766246000
Marburg acute multiple sclerosis (disorder)
SNOMEDCT
816984002
Progressive multiple sclerosis (disorder)
Numerator – Screening Component^
ICD-10
R37
Sexual Dysfunction
ICD-10
K59
Constipation
ICD-10
N32.81
Overactive Bladder
ICD-10
R32
Urinary Incontinence
ICD-10
R35
Nocturia
ICD-10
F52
Erectile Dysfunction
^New ICD-10 diagnostic code may meet the numerator if added on date of encounter
SNOMEDCT
777147011
Bladder dysfunction (finding)
SNOMEDCT
67519012
Bladder dysfunction
SNOMEDCT
639699011
Must urinate repeatedly to empty bladder (finding)
SNOMEDCT
371974010
Must urinate repeatedly to empty bladder
SNOMEDCT
1765650018
Neurogenic dysfunction of the urinary bladder (finding)
SNOMEDCT
1777342015
Neurogenic dysfunction of the urinary bladder
SNOMEDCT
1765983010
Neurogenic bladder (finding)
SNOMEDCT
1777632010
Neurogenic bladder
SNOMEDCT
624071012
Bowel dysfunction (disorder)
SNOMEDCT
353134013
Bowel dysfunction
SNOMEDCT
353133019
BD - Bowel dysfunction
SNOMEDCT
118202007
Finding of sexual function
SNOMEDCT
697616014
Finding of sexual function (finding)
SNOMEDCT
443249016
Finding of sexual function
SNOMEDCT
1220367013
Observation of sexual function
SNOMEDCT
795491010
Abnormal sexual function (finding)
SNOMEDCT
94664018
Abnormal sexual function
SNOMEDCT
1231659011
Sexual dysfunction

26
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
SNOMEDCT
613002013
Decreased sexual function (finding)
SNOMEDCT
339169010
Decreased sexual function
SNOMEDCT
339168019
Impaired sexual function
SNOMEDCT
65210015
Normal sexual function (finding)
SNOMEDCT
19904012
Normal sexual function
SNOMEDCT
788443011
Normal female sexual function (finding)
SNOMEDCT
84366014
Normal female sexual function
SNOMEDCT
823566017
Normal male sexual function (finding)
SNOMEDCT
136328013
Normal male sexual function
SNOMEDCT
758802014
Abnormal female sexual function (finding)
SNOMEDCT
47132019
Abnormal female sexual function
SNOMEDCT
792328019
Abnormal male sexual function (finding)
SNOMEDCT
10048011
Abnormal male sexual function
SNOMEDCT
3035843015
Male sexual dysfunction
Presence of key phrases in clinical note may meet numerator component for Axon Registry.
Suggested key phrases to locate numerator components via Axon Registry
®
are included below. This list is not
exhaustive and will be updated annually if adopted into the Axon Registry:
• “Screened for GI symptoms; no follow-up needed”
• “Screened for GI symptoms; follow-up needed”
• “Screened for bladder symptoms; follow-up needed”
• “Screened for bladder symptoms; no follow-up needed”
• “Screened for bowel symptoms; follow-up needed”
• “Screened for bowel symptoms; no follow-up needed”
• “Screened for sexual dysfunction; follow-up needed”
• “Screened for sexual dysfunction; no follow-up needed”
Numerator – Follow-Up Component
SNOMEDCT
1779009018
Development of care plan
SNOMEDCT
1767604017
Development of care plan (procedure)
SNOMEDCT
1196083017
Development of individualized plan of care (procedure)
SNOMEDCT
1209518012
Development of individualized plan of care
SNOMEDCT
1228792012
Develops individualized plan of care
SNOMEDCT
566252018
Change of medication (procedure)
SNOMEDCT
282660014
Change of medication
SNOMEDCT
282659016
Medication changed
SNOMEDCT
750861000124112
Recommendation to change medication to lower cost therapeutic equivalent
(procedure)
SNOMEDCT
750871000124117
Recommendation to change medication to lower cost therapeutic equivalent
SNOMEDCT
616161000124116
Recommendation to change medication dose form (procedure)
SNOMEDCT
616171000124111
Recommendation to change medication dose form
SNOMEDCT
616181000124114
Advice to change medication dose form
SNOMEDCT
616281000124118
Recommendation to change medication dose (procedure)
SNOMEDCT
616291000124115
Recommendation to change medication dose
SNOMEDCT
616301000124119
Advice to change medication dose
SNOMEDCT
616161000124116
Recommendation to change medication dose form (procedure)
SNOMEDCT
616171000124111
Recommendation to change medication dose form
SNOMEDCT
616181000124114
Advice to change medication dose form
SNOMEDCT
223415003
Recommendation regarding activity (procedure)
SNOMEDCT
223440005
Recommendation to undertake activity (procedure)
SNOMEDCT
223469001
Discussion about activity (procedure)

27
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
SNOMEDCT
223415003
Recommendation regarding activity (procedure)
SNOMEDCT
566927011
Referral for further care (procedure)
SNOMEDCT
283512014
Referral for further care
SNOMEDCT
183444007
Referral for further care (procedure)
SNOMEDCT
709318013
Provision of specialist further education (procedure)
SNOMEDCT
456380014
Provision of specialist further education
SNOMEDCT
706904013
Further opinion sought (finding)
SNOMEDCT
453917017
Further opinion sought
SNOMEDCT
2463898019
Sexual behavior management (regime/therapy)
SNOMEDCT
1477096017
Sexual behavior management
SNOMEDCT
1490387018
Behavior management: sexual
SNOMEDCT
1490158013
Behaviour management: sexual
SNOMEDCT
1476135011
Sexual behaviour management
SNOMEDCT
567047012
Referred to urologist (finding)
SNOMEDCT
283665014
Referred to urologist
SNOMEDCT
705017017
Referral to gastroenterologist (procedure)
SNOMEDCT
451830012
Referral to gastroenterologist
SNOMEDCT
567043011
Referral to gynecology service (procedure)
SNOMEDCT
283659014
Referral to gynecology service
SNOMEDCT
283658018
Gynaecological referral
SNOMEDCT
283656019
Gynecological referral
SNOMEDCT
283657011
Referral to gynaecology service
SNOMEDCT
702506019
Referral to obstetrics and gynecology service (procedure)
SNOMEDCT
449028018
Referral to obstetrics and gynecology service
SNOMEDCT
449029014
Referral to obstetrics and gynaecology service
SNOMEDCT
2572866010
Urinary catheter care education (procedure)
SNOMEDCT
2471974018
Urinary catheter care education
SNOMEDCT
2477933010
Teach urinary catheter care
SNOMEDCT
2572868011
Urinary catheter irrigation education (procedure)
SNOMEDCT
2471980014
Urinary catheter irrigation education
SNOMEDCT
2477939014
Teach urinary catheter irrigation
Presence of key phrases in clinical note may meet numerator follow-up component for Axon Registry.
Suggested key phrases to locate follow-up via Axon Registry
®
are included below. This list is not exhaustive and will
be updated annually if adopted into the Axon Registry:
• “Behavioral modification”
• “Treatment plan updated”
• “Treatment changed”
• “Lifestyle changes”
• “Referral to urologist”
• “Referral to GI specialist”
• “Referral to OB/GYN”
• “Further testing conducted”
• “Additional tests ordered”
• “Pharmacological updates made”
• “Medication adjusted”
• “Catheter guidance provided”
• “Care instructions provided for catheter”
• “Clean intermittent self-catheterization”
Allowable Exclusions

28
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
SNOMEDCT
746791000124111
Recommendation refused by patient (situation)
SNOMEDCT
746801000124112
Recommendation refused by patient
SNOMEDCT
2608177018
Refused procedure - after thought (situation)
SNOMEDCT
284171012
Refused procedure - after thought
SNOMEDCT
183947005
Refused procedure - after thought (situation)
SNOMEDCT
2606319010
Refusal of treatment by patient (situation)
SNOMEDCT
169559019
Refusal of treatment by patient
SNOMEDCT
105480006
Refusal of treatment by patient (situation)
SNOMEDCT
2612741019
Refusal of treatment by parents (situation)
SNOMEDCT
1209841012
Refusal of treatment by parents
SNOMEDCT
2608092019
Refused procedure - parent's wish (situation)
SNOMEDCT
284172017
Refused procedure - parent's wish
SNOMEDCT
183948000
Refused procedure - parent's wish (situation)
SNOMEDCT
183944003
Procedure refused (situation)
SNOMEDCT
183945002
Procedure refused for religious reason (situation)
SNOMEDCT
413310006
Patient non-compliant - refused access to services (situation)
SNOMEDCT
413311005
Patient non-compliant - refused intervention / support (situation)
SNOMEDCT
413312003
Patient non-compliant - refused service (situation)
SNOMEDCT
183948000
Refused procedure - parent's wish (situation)
SNOMEDCT
416432009
Procedure not wanted (situation)
SNOMEDCT
443390004
Refused (qualifier value)
Presence of key phrases in clinical note may meet allowable exclusion component for Axon Registry.
Suggested key phrases to locate exclusions via Axon Registry
®
are included below. This list is not exhaustive and will
be updated annually if adopted into the Axon Registry:
• “Patient has declined screening”
• “Patient declines to discuss bladder, bowel, and sexual function”
• “Patient refuses to discuss bladder, bowel, and sexual function”
• “Patient refuses screening”
• “Patient declines screening”

29
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Yes
Yes
No
No
No
No
Yes
No
No
Yes
Flow Chart Diagram: Bladder, Bowel, and Sexual Dysfunction Screening and Follow-Up for Patients with MS
Patient met
numerator
criteria
Patient did
NOT meet
numerator
criteria
On date of the encounter, was patient
screened for bladder, bowel, or sexual
dysfunction, and if screen positive, had
appropriate follow-up care?
Patient
INCLUDED in
Eligible
Population
Patient NOT
Included in
Eligible
Population
Did the patient have at least one new or
established visit with an eligible provider
during the measurement period?
Did patient have a MS diagnosis on the date
of the visit?
Patient
INCLUDED in
Denominator
On date of encounter, did patient decline or
refuse screening?
Remove from
denominator*
*Do not remove/exclude
if patient meets the
numerator

30
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Cognitive Impairment Screening and Follow-Up for Patients with Multiple Sclerosis (MS)
Measure Title
Cognitive Impairment Screening and Follow-Up for Patients with MS
Description
Percentage of patients with MS who were screened* for cognitive impairment in the past 12
months and if screening positive, patient was referred appropriately for further evaluation and
management.
Measurement
Period
January 1, 20xx to December 31, 20xx
Eligible
Population
Eligible Providers
Medical Doctor (MD), Doctor of Osteopathy (DO), Pharmacist (PharmD),
Assistant (PA), Advanced Practice Registered Nurse (APRN), Physical
Therapy (PT), Occupational Therapy (OT)
Care Setting(s)
Outpatient Care
Ages
Any
Event
Office or telehealth encounter
Diagnosis
Multiple Sclerosis
Denominator
All patients with a diagnosis of MS.
Numerator
Patients with MS were screened* for cognitive impairment in past 12 months, and if screening
positive, patient was referred appropriately for further evaluation and management**.
Definitions:
* Screened is defined as administering any one of the following tools:
• Brief International Assessment of Cognition for MS (BICAMS),
1
• Symbol Digit Modalities Test (SDMT),
2
• MS Neuropsychological Screening Questionnaire (MSNQ) Observer version,
3
• Computerized Speed Cognitive Test (CST),
3
• Processing Speed Test (PST),
3
• Verbal fluency (phonemic and semantic),
4
• Paced Auditory Serial Addition Test (PASAT),
5
• Rao Brief Repeatable Neuropsychological Battery (BRNB),
5
• Minimal Assessment of Cognitive Function in MS (MACFIMS),
5
• PROMIS,
6
or
• Montreal Cognitive Assessment (MoCA).
7-9
**Further evaluation and management is defined as referral to:
• MS neuropsychological rehabilitation
• Neuropsychologist or psychologist,
• Speech/language pathologist, or
• Occupational therapist.
Neuropsychological evaluation in the past 12 months, may be used to meet the
numerator for screening and evaluation and management.
Required
Exclusions
• Patient not seen in past 12 months
Allowable
Exclusions
• Patient declines to complete a cognitive assessment on date of encounter.
• Patient is not able to complete a cognitive assessment on date of encounter.
• Patient currently receiving treatment to address cognitive impairment.
Allowable
Exclusion
Inclusion Logic
Allowable exclusions can only help measure performance. If a patient has an allowable
exclusion but is found to meet the numerator that patient is included in the count to meet the
measure.
Exclusion
Rationale
• Patients who have not been seen in the past 12 months are appropriate to exclude as
cognitive screening could not be completed in the required timeframe.

31
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
• Patients need to be willing to complete the screening tool for the screening scores to be
valid.
• Patients may be unable to meaningfully participate in a cognitive assessment and
exclusion is appropriate for those in a coma, delirious, or severely cognitively impaired.
• Screening would not be needed for those currently receiving treatment for a cognitive
impairment previously identified.
Measure Scoring
Percentage
Interpretation of
Score
Higher Score Indicates Better Quality
Measure Type
Process
Level of
Measurement
Provider
Risk Adjustment
Not applicable
Opportunity to
Improve Gap in
Care
43-70% of people with MS have reported cognitive impairments.
10
Clinicians cannot detect
cognitive impairment unless there is regular assessment. Clinical interview and standard
neurological examination is not sufficiently sensitive to detect cognitive impairment in multiple
sclerosis, and suggests need for a brief, accurate cognitive screening.
11
Screening should start
early, given the potential for cognitive impairment to start early for patients with MS and
appropriate referrals provided for positive results or changes.
11, 3
The Mini Mental Status Exam was not included in the list of screening tools due to concerns the
tool is not sufficiently sensitive to the most common cognitive deficits seen in patients with
MS.
12
For Process
Measures
Relationship to
Desired Outcome
Cognitive functioning impacts life satisfaction and health-related quality of life. It is anticipated
that if assessed on an ongoing basis, cognitive deficits may be identified and addressed in a
timely manner. Once identified, such deficits could be treated (or patients referred to
appropriate resources) and thereby improve individuals’ quality of life.
Following evidence statements are quoted verbatim from the referenced clinical guidelines:
• For adults and children (8+ years of age) with clinical or magnetic resonance imaging
(MRI) evidence of neurologic damage consistent with MS: As a minimum, early
baseline screening with the Symbol Digit Modalities Test (SDMT) or similarly validated
test, when the patient is clinically stable” (Consensus statement)
3
• For adults and children (8+ years of age) with clinical or magnetic resonance imaging
(MRI) evidence of neurologic damage consistent with MS: Annual re-assessment with
the same instrument, or more often as needed to (1) detect acute disease activity; (2)
assess for treatment effects (e.g. starting/changing a disease modifying therapy) or for
relapse recovery; (3) evaluate progression of cognitive impairment; and/or (4) screen for
new-onset cognitive problems.” (Consensus statement)
3
• For adults (18+ years): more comprehensive assessment for anyone who tests positive
on initial cognitive screening or demonstrates significant cognitive decline, especially if
there are concerns about comorbidities or the individual is applying for disability due to
cognitive impairment.” (Consensus statement)
3
• “Appropriate management of cognitive dysfunction in MS includes education for people
with MS and their family members, early screening and ongoing monitoring throughout
the disease course, and interventions to remediate dysfunction and provide
compensatory strategies to optimize function and participation.”(Consensus statement)
3
• “…is recommended to assess areas of cognitive deficit and strength, as well as to
evaluate all factors that could be impacting cognitive functioning, such as cognitive
reserve, depression and/or anxiety fatigue, co-morbid health conditions, and
medications.”(Consensus statement)
3

32
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
• Patients who require rehabilitation to address cognitive changes impacting their
functioning at home or at work should be referred to a specialist. The optimal referral is
to a specialist in MS neuropsychological rehabilitation (neuropsychologist,
speech/language pathologist or occupational therapist)… .”(Consensus statement)
3
• “Tailor the comprehensive review to the needs of the person with MS assessing: MS
symptoms: …cognitive symptoms…”
13
• “Be aware that the symptoms of MS can include cognitive problems, including memory
problems that the person may not immediately recognise or associate with their MS.”
13
• “Talk to people with MS and their family members or carers about the possibility that
the condition might lead to cognitive problems.”
13
• “Consider referring people with MS and persisting memory or cognitive problems to
both an occupational therapist and a neuropsychologist to assess and manage these
symptoms.”
13
Harmonization
with Existing
Measures
There are no known cognitive impairment quality measures that incorporate patients with MS in
the denominator. A measure is needed to address the opportunity for improvement specific to
the cognitive impairments faced by the MS population.
References
1. Benedict RHB, Amato MP, Boringa J, et al. Brief International Cognitive Assessment for MS
(BICAMS): international standards for validation. BMC Neurology. 2012;12:55.
2. Smith A. The symbol-digit modalities test: a neuropsychologic test of learning and other cerebral
disorders. J. Helmuth (Ed.) Learning disorders, Special Child Publications, Seattle (1968), pp.
83-91.
3. Kalb R, Beier M, Benedict RH, et al. Recommendations for cognitive screening and management
in multiple sclerosis care. Multiple Sclerosis. 2018; 24(13):1665-1680.
4. Connick P, Kolappan M, Bak TH, et al. Verbal fluency as a rapid screening test for cognitive
impairment in progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83(3):346-
347.
5. Foley FW, Benedict RHB, Gromisch ES, et al. The Need for Screening, Assessment, and
Treatment for Cognitive Dysfunction in Multiple Sclerosis. Results of a Multidisciplinary CMSC
Consensus Conference, September 24, 2010. Int J MS Care. 2012;14:58–64.
6. Becker H, Stuifbergen A, Lee H, et al. Reliability and Validity of PROMIS Cognitive Abilities
and Cognitive Concerns Scales Among People with Multiple Sclerosis. Int J MS Care.
2014;16(1):1-8.
7. Freitas S, Batista S, Afonso A, et al. The Montreal Cognitive Assessment (MoCA) as a screening
test for cognitive dysfunction in MS. Appl Neuropsychol Adult. 2018;25(1):57-70.
8. Dagenais E, Roulou I, Demers M, et al. Value of the MoCA as a screening instrument in multiple
sclerosis. Can J Neurol Sci. 2013; 40(3):410-415.
9. Kaur D, Kumer G, Singh A. Quick screening of cognitive function in Indian multiple sclerosis
patients using Montreal cognitive assessment test-short version. Ann Indian Acad Neurol.
2013;16(4);585-589.
10. Langdon DW, Amato MP, Boringa J, et al. Recommendations for a Brief International
Cognitive Assessment for Multiple Sclerosis (BICAMS). Multiple Sclerosis. 2012;0(0);1-8.
11. Romero K, Shammi P, Feinstein A. Neurologist Accuracy and Predicting Cognitive Impairment
in Multiple Sclerosis. Multiple Sclerosis and Related Disorders. 2015;15(4):291–295.
Process
Cognitive impairment
screening completed
Intermediate
Outcome
Cognitive impairment
treatment initiated
Outcome
Improved quality of life

33
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
12. Beatty WW and Goodkin DE. Screening for Cognitive Impairment in Multiple Sclerosis: An
Evaluation of the Mini-Mental State Examination. Arch Neurol. 1990;47(3):297-301.
13. National Clinical Guideline Centre (NICE) (UK). Multiple Sclerosis: Management of Multiple
Sclerosis in Primary and Secondary Care. London: National Institute for Health and Care
Excellence; 2014 Oct. 2019 update available at: https://www.nice.org.uk/guidance/cg186
Accessed on November 13, 2020.
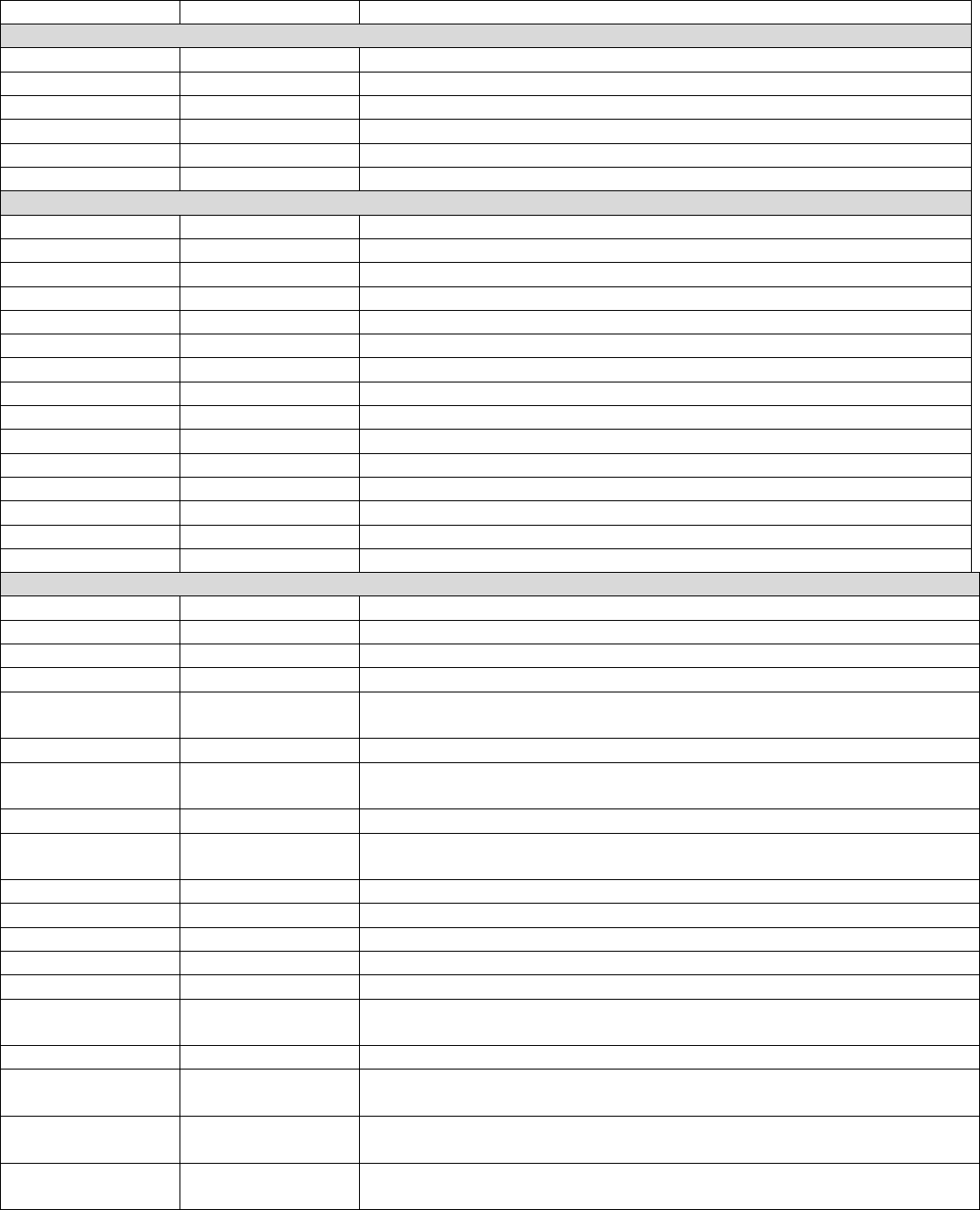
34
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Code System
Code
Code Description
Initial Population
CPT
99201-99205
Office or other outpatient visit, new patient
CPT
99211-99215
Office or other outpatient visit, established patient
CPT
99241-99245
Office or other outpatient consultation, new or established patient
CPT
97003, 97004
Occupational therapy, evaluation and re-evaluation
CPT
97161-97164
Physical therapy, evaluation and re-evaluation
CPT
Telehealth codes TBD
Denominator
ICD-10
G35
Multiple Sclerosis
SNOMEDCT
24700007
Multiple sclerosis (disorder)
SNOMEDCT
192929006
Exacerbation of multiple sclerosis (disorder)
SNOMEDCT
230372003
Acute relapsing multiple sclerosis (disorder)
SNOMEDCT
425500002
Secondary progressive multiple sclerosis (disorder)
SNOMEDCT
426373005
Relapsing remitting multiple sclerosis (disorder)
SNOMEDCT
428700003
Primary progressive multiple sclerosis (disorder)
SNOMEDCT
438511000
Benign multiple sclerosis (disorder)
SNOMEDCT
92926004
Multiple sclerosis of the brainstem (disorder)
SNOMEDCT
192927008
Multiple sclerosis of the spinal cord (disorder)
SNOMEDCT
439567002
Malignant multiple sclerosis (disorder)
SNOMEDCT
724778008
Progressive relapsing multiple sclerosis (disorder)
SNOMEDCT
733028000
Multiple sclerosis, ichthyosis, factor VIII deficiency syndrome (disorder)
SNOMEDCT
766246000
Marburg acute multiple sclerosis (disorder)
SNOMEDCT
816984002
Progressive multiple sclerosis (disorder)
Numerator – Screened component
LOINC
81529-0
PROMIS short form - cognitive function 6a - version 2.0
LOINC
81532-4
PROMIS short form - cognitive function 6a - version 2.0 raw score
LOINC
81530-8
PROMIS short form - cognitive function 8a - version 2.0
LOINC
81531-6
PROMIS short form - cognitive function 8a - version 2.0 raw score
LOINC
81534-0
PROMIS short form - cognitive function - abilities subset 4a - version 2.0
raw score
LOINC
81526-6
PROMIS short form - cognitive function - abilities subset 6a - version 2.0
LOINC
81535-7
PROMIS short form - cognitive function - abilities subset 6a - version 2.0
raw score
LOINC
81527-4
PROMIS short form - cognitive function - abilities subset 8a - version 2.0
LOINC
81536-5
PROMIS short form - cognitive function - abilities subset 8a - version 2.0
raw score
LOINC
81528-2
PROMIS short form - cognitive function 4a - version 2.0
LOINC
81525-8
PROMIS short form - cognitive function - abilities subset 4a - version 2.0
LOINC
72172-0
Total score [MoCA]
LOINC
72133-2
Montreal Cognitive Assessment [MoCA]
LOINC
84436-5
Pattern Comparison Processing Speed Test [NIH Toolbox]
LOINC
84483-7
Pattern Comparison Processing Speed Test - national percentile [NIH
Toolbox]
LOINC
84486-0
Pattern Comparison Processing Speed Test - raw score [NIH Toolbox]
LOINC
84484-5
Pattern Comparison Processing Speed Test - scale score age adjusted [NIH
Toolbox]
LOINC
84485-2
Pattern Comparison Processing Speed Test - scale score fully adjusted
[NIH Toolbox]
LOINC
84487-8
Pattern Comparison Processing Speed Test - unadjusted scale score [NIH
Toolbox]

35
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
SNOMEDCT
666547011
Symbol digit modalities test (assessment scale)
SNOMEDCT
409521019
Symbol digit modalities test
SNOMEDCT
409522014
SDMT - Symbol digit modalities test
SNOMEDCT
3310099013
Symbol Digit Modalities Test score (observable entity)
SNOMEDCT
3310105010
Symbol Digit Modalities Test score
SNOMEDCT
3311106017
SDMT (Symbol Digit Modalities Test) score
SNOMEDCT
3305110019
Assessment using Symbol Digit Modalities Test (procedure)
SNOMEDCT
3305111015
Assessment using Symbol Digit Modalities Test
SNOMEDCT
3307589015
Assessment using SDMT (Symbol Digit Modalities Test)
SNOMEDCT
708044014
Paced auditory serial addition test (assessment scale)
SNOMEDCT
455064017
Paced auditory serial addition test
SNOMEDCT
455063011
PASAT - Paced auditory serial addition test
SNOMEDCT
1785144016
PASAT - Paced auditory stimulation test
SNOMEDCT
1785145015
Paced auditory stimulation test
SNOMEDCT
3725598019
Paced Auditory Serial Addition Test score (observable entity)
SNOMEDCT
3725600013
Paced Auditory Serial Addition Test score
SNOMEDCT
3725752017
PASAT-Paced Auditory Serial Addition Test score
SNOMEDCT
3725599010
Paced Auditory Stimulation Test score
SNOMEDCT
3725598019
Paced Auditory Serial Addition Test score (observable entity)
SNOMEDCT
790891000124112
Assessment using Montreal cognitive assessment (procedure)
SNOMEDCT
790901000124111
Assessment using Montreal cognitive assessment
SNOMEDCT
790871000124111
MoCA Assessment
SNOMEDCT
790911000124114
Montreal cognitive assessment
SNOMEDCT
790831000124113
Montreal cognitive assessment score (observable entity)
SNOMEDCT
790821000124110
Montreal cognitive assessment score
SNOMEDCT
790841000124115
MoCA score
SNOMEDCT
1460984013
Impaired cognition (finding)
SNOMEDCT
1480926019
Impaired cognition
SNOMEDCT
1491798012
Cognitive decline
SNOMEDCT
3289770014
Cognitive deficit
SNOMEDCT
1491796011
Cognitive disturbance
SNOMEDCT
1491797019
Cognitive dysfunction
SNOMEDCT
1491795010
Cognitive impairment
SNOMEDCT
621379018
Minimal cognitive impairment (finding)
SNOMEDCT
175146011
Minimal cognitive impairment
SNOMEDCT
3006637011
Moderate cognitive impairment (finding)
SNOMEDCT
3006646017
Moderate cognitive impairment
SNOMEDCT
3006674010
Severe cognitive impairment (finding)
SNOMEDCT
3006632017
Severe cognitive impairment
SNOMEDCT
751741000124113
Cognitive impairment due to multiple sclerosis (disorder)
SNOMEDCT
751781000124119
Cognitive impairment due to multiple sclerosis
SNOMEDCT
751811000124117
Cognitive deficit due to multiple sclerosis
Presence of key phrases in clinical note may meet numerator screening component for Axon Registry.
Suggested key phrases to locate screening component via Axon Registry
®
are included below. This list is not
exhaustive and will be updated annually if adopted into the Axon Registry:
• “Brief International Assessment of Cognition for MS completed and f/u needed”
• “BICAMS completed and f/u needed”
• “Brief International Assessment of Cognition for MS completed and f/u not needed”
• “BICAMS completed and f/u not needed”
36
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
• “Symbol Digit Modalities Test completed and f/u needed”
• “SDMT completed and f/u needed”
• “Symbol Digit Modalities Test completed and f/u not needed”
• “SDMT completed and f/u not needed”
• “MS Neuropsychological Screening Questionnaire - Observer version completed and f/u needed”
• “MSNQ completed and f/u needed”
• “MS Neuropsychological Screening Questionnaire - Observer version completed and f/u not needed”
• “MSNQ completed and f/u not needed”
• “Computerized Speed Cognitive Test completed and f/u needed”
• “CST completed and f/u needed”
• “Computerized Speed Cognitive Test completed and f/u not needed”
• “CST completed and f/u not needed”
• “Processing Speed Test completed and f/u needed”
• “PST completed and f/u needed”
• “Processing Speed Test completed and f/u not needed”
• “PST completed and f/u not needed”
• “Verbal fluency completed and f/u needed”
• “Verbal fluency completed and f/u not needed”
• “Paced Auditory Serial Addition Test completed and f/u needed”
• “PASAT completed and f/u needed”
• “Paced Auditory Serial Addition Test completed and f/u not needed”
• “PASAT completed and f/u not needed”
• “Rao Brief Repeatable Neuropsychological Battery completed and f/u needed”
• “BRNB completed and f/u needed”
• “Rao Brief Repeatable Neuropsychological Battery completed and f/u not needed”
• “BRNB completed and f/u not needed”
• “Minimal Assessment of Cognitive Function in MS completed and f/u needed”
• “MACFIMS completed and f/u needed”
• “Minimal Assessment of Cognitive Function in MS completed and f/u not needed”
• “MACFIMS completed and f/u not needed”
• “PROMIS completed and f/u needed”
• “PROMIS completed and f/u not needed”
• “Montreal Cognitive Assessment completed and f/u needed”
• “Montreal Cognitive Assessment completed and f/u not needed”
• “Neuropsychological exam results reviewed”
Numerator – Follow-up component
CPT
99483
Cognitive Impairment and Care Plan Assessment
CPT
96136, 96138,
96146
Neuropsychological testing
CPT
96116
Neurobehavioral status exam
SNOMEDCT
308459004
Referral to psychologist (procedure)
SNOMEDCT
308477009
Referral to psychiatrist (procedure)
SNOMEDCT
81294000
Patient referral for psychotherapy (procedure)
SNOMEDCT
88848003
Psychiatric follow-up (procedure)
SNOMEDCT
309627007
Child referral - clinical psychologist (procedure)
SNOMEDCT
2546404011
Referral for neuropsychological testing (procedure)
SNOMEDCT
2548695019
Referral for neuropsychological testing
SNOMEDCT
704991010
Referral to speech and language therapist (procedure)
SNOMEDCT
451792012
Referral to speech and language therapist
SNOMEDCT
451791017
Refer to speech therapist

37
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
SNOMEDCT
702758011
Referral to community-based speech and language therapist (procedure)
SNOMEDCT
449359011
Referral to community-based speech and language therapist
SNOMEDCT
449360018
Referral to community speech and language therapist
SNOMEDCT
702551019
Referral to speech and language therapy service (procedure)
SNOMEDCT
449090019
Referral to speech and language therapy service
SNOMEDCT
702552014
Referral to community-based speech and language therapy service
(procedure)
SNOMEDCT
449091015
Referral to community-based speech and language therapy service
SNOMEDCT
449092010
Referral to community speech and language therapy service
SNOMEDCT
750281000124115
Referral for occupational therapy (procedure)
SNOMEDCT
750291000124117
Referral for occupational therapy
SNOMEDCT
702543017
Referral to occupational therapy service (procedure)
SNOMEDCT
449079012
Referral to occupational therapy service
SNOMEDCT
2788042017
Referral to pediatric occupational therapy service (procedure)
SNOMEDCT
2792715016
Referral to pediatric occupational therapy service
SNOMEDCT
2792714017
Referral to paediatric occupational therapy service
SNOMEDCT
702544011
Referral to community-based occupational therapy service (procedure)
SNOMEDCT
449080010
Referral to community-based occupational therapy service
SNOMEDCT
449081014
Referral to community occupational therapy service
Presence of key phrases in clinical note may meet numerator follow-up component for Axon Registry.
Suggested key phrases to locate follow-up component via Axon Registry
®
are included below. This list is not
exhaustive and will be updated annually if adopted into the Axon Registry:
• “Referral to MS neuropsychological rehabilitation”
• “Referral to neuropsychologist”
• “Referral to psychologist”
• “Referral to speech pathologist”
• “Referral to speech/language pathologist”
• “Referral to occupational therapist”
• “Referred to MS neuropsychological rehabilitation”
• “Referred to neuropsychologist”
• “Referred to psychologist”
• “Referred to speech pathologist”
• “Referred to speech/language pathologist”
• “Referred to occupational therapist”
• “Neuropsychological exam results reviewed”
Required Exclusions
A required exclusion for patient not seen in prior 12 months before encounter would be calculated using CPT codes
above in initial population.
Allowable Exclusions
SNOMEDCT
746791000124111
Recommendation refused by patient (situation)
SNOMEDCT
746801000124112
Recommendation refused by patient
SNOMEDCT
2608177018
Refused procedure - after thought (situation)
SNOMEDCT
284171012
Refused procedure - after thought
SNOMEDCT
183947005
Refused procedure - after thought (situation)
SNOMEDCT
2606319010
Refusal of treatment by patient (situation)
SNOMEDCT
169559019
Refusal of treatment by patient
SNOMEDCT
105480006
Refusal of treatment by patient (situation)
SNOMEDCT
2612741019
Refusal of treatment by parents (situation)
SNOMEDCT
1209841012
Refusal of treatment by parents

38
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
SNOMEDCT
2608092019
Refused procedure - parent's wish (situation)
SNOMEDCT
284172017
Refused procedure - parent's wish
SNOMEDCT
183948000
Refused procedure - parent's wish (situation)
SNOMEDCT
183944003
Procedure refused (situation)
SNOMEDCT
183945002
Procedure refused for religious reason (situation)
SNOMEDCT
413310006
Patient non-compliant - refused access to services (situation)
SNOMEDCT
413311005
Patient non-compliant - refused intervention / support (situation)
SNOMEDCT
413312003
Patient non-compliant - refused service (situation)
SNOMEDCT
183948000
Refused procedure - parent's wish (situation)
SNOMEDCT
416432009
Procedure not wanted (situation)
SNOMEDCT
443390004
Refused (qualifier value)
Presence of key phrases in clinical note may meet allowable exclusion for Axon Registry.
Suggested key phrases to locate allowable exclusions via Axon Registry
®
are included below. This list is not exhaustive
and will be updated annually if adopted into the Axon Registry:
• “Patient declines cognitive assessment”
• “Patient is not able to complete a cognitive assessment”
• “Patient unable to complete cognitive assessment”
• “Patient currently receiving treatment to address cognitive impairment.”
• “Patient currently receiving treatment for cognitive impairment”
• “Patient receiving cognitive impairment care from other provider”

39
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
No
No
Yes
No
Yes
Yes
No
No
Yes
No
Yes
Yes
No
No
Yes
No
No
Flow Chart Diagram: Cognitive Impairment Screening and Follow-Up for Patients with MS
Patient
INCLUDED in
Eligible
Population
Patient
INCLUDED in
Denominator
On date of encounter, did patient decline or
refuse a cognitive screening?
Did patient have a MS diagnosis on the date
of the visit?
Patient NOT
Included in
Eligible
Population
Patient met
numerator
criteria
Patient did
NOT meet
numerator
criteria
Was patient screened for cognitive impairment in
past 12 months, and if screening positive were
provided appropriate follow-up?
Did the patient have at least one new or
established visit with an eligible provider
during the measurement period?
On the date of the encounter, was the
patient unable to complete a cognitive
screening?
Remove from
denominator*
*Do not remove/exclude
if patient meets the
numerator
Is the patient currently receiving treatment
for a cognitive impairment?
Was patient seen previously in the 12
months prior to the date of the visit?

40
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Fatigue Screening and Follow-Up for Patients with Multiple Sclerosis (MS)
Measure Title
Fatigue Screening and Follow-Up for Patients with MS
Description
Percentage of patients 18 years and older with diagnosis of MS who were screened for fatigue in
past 12 months, and if screening positive were provided appropriate follow-up.
Measurement
Period
January 1, 20xx to December 31, 20xx
Eligible
Population
Eligible Providers
Medical Doctor (MD), Doctor of Osteopathy (DO), Pharmacist (PharmD),
Physician Assistant (PA), Advanced Practice Registered Nurse (APRN),
Physical Therapy (PT), Occupational Therapy (OT)
Care Setting(s)
Outpatient Care
Ages
18 and older
Event
Office or telehealth encounter
Diagnosis
Multiple Sclerosis
Denominator
Patients 18 years and older with a diagnosis of MS.
Numerator
Patients with MS who were screened* for fatigue in past 12 months, and if screening positive
were provided appropriate follow-up.**
Definitions
*Screened is defined as use of one of the following validated fatigue rating instruments:
• the Fatigue Severity Scale (FSS)
1-4
or
• Modified Fatigue Impact Scale
5,6
or
• Shortened Modified Fatigue Impact Scale
6
**Follow-up for this measure is defined as adjustment to the treatment plan, adjustment or
initiation of appropriate medication, further testing, referral to PT/OT, exercise program,
lifestyle modification program, or referral to an appropriate healthcare provider.
Required
Exclusions
None
Allowable
Exclusions
• Patients unable to complete a fatigue screening on date of encounter
• Patient declines to complete a fatigue screening on date of encounter
Allowable
Exclusion
Inclusion Logic
Allowable exclusions can only help measure performance. If a patient has an allowable
exclusion but is found to meet the numerator that patient is included in the count to meet the
measure.
Exclusion
Rationale
• Fatigue is a subjective symptom that requires patient cooperation to assess.
Measure Scoring
Percentage
Interpretation of
Score
Higher Score Indicates Better Quality
Measure Type
Process
Level of
Measurement
Provider
Risk Adjustment
Not Applicable
Opportunity to
Improve Gap in
Care
Fatigue occurs in about 80% of patients with MS reducing physical activity and level of daily
functioning.
7
It is anticipated that by addressing fatigue, quality of life will improve as
individuals have decreased fatigue and increased ability to function at work and home.
For Process
Measures
Relationship to
Desired Outcome
The desired outcome is to reduce or eliminate fatigue in MS patients. The measure will provide
an incentive for providers to identify and manage fatigue in MS patients.
The following evidence statements are quoted verbatim from the referenced clinical guidelines:

41
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
• “Assess and offer treatment to people with MS who have fatigue for anxiety, depression,
difficulty in sleeping, and any potential medical problems such as anaemia or thyroid
disease.”
8
• “Explain that MS-related fatigue may be precipitated by heat, overexertion and stress or
may be related to the time of day.”
8
• “Advise people that aerobic, balance and stretching exercises including yoga may be
helpful in treating MS-related fatigue.
”8
Harmonization
with Existing
Measures
There are currently no other comparable fatigue measures in national measurement programs or
endorsed by the National Quality Forum.
References
1
Krupp LB, LaRocca NG, Nuir-Nash J, et al. The Fatigue Severity Scale: Application to Patients with
Multiple Sclerosis and Systemic Lupus Erythematosus. Arch Neurol. 1989;46(10):1121-1123.
2
Christodoulou C, MacAllister WS, Krupp LB: Psychiatry for Neurologists: Fatigue 295-306
Philadelphia: Elsevier Science; 2003.
3
Schwartz JE, Jandorf L, Krupp LB. The measurement of fatigue: A new instrument. Journal of
Psychosomatic Research. 1993; 37(7):753-762.
4
Téllez N, Río J, Tintoré M, et al. Does the Modified Fatigue Impact Scale offer a more comprehensive
assessment of fatigue in MS? Mult Scler. 2005 11: 198.
5 Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with
multiple sclerosis. Can J Neurol Sci. 1994; 21: 9-14.
6
Ritvo PG, Fischer JS, Miller DM, et al. Multiple Sclerosis Quality of Life Inventory (MSQLI): A User’s
Manual. New York: The Consortium of Multiple Sclerosis Centers Health Services Research
Subcommittee, National Multiple Sclerosis Society, New York, 1997.
7
Meads DM, Doward LC, McKenna SP, et al. The development and validation of the Unidimensional
Fatigue Impact Scale (U-FIS). Multiple Sclerosis. 2009; 15(10):1228-1238.
8
National Clinical Guideline Centre (NICE) (UK). Multiple Sclerosis: Management of Multiple Sclerosis
in Primary and Secondary Care. London: National Institute for Health and Care Excellence; 2014 Oct.
2019 update available at: https://www.nice.org.uk/guidance/cg186 Accessed on November 13, 2020.
Process
Fatigue screening
completed
Intermediate
Outcome
Fatigue symptoms
identified
Treatment initiated for
fatigue symptoms
Outcome
Reduction of fatigue
symptoms
Improved quality of life

42
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Code System
Code
Code Description
Initial Population
CPT
99201-99205
Office or other outpatient visit, new patient
CPT
99211-99215
Office or other outpatient visit, established patient
CPT
99241-99245
Office or other outpatient consultation, new or established patient
CPT
97003, 97004
Occupational therapy, evaluation and re-evaluation
CPT
97161-97164
Physical therapy, evaluation and re-evaluation
CPT
Telehealth codes TBD
Denominator
ICD-10
G35
Multiple Sclerosis
SNOMEDCT
24700007
Multiple sclerosis (disorder)
SNOMEDCT
192929006
Exacerbation of multiple sclerosis (disorder)
SNOMEDCT
230372003
Acute relapsing multiple sclerosis (disorder)
SNOMEDCT
425500002
Secondary progressive multiple sclerosis (disorder)
SNOMEDCT
426373005
Relapsing remitting multiple sclerosis (disorder)
SNOMEDCT
428700003
Primary progressive multiple sclerosis (disorder)
SNOMEDCT
438511000
Benign multiple sclerosis (disorder)
SNOMEDCT
92926004
Multiple sclerosis of the brainstem (disorder)
SNOMEDCT
192927008
Multiple sclerosis of the spinal cord (disorder)
SNOMEDCT
439567002
Malignant multiple sclerosis (disorder)
SNOMEDCT
724778008
Progressive relapsing multiple sclerosis (disorder)
SNOMEDCT
733028000
Multiple sclerosis, ichthyosis, factor VIII deficiency syndrome (disorder)
SNOMEDCT
766246000
Marburg acute multiple sclerosis (disorder)
SNOMEDCT
816984002
Progressive multiple sclerosis (disorder)
Numerator -Screened component
LOINC
28100-6
Fatigue
SNOMEDCT
3039531019
Assessment of fatigue (procedure)
SNOMEDCT
3039553017
Assessment of fatigue
SNOMEDCT
2879783014
Fatigue impact scale score (observable entity)
SNOMEDCT
2883110010
Fatigue impact scale score
Presence of key phrases in clinical note may meet numerator component for Axon Registry.
Suggested key phrases to locate numerator component via Axon Registry
®
are included below. This list is not
exhaustive and will be updated annually if adopted into the Axon Registry:
• “FSS results indicate f/u needed”
• “FSS results indicate no f/u needed”
• “Modified Fatigue Impact Scale
results indicate f/u needed”
• “Modified Fatigue Impact Scale
results indicate no f/u needed”
• “MFIS
results indicate f/u needed”
• “MFIS
results indicate no f/u needed”
• “MFIS-5 results indicate f/u needed”
• “MFIS-5 results indicate no f/u needed”
Numerator -Follow-up needed for positive screening component
Follow-up is required when the FSS score is greater than or equal to 5 and when the MFIS score is greater than or
equal to 39. The work group will evaluate adding future cutoffs for the SMFIS should this data be published.
SNOMEDCT
826028019
Fatigue (finding)
Presence of key phrases in clinical note may meet numerator component for Axon Registry.
Suggested key phrases to locate numerator component via Axon Registry
®
are included below. This list is not
exhaustive and will be updated annually if adopted into the Axon Registry:
• “FSS results indicate f/u needed”

43
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
• “FSS score is 5 [or greater]”
• “Modified Fatigue Impact Scale
results indicate f/u needed”
• “MFIS
results indicate f/u needed”
• “MFIS score is 39 [or greater]”
• “Shortened Modified Fatigue Impact Scale
results indicate f/u needed”
• “MFIS-5 indicates f/u needed”
Numerator -Follow-up component
SNOMEDCT
1779009018
Development of care plan
SNOMEDCT
1767604017
Development of care plan (procedure)
SNOMEDCT
1196083017
Development of individualized plan of care (procedure)
SNOMEDCT
1209518012
Development of individualized plan of care
SNOMEDCT
1228792012
Develops individualized plan of care
SNOMEDCT
566252018
Change of medication (procedure)
SNOMEDCT
282660014
Change of medication
SNOMEDCT
282659016
Medication changed
SNOMEDCT
750861000124112
Recommendation to change medication to lower cost therapeutic
equivalent (procedure)
SNOMEDCT
750871000124117
Recommendation to change medication to lower cost therapeutic
equivalent
SNOMEDCT
616161000124116
Recommendation to change medication dose form (procedure)
SNOMEDCT
616171000124111
Recommendation to change medication dose form
SNOMEDCT
616181000124114
Advice to change medication dose form
SNOMEDCT
616281000124118
Recommendation to change medication dose (procedure)
SNOMEDCT
616291000124115
Recommendation to change medication dose
SNOMEDCT
616301000124119
Advice to change medication dose
SNOMEDCT
616161000124116
Recommendation to change medication dose form (procedure)
SNOMEDCT
616171000124111
Recommendation to change medication dose form
SNOMEDCT
616181000124114
Advice to change medication dose form
SNOMEDCT
223415003
Recommendation regarding activity (procedure)
SNOMEDCT
223440005
Recommendation to undertake activity (procedure)
SNOMEDCT
223469001
Discussion about activity (procedure)
SNOMEDCT
223415003
Recommendation regarding activity (procedure)
SNOMEDCT
566927011
Referral for further care (procedure)
SNOMEDCT
283512014
Referral for further care
SNOMEDCT
183444007
Referral for further care (procedure)
SNOMEDCT
709318013
Provision of specialist further education (procedure)
SNOMEDCT
456380014
Provision of specialist further education
SNOMEDCT
706904013
Further opinion sought (finding)
SNOMEDCT
453917017
Further opinion sought
SNOMEDCT
223415003
Recommendation regarding activity (procedure)
SNOMEDCT
223440005
Recommendation to undertake activity (procedure)
SNOMEDCT
223469001
Discussion about activity (procedure)
SNOMEDCT
390893007
Referral to physical activity program (procedure)
SNOMEDCT
410289001
Exercises education, guidance, and counseling (procedure)
SNOMEDCT
223415003
Recommendation regarding activity (procedure)
SNOMEDCT
762227003
Provision of advice about aerobic exercise (procedure)
SNOMEDCT
710138000
Promotion of adherence to exercise regime (procedure)
SNOMEDCT
410335001
Exercises case management (procedure)
SNOMEDCT
410289001
Exercises education, guidance, and counseling (procedure)
SNOMEDCT
386292004
Exercise promotion: stretching (procedure)

44
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
SNOMEDCT
386291006
Exercise promotion: strength training (procedure)
SNOMEDCT
370873006
Ambulation therapy management (procedure)
SNOMEDCT
370872001
Ambulation therapy education (procedure)
SNOMEDCT
370870009
Ambulation therapy assessment (procedure)
SNOMEDCT
304549008
Giving encouragement to exercise (procedure)
SNOMEDCT
304507003
Exercise education
SNOMEDCT
308477009
Referral to psychiatrist (procedure)
SNOMEDCT
703978000
Referral to primary care service (procedure)
SNOMEDCT
183851006
Referral to clinic (procedure)
SNOMEDCT
81294000
Patient referral for psychotherapy (procedure)
SNOMEDCT
88848003
Psychiatric follow-up (procedure)
SNOMEDCT
309627007
Child referral - clinical psychologist (procedure)
SNOMEDCT
444831000124102
Referral for physical therapy (procedure)
SNOMEDCT
444911000124101
Referral for office-based physical therapy (procedure)
SNOMEDCT
416790000
Referral for home physical therapy (procedure)
SNOMEDCT
750281000124115
Referral for occupational therapy (procedure)
SNOMEDCT
750291000124117
Referral for occupational therapy
SNOMEDCT
702543017
Referral to occupational therapy service (procedure)
SNOMEDCT
449079012
Referral to occupational therapy service
SNOMEDCT
2788042017
Referral to pediatric occupational therapy service (procedure)
SNOMEDCT
2792715016
Referral to pediatric occupational therapy service
SNOMEDCT
2792714017
Referral to paediatric occupational therapy service
SNOMEDCT
702544011
Referral to community-based occupational therapy service (procedure)
SNOMEDCT
449080010
Referral to community-based occupational therapy service
SNOMEDCT
449081014
Referral to community occupational therapy service
Presence of key phrases in clinical note may meet numerator follow-up component for Axon Registry.
Suggested key phrases to locate follow-up via Axon Registry
®
are included below. This list is not exhaustive and will
be updated annually if adopted into the Axon Registry:
• “Behavioral modification”
• “Treatment plan updated”
• “Treatment changed”
• “Lifestyle changes”
• “Referral to physical therapy”
• “Referral to occupational therapy”
• “Referral to exercise program”
• “Referral to sleep study”
• “Referral to polysomnography”
• “Further testing conducted”
• “Additional tests ordered”
• “Pharmacological updates made”
• “Medication adjusted”
Allowable Exclusions
SNOMEDCT
746791000124111
Recommendation refused by patient (situation)
SNOMEDCT
746801000124112
Recommendation refused by patient
SNOMEDCT
2608177018
Refused procedure - after thought (situation)
SNOMEDCT
284171012
Refused procedure - after thought
SNOMEDCT
183947005
Refused procedure - after thought (situation)
SNOMEDCT
2606319010
Refusal of treatment by patient (situation)
SNOMEDCT
169559019
Refusal of treatment by patient

45
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
SNOMEDCT
105480006
Refusal of treatment by patient (situation)
SNOMEDCT
2612741019
Refusal of treatment by parents (situation)
SNOMEDCT
1209841012
Refusal of treatment by parents
SNOMEDCT
2608092019
Refused procedure - parent's wish (situation)
SNOMEDCT
284172017
Refused procedure - parent's wish
SNOMEDCT
183948000
Refused procedure - parent's wish (situation)
SNOMEDCT
183944003
Procedure refused (situation)
SNOMEDCT
183945002
Procedure refused for religious reason (situation)
SNOMEDCT
413310006
Patient non-compliant - refused access to services (situation)
SNOMEDCT
413311005
Patient non-compliant - refused intervention / support (situation)
SNOMEDCT
413312003
Patient non-compliant - refused service (situation)
SNOMEDCT
183948000
Refused procedure - parent's wish (situation)
SNOMEDCT
416432009
Procedure not wanted (situation)
SNOMEDCT
443390004
Refused (qualifier value)
Presence of key phrases in clinical note may meet allowable exclusion for Axon Registry.
Suggested key phrases to locate allowable exclusions via Axon Registry
®
are included below. This list is not
exhaustive and will be updated annually if adopted into the Axon Registry:
• “Patient unable to complete a fatigue screening”
• “Patient declines to complete a fatigue screening”

46
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Yes
No
No
Yes
Yes
No
No
No
Yes
No
Yes
No
Yes
No
Flow Chart Diagram: Fatigue Screening and Follow-Up for Patients with MS
Patient met
numerator
criteria
Patient did
NOT meet
numerator
criteria
Was patient screened for fatigue in past 12
months, and if screening positive were provided
appropriate follow-up?
Patient
INCLUDED in
Denominator
On date of encounter, did patient decline
or refuse fatigue screening?
Remove from
denominator*
*Do not remove/exclude
if patient meets the
numerator
On date of encounter, was patient unable
to complete fatigue screening?
Patient
INCLUDED in
Eligible
Population
Patient NOT
Included in
Eligible
Population
Did the patient have at least one new or
established visit with an eligible provider
during the measurement period?
Did patient have a MS diagnosis on the
date of the visit?
Was patient 18 years or older on the date
of the visit?

47
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Exercise and Appropriate Physical Activity Counseling for Patients with Multiple Sclerosis (MS)
Measure Title
Exercise and Appropriate Physical Activity Counseling for Patients with MS
Description
Percentage of patients with MS who are counseled* on the benefits of exercise and appropriate
physical activity for patients with MS in the past 12 months.
Measurement
Period
January 1, 20xx to December 31, 20xx
Eligible
Population
Eligible Providers
Medical Doctor (MD), Doctor of Osteopathy (DO), Clinical Psychologist
(PhD & PsyD), Physician Assistant (PA), Advanced Practice Registered
Nurse (APRN), Physical Therapy (PT), Occupational Therapy (OT)
Care Setting(s)
Outpatient Care
Ages
Any
Event
Office or telehealth encounter
Diagnosis
Multiple Sclerosis
Denominator
Patients with a diagnosis of MS.
Numerator
Patients with MS counseled* on the benefits of exercise and appropriate physical activity for
patients with MS in past 12 months.
*Counseled: to advise seriously and formally after consultation
1-2
Required
Exclusions
None
Allowable
Exclusions
None**
**All patients including those unable to exercise should be provided information on appropriate
range of motion and activity.
Exclusion
Rationale
Not Applicable
Measure Scoring
Percentage
Interpretation of
Score
Higher Score Indicates Better Quality
Measure Type
Process
Level of
Measurement
Provider
Risk Adjustment
Not Applicable
Opportunity to
Improve Gap in
Care
CMS utilized Axon Registry data to establish benchmarks for this measure in 2019. Average
performance for 2019 Axon Registry users reporting on the measure for Merit-based Incentive
Payment System reporting was 73.063%.
3
Despite known benefits of exercise and physical activity, persons with MS remain inactive.
4-5
The Work Group encourages referral to rehabilitation services, including physical therapy, when
clinically appropriate given the evidence supporting improved outcomes for patients.
6-8
For Process
Measures
Relationship to
Desired Outcome
Increased rates of physical activity and exercise improve the physical functioning levels and
quality of life for patients with MS.
2,9-10
Therefore, healthcare providers should encourage
patients with MS to perform ⩾150 min/week of exercise or lifestyle physical activity.
2
The following evidence statements are quoted verbatim from the referenced clinical guidelines:
o “Evidence-based treatment interventions for mobility optimization include exercise
promotion (Level 1).”
11
o “Encourage participation in a regular pattern of exercise to improve mood, fatigue,
quality of life (Level 1).”
2,9,11
o “Encourage people with MS to exercise. Advise them that regular exercise have
beneficial effects on their MS and does not have any harmful effects on their MS.”
2, 9, 12

48
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
o “Encourage to perform ⩾150 min/week of exercise and/or lifestyle physical activity”
2
o "Healthcare providers should endorse & promote the benefits/safety of exercise &
lifestyle physical activity for every person with MS."
2
o “Ensure all people with MS have a tailored comprehensive review of all aspects of their
care at least once a year.”
12
Harmonization
with Existing
Measures
There are currently no other comparable measures in national measurement programs or
endorsed by the National Quality Forum.
References
1. Merriam Webster. Available at: http://www.merriam-webster.com/medical/counsel Accessed on
November 13, 2020.
2. Kalb R, Brown T, Coote S, et al. Exercise and lifestyle physical activity recommendations for
people with multiple sclerosis throughout the disease course. Mult Scler. 2020;26(12):1459-
1469.
3. CMS 2020 MIPS Historical Quality Benchmarks. AAN8 Exercise and Appropriate Physical
Activity counseling for Patients with MS. Available at: https://qpp.cms.gov/about/resource-
library Accessed on November 13, 2020.
4. Mayo NE, Bayley M, Duquette P, et. Al. The role of exercise in modifying outcomes for people
with multiple sclerosis: a randomized trial. BMC Neurology. 2013;13:69.
5. Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult
Scler. 2005; 11(4):459-463.
6. Khan F, Turner-Stokes L, Ng L, et al. Multidisciplinary rehabilitation for adults with multiple
sclerosis. Cochrane Database of Systematic Reviews. 2007, Issue 2. Art. No.: CD006036.
7. Rietberg MB, Brooks D, Uitdehaag BMJ, et al. Exercise therapy for multiple sclerosis. Cochrane
Database of Systematic Reviews. 2004, Issue 3. Art. No.: CD003980.
8. Döring A, Caspar FP, Friedemann P, et al. Exercise in multiple sclerosis – an integral component
of disease management. The EPMA Journal. 2012;3:2-13.
9. Mayo C, Miksche K, Atwell-Pope K, et al. The relationship between physical activity and
symptoms of fatigue, mood, and perceived cognitive impairment in adults with multiple
sclerosis. J Clin Exp Neuropsychol. 2019 Sep;41(7):715-722.
10. American College of Sports Medicine: ACSM’s Resource Manual for Guidelines for Exercise
Testing and Prescription, 6
th
edition edn. Baltimore, MD: Lippincott Williams & Wilkins; 2010.
11. American Association of Neuroscience Nurses (AANN), Association of Rehabilitation Nurses
(ARN), International Organization of Multiple Sclerosis Nurses (IOMSN). Nursing management
of the patient with multiple sclerosis. Glenview (IL): American Association of Neuroscience
Nurses (AANN); 2011. 49 p.
12. National Clinical Guideline Centre (NICE) (UK). Multiple Sclerosis: Management of Multiple
Sclerosis in Primary and Secondary Care. London: National Institute for Health and Care
Excellence; 2014 Oct. 2019 update available at: https://www.nice.org.uk/guidance/cg186
Accessed on November 13, 2020.
Process
Excercise and
appropriate
physical activity
counseling provided
Intermediate
Outcome
Excercise and
physical activity
initiated
Outcome
Improved quality of
life
Reduction of
comorbid
symptoms &
chronic conditions

49
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Code System
Code
Code Description
Initial Population
CPT
99201-99205
Office or other outpatient visit, new patient
CPT
99211-99215
Office or other outpatient visit, established patient
CPT
99241-99245
Office or other outpatient consultation, new or established patient
CPT
97003, 97004
Occupational therapy, evaluation and re-evaluation
CPT
97161-97164
Physical therapy, evaluation and re-evaluation
CPT
96156, 96158,
96164, 96167,
96170
Health and behavior visit
CPT
Telehealth codes TBD
Denominator
ICD-10
G35
Multiple Sclerosis
SNOMEDCT
24700007
Multiple sclerosis (disorder)
SNOMEDCT
192929006
Exacerbation of multiple sclerosis (disorder)
SNOMEDCT
230372003
Acute relapsing multiple sclerosis (disorder)
SNOMEDCT
425500002
Secondary progressive multiple sclerosis (disorder)
SNOMEDCT
426373005
Relapsing remitting multiple sclerosis (disorder)
SNOMEDCT
428700003
Primary progressive multiple sclerosis (disorder)
SNOMEDCT
438511000
Benign multiple sclerosis (disorder)
SNOMEDCT
92926004
Multiple sclerosis of the brainstem (disorder)
SNOMEDCT
192927008
Multiple sclerosis of the spinal cord (disorder)
SNOMEDCT
439567002
Malignant multiple sclerosis (disorder)
SNOMEDCT
724778008
Progressive relapsing multiple sclerosis (disorder)
SNOMEDCT
733028000
Multiple sclerosis, ichthyosis, factor VIII deficiency syndrome (disorder)
SNOMEDCT
766246000
Marburg acute multiple sclerosis (disorder)
SNOMEDCT
816984002
Progressive multiple sclerosis (disorder)
Numerator
SNOMEDCT
281090004
Recommendation to exercise (procedure)
SNOMEDCT
304549008
Giving encouragement to exercise (procedure)
SNOMEDCT
304558001
Reassuring about exercise (procedure)
SNOMEDCT
310882002
Exercise on prescription (regime/therapy)
SNOMEDCT
386291006
Exercise promotion: strength training (procedure)
SNOMEDCT
386292004
Exercise promotion: stretching (procedure)
SNOMEDCT
386463000
Prescribed activity/exercise education (procedure)
SNOMEDCT
390893007
Referral to physical activity program (procedure)
SNOMEDCT
429778002
Patient given written advice on benefits of physical activity (situation)
SNOMEDCT
710138000
Promotion of adherence to exercise regime (procedure)
SNOMEDCT
710883002
Education about increasing activity tolerance (procedure)
SNOMEDCT
410289001
Exercises education, guidance, and counseling (procedure)
SNOMEDCT
304507003
Exercise education (procedure)
SNOMEDCT
762227003
Provision of advice about aerobic exercise (procedure)
SNOMEDCT
819961005
Physical activity guidance (procedure)
SNOMEDCT
435551000124105
Counseling about physical activity (procedure)
SNOMEDCT
183073003
Patient advised about exercise (situation)
SNOMEDCT
386463000
Prescribed activity/exercise education (procedure)
ICD10CM
Z71.89
Exercise counseling
Exclusions

50
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
None

51
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Yes
No
No
Yes
Yes
No
No
Chart Diagram: Exercise and Appropriate Physical Activity Counseling for Patients with MS
Patient
INCLUDED in
Eligible
Population
Patient NOT
Included in
Eligible
Population
Did the patient have at least one new or
established visit with an eligible provider
during the measurement period?
Patient met
numerator
criteria
Patient did
NOT meet
numerator
criteria
In the past 12 months, was patient counseled on
the benefits of exercise and appropriate physical
activity for patients with MS?
Patient
INCLUDED in
Denominator
Did patient have a MS diagnosis on the date
of the visit?

53
©2020. American Academy of Neurology Institute. All Rights Reserved.
CPT Copyright 2004-2020 American Medical Association.
Appendix A: Disclosures
Work Group Member
Disclosures
Lilyana Amezcua, MD, MS, FAAN
Reports active research support from the National MS Society,
NIH NINDS, Bristol Myers Squibb Foundation, and Biogen Idec.;
has served as a consultant to Biogen Idec, Novartis, Alexion
Pharmaceuticals, Genentech, EMD Serono and AbbVie; has
served as primary investigator for clinical trials with MedDay,
Genenetch and PCORI.
Tracie Caller, MD
(non-voting member)
Reports no relevant disclosures for this project.
Jeffrey English, MD
Reports serving as a Board of Directors for the Consortium of
Multiple Sclerosis Centers; has served as consultant to Biogen-
Idec, Novartis, Sanofi-Genzyme, Genetech, EMD-Serono, Teva,
Raptor, Abbvie; served as speaker consultant for the MS
Association of America and National MS Society; is a founding
member of Healthcare Impact Partners and HIP Nation.
Neeta Garg, MD
Reports no disclosures.
Barbara Giesser, MD, FAAN
Reports royalties from 2 multiple sclerosis publications and has
received a consulting fee from Greenwich Biosciences.
Adam G. Kelly, MD, FAAN
(non-voting member)
Reports no relevant disclosures for this project.
Iris Vanessa Marin Collazo, MD
Reports no relevant disclosures for this project.
Amanda Montague, EdM
Reports no disclosures.
Michael Olek, DO
Reports no disclosures.
Elizabeth Page
Reports no disclosures.
Alex Rae-Grant, MD, FRCPC, FAAN
(Chair)
Reports currently serving as neurology editor for DynaMed a
subscription based point of care tool for clinicians with no
industry support or advertising; royalties from 2 textbooks he has
published, 1 on neurology and 1 on multiple sclerosis; organizes
neurology review courses.

