
BinaxNOW
™
COVID19 Ag
CARD HOME TEST
BinaxNOW
™
COVID19 Ag
CARD HOME TEST
Healthcare Provider Instructions for Use
For Use Under an Emergency Use Authorization (EUA) Only
For use with anterior nasal swab specimens
For in vitro Diagnostic Use Only
INTENDED USE
The BinaxNOW
™
COVID-19 Ag Card Home Test is a lateral flow immunoassay device intended for the qualitative
detection of nucleocapsid protein antigen from SARS-CoV-2. This test is authorized for non-prescription home use with
self-collected observed anterior nasal (nares) swab samples from individuals aged 15 years or older or adult collected anterior
nasal (nares) swab samples from individuals aged 2 years or older. This test is authorized for individuals with symptoms of
COVID-19 within the first seven days of symptom onset when tested at least twice over three days with at least 48 hours
between tests, and for individuals without symptoms or other epidemiological reasons to suspect COVID-19, when tested
at least three times over five days with at least 48 hours between tests.
The BinaxNOW COVID-19 Ag Card Home Test is to be performed only with the supervision of a telehealth proctor.
The BinaxNOW COVID-19 Ag Card Home Test does not dierentiate between SARS-CoV and SARS-CoV-2.
Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen which is generally detectable in anterior
nasal (nares) swabs during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical
correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results
do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of
disease. Individuals who test positive with the BinaxNOW COVID-19 Ag Card Home Test should self-isolate and seek
follow up care with their physician or healthcare provider as additional testing may be necessary.
All negative results are presumptive and confirmation with a molecular assay, if necessary, for patient management, may be
performed. Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment
or patient management decisions, including infection control measures such as isolating from others and wearing masks.
Negative results should be considered in the context of a patient’s recent exposures, history and the presence of clinical
signs and symptoms consistent with COVID-19.
Individuals who test negative and continue to experience COVID-like symptoms of fever, cough and/or shortness of breath
may still have SARS-CoV-2 infection and should seek follow up care from their healthcare provider.
Individuals should provide all results obtained with this product to their healthcare provider for public health reporting and
to receive appropriate medical care. All healthcare providers will report all test results they receive from individuals who use
the authorized product to relevant public health authorities in accordance with local, state, and federal requirements using
appropriate LOINC and SNOMED codes, as defined by the Laboratory In Vitro Diagnostics (LIVD) Test Code Mapping
for SARS-CoV-2 Tests provided by CDC.
The BinaxNOW COVID-19 Ag Card Home Test is intended for non-prescription self-use and/or, as applicable for an adult
lay user testing another person aged 2 years or older in a non-laboratory setting. The BinaxNOW COVID-19 Ag Card
Home Test is only for in vitro diagnostic use under the Food and Drug Administration’s Emergency Use Authorization. This
product has not been FDA cleared or approved.
SUMMARY and EXPLANATION of the TEST
Coronaviruses are a large family of viruses which may cause illness in animals or humans. SARS-CoV-2 is an enveloped,
single-stranded RNA virus of the β genus. The virus can cause mild to severe respiratory illness and has spread globally,
including the United States.
The BinaxNOW COVID-19 Ag Card Home Test is a rapid lateral flow immunoassay for the qualitative detection of
SARS-CoV-2 directly from nasal swabs, without viral transport media. The BinaxNOW COVID-19 Ag Card Home Test kit
contains all components required to carry out an assay for SARS-CoV-2.
PRINCIPLES of the PROCEDURE
The BinaxNOW COVID-19 Ag Card Home Test is an immunochromatographic membrane assay that uses highly sensitive
antibodies to detect SARS-CoV-2 nucleocapsid protein from nasal swab specimens. SARS-CoV-2 specific antibodies and
a control antibody are immobilized onto a membrane support as two distinct lines and combined with other reagents/pads
to construct a test strip. This test strip and a well to hold the swab specimen are mounted on opposite sides of a cardboard,
book-shaped hinged test card.
To perform the test, a nasal swab specimen is collected under observation by or from the patient, then 6 drops of extraction
reagent from a dropper bottle are added to the top hole of the swab well. The patient sample is inserted into the test card
through the bottom hole of the swab well, and firmly pushed upwards until the swab tip is visible through the top hole. The
swab is rotated 3 times clockwise and the card is closed, bringing the extracted sample into contact with the test strip. Test
results are interpreted visually at 15 minutes based on the presence or absence of visually detectable pink/purple colored
lines. Results should not be read after 30 minutes.
REAGENTS and MATERIALS
Materials Provided (Your box may contain more than one test kit set)
Test Cards (1 or 2): A cardboard, book-shaped hinged test card containing the test strip
Extraction Reagent (1 or 2): Bottle containing <1 mL of extraction reagent
Nasal Swabs (1 or 2): Sterile swab for use with BinaxNOW COVID-19 Ag Card Home test
1 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use

Materials Required but not Provided
Clock, timer or stopwatch
Smart Phone:* Apple is ios11 or newer
Android is version 8 or newer
*Required to download the NAVICA app from the Google play store or Apple app store
WARNINGS, PRECAUTIONS and SAFETY INFORMATION
1. Read all instructions carefully before performing the test. Failure to follow the instructions may result in inaccurate
test results.
2. For in vitro diagnostic use.
3. In the USA, this product has not been FDA cleared or approved but has been authorized by FDA under an Emergency
Use Authorization. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any
other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration
that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or
diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-
3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
4. Wear safety mask or other face covering when collecting anterior nares swab specimen from a child or another
individual.
5. Use of gloves is recommended when conducting testing.
6. Keep testing kit and kit components out of the reach of children and pets before and after use.
7. Incorrect test results may occur if a specimen is incorrectly collected or handled.
8. Leave test card sealed in its foil pouch until just before use.
9. Do not use if any of the test kit contents or packaging is damaged.
10. Once opened, the test card should be used immediately.
11. Do not dip the swab into the liquid reagent or other liquid before inserting the swab into the nose.
12. Do not touch swab tip when handling the swab sample.
13. Do not use kit past its expiration date.
14. Do not mix components from dierent kit lots.
15. All kit components are single use items. Do not use with multiple specimens. Do not reuse the used test card or swab.
16. Wash hands thoroughly or use hand sanitizer after handling.
17. Dispose of kit components and patient samples in household trash.
18. INVALID RESULTS can occur when an insucient volume of extraction reagent is added to the test card. To ensure
delivery of adequate volume, hold vial vertically, 1/2 inch above the swab well, and add drops slowly.
19. Serial testing should be performed in individuals with negative results at least twice over three days (with 48 hours
between tests) for symptomatic individuals and three times over five days (with at least 48 hours between tests) for
asymptomatic individuals. You may need to purchase additional tests to perform this serial (repeat) testing.
20. An anterior nasal swab sample can be self-collected by an individual age 15 years and older. Children age 2 to 15 years
should be tested by an adult.
21. Do not use on anyone under 2 years of age.
22. If you have had symptoms longer than 7 days you should consider testing at least three times over five days with at least
48 hours between tests.
23. Do not read test results before 15 minutes or after 30 minutes. Results read before 15 minutes or after 30 minutes
may lead to a false positive, false negative, or invalid result.
The Reagent Solution contains a harmful chemical (see table below). Do not ingest any kit components. If the solution
contacts the skin or eye, flush with copious amounts of water. If irritation persists, seek medical advice: www.poisonhelp.org
or 1-800-222-1222.
Chemical Name/CAS
Sodium Azide/26628-22-8
GHS Code for each Ingredient
Acute Tox. 2 (Oral), H300
Acute Tox. 1 (Dermal), H310
Concentration
0.0125%
There is a higher chance of false negative results with antigen tests than with laboratory-based molecular tests. This means
that there is a higher chance this test will give you a negative result when you have COVID-19.
For more information on EUAs please visit: https://www.fda.gov/emergencypreparedness-and-response/mcm-legal-
regulatory-and-policy-framework/emergencyuse-authorization
For the most up to date information on COVID-19, please visit: www.cdc.gov/COVID19
STORAGE and STABILITY
Store kit between 35.6-86°F (2-30°C). Ensure all test components are at room temperature before use. The BinaxNOW
COVID-19 Ag Card Home Test is stable until the expiration date marked on the outer packaging and containers. For
information about current expiration dates for at-home OTC COVID-19 diagnostic tests, visit http://www.fda.gov/
covid-tests.
INITIATING the TELEHEALTH VISIT
Upon receipt of the BinaxNOW COVID-19 Ag Home Test, the patient logs into NAVICA and selects, “I Already Have
a Test Kit”. The home user then visits the telehealth provider website to start testing and waits in queue to connect to the
telehealth proctor.
2 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use

DIRECTIONS for RUNNING the BinaxNOW
™
COVID-19 Ag CARD HOME TEST
DO NOT OPEN ITEMS UNTIL INSTRUCTED TO DO SO
Wash or sanitize your hands. Make sure they are dry
before starting.
1
Set Up
It is recommended gloves (not provided) also be used during testing. Your box may contain
more than one test kit. Use only 1 of each of the following for each test:
DO NOT open items until instructed.
1 Swab 1 Test Card 1 Bottle
B
i
nax
NOW
™
C
O
V
O
O
I
D
19
A
g
C
A
RD
S
AMPLE
CON
TR
OL
or
2
Open Pouch and Scan QR Code on Card
If using a mobile device:
If using a computer:
BinaxNOW
™
COVID-19 Ag
CARD
SAMPLE
CONTROL
3 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
3
Open Card
Card must stay FLAT on table for entire test.
Top Hole
Bottom Hole
Result Window
Test Strip
DO NOT touch
any
parts on
inside of card.

4
Apply Fluid to Top Hole
A. Remove dropper
bottle cap.
B. Hold dropper bottle
straight over TOP HOLE,
not at an angle.
C. Put 6 DROPS into
TOP HOLE. Do not
touch card with tip.
6 drops
Note: False negative results may occur if less than 6 drops of fluid is used.
5
Open Swab
Keep fingers away from swab end.
A. Open swab package at stick end. B. Take swab out.
6
Swab Left Nostril
A. Insert the entire absorbent tip
of the swab (usually 1/2 to 3/4 of
an inch) into left nostril.
Up to 3/4 of
an inch.
B. Firmly brush against insides of
nostril in a circular motion 5 times
or more for at least 15 seconds.
x5
7
Swab Right Nostril
A. Remove swab and insert it
into right nostril.
B. Firmly brush against insides of
nostril in a circular motion 5 times
or more for at least 15 seconds.
x5
Note: False negative results may
occur if the nasal swab is not properly
collected.
4 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use

8
Insert Swab Into Bottom Hole
Keep card FLAT on table.
Insert swab tip into BOTTOM HOLE and firmly push up until tip fills TOP HOLE.
9
Turn Swab 3 Times
Keep card FLAT on table.
Turn swab to right 3 times in card and leave it in place.
x3
Note: False negative results can occur if the sample swab is not turned prior to closing the card.
10
Peel Strip
DO NOT remove swab.
Keep card FLAT on table.
Keep swab in place. Peel adhesive liner off.
11
Close Card and Seal
DO NOT remove swab.
Keep card FLAT on table.
Close left side of card over swab to seal it. Keep card face up on table.
5 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use

12
Wait 15 Minutes
DO NOT disturb card during this time.
15:00
Note: False results can occur if the card is disturbed/moved or test results are read before 15 minutes.
13
Scan QR Code
If using a mobile device: If using a computer:
BinaxNOW
™
COVID-19 Ag
CARD
SAMPLE
CONTROL
6 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
14
Show Result to Your Proctor
BinaxNOW
™
COVID-19 Ag
CARD
SAMPLE
CONTROL
SAMPLE
CONTROL

RESULT INTERPRETATION
There are three types of results possible. You will be instructed how to read each type in a specific order. Follow this order
with your proctor:
1. Check for a Positive Result
2. Check for a Negative Result
3. Check for an Invalid Result
Repeat testing is needed to improve test accuracy. Please follow the table below when interpreting test results for
COVID-19.
Status on First
Day of Testing
First
Result Day 1
Second
Result Day 3
Third
Result Day 5
Interpretation
With
Symptoms
Positive N/A N/A Positive for COVID-19
Negative Positive N/A Positive for COVID-19
Negative Negative N/A Negative for COVID-19
Without
Symptoms
Positive N/A N/A Positive for COVID-19
Negative Positive N/A Positive for COVID-19
Negative Negative Positive Positive for COVID-19
Negative Negative Negative Negative for COVID-19
Results should be considered in the context of an individual’s recent exposures, history, and the presence of clinical signs
and symptoms consistent with COVID-19.
Check for Positive COVID-19 Result
Find result window and look carefully for two pink/purple lines in window.
• Positive Result: Two pink/purple lines will appear. One on the top half and one on the bottom half.
COVID-19 was detected.
Positive ositive
CONTROL
OR
SAMPLE
Solid Line Faint Line
P
Look very closely!
The bottom line can be very
faint. Any pink/purple line
visible here is positive.
Her
e are photos of actual positive tests. On the right, note how faint the bottom line can get.
A positive test result means that the virus that causes COVID-19 was detected in the sample and it is very likely the
individual has COVID-19 and is contagious. Please contact the doctor/primary care physician (if applicable) and the
local health authority immediately and instruct your patient to adhere to the local guidelines regarding self-isolation.
There is a very small chance that this test can give a positive result that is incorrect (a false positive result).
Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be
the definite cause of disease. Individuals who test positive with the BinaxNOW COVID-19 Antigen Self Test should
self-isolate and seek follow up care with their physician or healthcare provider as additional confirmatory testing with a
molecular test for positive results may also be necessary, if there is a low likelihood of COVID-19, such as in individuals
without known exposures to COVID-19 or residing in communities with low prevalence of infection.
Repeat testing does not need to be performed if patients have a positive result at any time.
7 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use

Check for Negative COVID-19 Result
Find result window and look for a single pink/purple line in window.
• Negative Result: A single pink/purple line on the top half where it says "Control." COVID-19 was not detected.
To increase the chance that the negative result for
COVID-19 is accurate, you should:
•
Test again in 48 hours if you have symptoms on the
first day of testing.
•
Test 2 more times at least 48 hours apart if you do not
have symptoms on the first day of testing.
A negative test result indicates that the virus that
causes COVID-19 was not detected in the sample. A
negative result does not rule out COVID-19. There is
a higher chance of false negative results with antigen
tests compared to laboratory-based tests such as
PCR. If the test is negative but COVID-19-like
symptoms, e.g., fever, cough, and/or shortness of
breath continue, follow up testing for SARS-CoV-2
with a molecular test or testing for other respiratory
disease should be considered. If applicable, seek
follow up care with the primary health care provider.
All negative results should be treated as presumptive
and confirmation with a molecular assay may be
necessary if there is a high likelihood of SARS-CoV-2
infection, such as in an individual with a close contact
with COVID-19 or with suspected exposure to
COVID-19 or in communities with high prevalence
of infection. Negative results do not rule out SARS-
CoV-2 infection and should not be used as the sole
basis for treatment or patient management decisions,
including infection control decisions.
Negative
CONTROL
SAMPLE
No Line
Check for Invalid Result
If you see any of these, the test is invalid.
Re-test with a new swab and new test device.
Blue control Pink/purple Blue control line AND
No lines seen
line only sample line only pink/purple sample line
CONTROL
SAMPLE
Dispose In Trash
8 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
BinaxNOW
™
COVID-19 Ag
CARD
SAMPLE
CONTROL

Reporting Patient Results Using the NAVICA app
Upon completion of the test and result interpretation by the user, the telehealth proctor will send the results to the
user via the NAVICA app and the telehealth provider will report results to relevant public health authorities. The user
will be notified by email and on their mobile device that their results are ready. The user will go to the results screen in
NAVICA to obtain their results.
If the BinaxNOW COVID-19 Ag Card Home Test result is Negative, the user will receive the following:
If the BinaxNOW COVID-19 Ag Card Home Test result is Positive, the user will receive the following:
9 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use

If the BinaxNOW COVID-19 Ag Card Home Test result is Invalid, the user will receive the following:
LIMITATIONS
• This test detects both viable (live) and non-viable, SARS-CoV, and SARS-CoV-2. Test performance depends on the
amount of virus (antigen) in the sample and may or may not correlate with viral culture results performed on the same
sample.
• A negative test result may occur if the level of antigen in a sample is below the detection limit of the test.
• The performance of the BinaxNOW COVID-19 Ag Card Home Test was evaluated using the procedures provided in
this product insert only. Modifications to these procedures may alter the performance of the test.
• Incorrect test results may occur if a specimen is improperly collected or handled.
• False negative results may occur if inadequate extraction buer is used (e.g., <6 drops).
• False negative results may occur if specimen swabs are not twirled within the test card.
• False negative results may occur if swabs are stored in their paper sheath after specimen collection.
• Positive test results do not rule out co-infections with other pathogens.
• Positive test results do not dierentiate between SARS-CoV and SARS-CoV-2.
• Negative test results are not intended to rule in other non-SARS viral or bacterial infections.
• The presence of mupirocin may interfere with the BinaxNOW COVID-19 Ag test and may cause false negative results.
• All COVID-19 antigen test negative results are presumptive and confirmation with a molecular assay may be
necessary.
• If the patient continues to have symptoms of COVID-19, and both the patient’s first and second tests are negative, the
patient may not have COVID-19, however additional follow-up may be needed.
• If the test is positive, then proteins from the virus that causes COVID-19 have been found in the sample and the
individual likely has COVID-19.
• There is a higher chance of false negative results with antigen tests than with laboratory-based molecular tests due to
the sensitivity of the test technology. This means that there is a higher chance this test will give a false negative result in
an individual with COVID-19 compared to a molecular test, especially in samples with low viral load.
• If the dierentiation of specific SARS viruses and strains is needed, additional testing, in consultation with state or local
public health departments, is required.
• The performance of this test was established based on the evaluation of a limited number of clinical specimens collected
between January, 2021, and May, 2022. The clinical performance has not been established in all circulating variants but
is anticipated to be reflective of the prevalent variants in circulation at the time and location of the clinical evaluation.
Performance at the time of testing may vary depending on the variants circulating, including newly emerging strains of
SARS-CoV-2 and their prevalence, which change over time.
• This test is read visually and has not been validated for use by those with impaired vision or color-impaired vision.
10 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use

PERFORMANCE CHARACTERISTICS
CLINICAL PERFORMANCE
A prospective clinical study was conducted between January 2021 and May 2022 as a component of the Rapid
Acceleration of Diagnostics (RADx) initiative from the National Institutes of Health (NIH). A total of 7,361 individuals
were enrolled via a decentralized clinical study design, with a broad geographical representation of the United States. Per
inclusion criteria, all individuals were asymptomatic upon enrollment in the study and at least 14 days prior to it and did not
have a SARS-CoV-2 infection in the three months prior to enrollment. Participants were assigned to one of three EUA
authorized SARS-CoV-2 OTC rapid antigen tests to conduct serial testing (every 48 hours) for 15 days. If an antigen test
was positive, the serial-antigen testing result is considered positive.
At each rapid antigen testing time point, study subjects also collected a nasal swab for comparator testing using a home
collection kit (using a 15-minute normalization window between swabs). SARS-CoV-2 infection status was determined by
a composite comparator method on the day of the first antigen test, using at least two highly sensitive EUA RT-PCRs. If
results of the first two molecular test were discordant a third highly sensitive EUA RT-PCR test was performed, and the
final test result was based upon the majority rule.
Study participants reported symptom status throughout the study using the MyDataHelps app. Two-day serial antigen
testing is defined as performing two antigen tests 36 – 48 hours apart. Three-day serial antigen testing is defined as
performing three antigen tests over five days with at least 48 hours between each test.
Out of the 7,361 participants enrolled in the study, 5,609 were eligible for analysis. Among eligible participants, 154 tested
positive for SARS-CoV-2 infection based on RTPCR, of which 97 (62%) were asymptomatic on the first day of their
infection, whereas 57 (39%) reported symptoms on the first day of infection. Pre-symptomatic subjects were included in
the positive percent agreement (PPA) of asymptomatic individuals, if they were asymptomatic on the first day of antigen
testing, regardless of whether they developed symptoms at any time after the first day of testing.
Performance of the antigen test with serial testing in individuals is described in the table below.
Data establishing PPA of COVID-19 antigen serial testing compared to the molecular comparator single day testing
throughout the course of infection with serial testing. Data is from all antigen tests in study combined.
Days After First
PCR Positive
Test Result
Asymptomatic On First Day Of Testing Symptomatic On First Day Of Testing
Ag Positive / PCR Positive
(Antigen Test Performance % PPA)
1 Test 2 Tests 3 Tests 1 Test 2 Tests 3 Tests
0
9/97
(9.3%)
35/89
(39.3%)
44/78
(56.4%)
34/57
(59.6%)
47/51
(92.2%)
44/47
(93.6%)
2
17/34
(50.0%)
23/34
(67.6%)
25/32
(78.1%)
58/62
(93.5%)
59/60
(98.3%)
43/43
(100%)
4
16/21
(76.2%)
15/20
(75.0%)
13/15
(86.7%)
55/58
(94.8%)
53/54
(98.1%)
39/40
(97.5%)
Days After First
PCR Positive
Test Result
Asymptomatic On First Day Of Testing Symptomatic On First Day Of Testing
Ag Positive / PCR Positive
(Antigen Test Performance % PPA)
1 Test 2 Tests 3 Tests 1 Test 2 Tests 3 Tests
6
20/28
(71.4%)
21/27
(77.8%)
16/18
(88.9%)
27/34
(79.4%)
26/33
(78.8%)
22/27
(81.5%)
8
13/23
(56.5%)
13/22
(59.1%)
4/11
(36.4%)
12/17
(70.6%)
12/17
(70.6%)
7/11
(63.6%)
10
5/9
(55.6%)
5/8
(62.5%)
4/9
(44.4%)
3/7
(42.9%)
1 Test = one (1) test performed on the noted days after the first PCR positive test result. Day 0 is the first day of
documented infection with SARS-CoV-2.
2 Tests = two (2) tests performed an average of 48 hours apart. The first test performed on the indicated day and the
second test performed 48 hours later.
3 Tests = three (3) tests performed an average of 48 hours apart. The first test performed on the indicated day, the
second test performed 48 hours later, and a final test performed 48 hours after the second test.
Clinical performance characteristics of BinaxNOW COVID-19 Ag Card Home Test was evaluated in a multi-site
prospective study in the U.S. A total of five (5) investigational sites throughout the U.S. participated in the study. To
be enrolled in the study, patients had to be presenting at the participating study centers with suspected COVID-19
within 7 days of symptom onset. Each Subject was provided a BinaxNOW COVID-19 Ag Card Home Test. Under the
observation and coaching of a clinical site sta member trained as a proctor, the Subject self-collected one (1) nasal swab
and performed the BinaxNOW COVID-19 Ag Card Home Test. Test results were interpreted and recorded by the
Subject or other home user and independently by the proctor. Parents of pediatric Subjects under the age of 14 or Legally
Authorized Representatives of adult Subjects unable to perform self-collection collected one (1) nasal swab from the
Subject, performed the BinaxNOW COVID-19 Ag Card Home Test, then interpreted and recorded the result for the
patient.
An FDA Emergency Use Authorized real-time Polymerase Chain Reaction (RT-PCR) assay for the detection of SARS-
CoV-2 was utilized as the comparator method for this study.
The performance of BinaxNOW COVID-19 Ag Card Home Test was established with 356 nasal swabs collected from
individual symptomatic patients (within 7 days of onset) who were suspected of COVID-19.
11 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use

BinaxNOW
™
COVID-19 Ag Card Home Test - Lay User Performance within 7 days of symptom onset against
the Comparator Method
BinaxNOW
™
COVID-19 Ag
Card Home Test
Comparator Method
Positive Negative Total
Positive 102 4 106
Negative 3
227 250
Total
125 231 356*
Positive Agreement: 102/125 81.6% (95% CI: 73.7% - 88.0%)
Negative Agreement: 227/231 98.3% (95% CI: 95.6% - 99.5%)
* 3 samples generated an invalid BinaxNOW COVID-19 Ag Card Home Test result [invalid rate of 3/397, or 0.76%
(95% CI from 0.16% to 2.19%)] and 38 samples were unevaluable for the method comparison to PCR.
BinaxNOW
™
COVID-19 Ag Card Home Test - Proctor Performance within 7 days of symptom onset against
the Comparator Method
BinaxNOW
™
COVID-19 Ag
Card Home Test
Comparator Method
Positive Negative Total
Positive 105 4 109
Negative 20
226 246
Total
125 230 355*
Positive Agreement: 105/125 84.0% (95% CI: 76.4% - 89.9%)
Negative Agreement: 226/230 98.3% (95% CI: 95.6% - 99.5%)
*4 samples generated an invalid BinaxNOW COVID-19 Ag Card Home Test result [invalid rate of 4/397,
or 1.01% (95% CI from 0.28% to 2.56%)] and 38 samples were unevaluable for the method comparison to PCR.
Hazardous Ingredients for the Reagent Solution
Chemical Name/CAS
Sodium Azide/26628-22-8
GHS Code for each Ingredient
Acute Tox. 2 (Oral), H300
Acute Tox. 1 (Dermal), H310
Concentration
0.0125%
The solution in the tube contains a hazardous ingredient (see table above). If the solution contacts the skin or eye, flush
with plen
ty of water. If irritation persists, seek medical advice. http://www.poisonhelp.org or 1-800-222-1222.
Patient demographics, time elapsed since onset of symptoms for all patients enrolled in the above study, are presented in
the table below. Positive results broken down by days since symptom onset:
Days Since
Symptom Onset
Cumulative RT-
PCR Positive (+)
Cumulative BinaxNOW
™
COVID-19 Ag Card Positive (+)
PPA
95 % Confidence
Interval
1 21 19 90.5% 69.6% 98.8%
2 57 47 82.5% 70.1% 91.3%
3 89 72 80.9% 71.2% 88.5%
4 104 86 82.7% 74.0% 89.4%
5 111 93 83.8% 75.6% 90.1%
6 119 100 84.0% 76.2% 90.1%
7 125 102 81.6% 73.7% 88.0%
12 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use

ANALYTICAL PERFORMANCE
Limit of Detection (Analytical Sensitivity)
BinaxNOW COVID-19 Ag Card Home Test limit of detection (LOD) was determined by evaluating dierent
concentrations of heat inactivated SARS-CoV-2 virus. Presumed negative natural nasal swab specimens were eluted
in PBS. Swab eluates were combined and mixed thoroughly to create a clinical matrix pool to be used as the diluent.
Inactivated SARS-CoV-2 virus was diluted in this natural nasal swab matrix pool to generate virus dilutions for testing.
Contrived nasal swab samples were prepared by absorbing 20 microliters of each virus dilution (1,125 TCID/mL) onto the
swab. The contrived swab samples were tested according to the test procedure.
The LOD was determined as the lowest virus concentration that was detected ≥ 95% of the time (i.e., concentration at
which at least 19 out of 20 replicates tested positive).
The BinaxNOW COVID-19 Ag Card Home Test LOD in natural nasal swab matrix was confirmed as 140.6 TCID/mL in the
test. Based upon the testing procedure for this study the LOD of 140.6 TCID/mL in the test equates to 22.5 TCID/swab.
Limit of Detection (LoD) Study Results
Concentration
TCID/mL
Number
Positive/Total
% Detected
140.6 20/20 100%
OMICRON TESTING
The performance of this test device in the detection of the Omicron variant of SARS-CoV-2 was evaluated in a dilution
series of clinical specimens which were positive for the Omicron variant. This testing was conducted by the National
Institutes of Health (NIH) as a component of the Rapid Acceleration of Diagnostics (RADx®) initiative. Specimen pools
were prepared by the RADx team using pooled clinical samples from currently circulating Omicron strains and tested
by RADx® to assess performance with the Omicron variant. Results from this dilution series cannot be compared to
any devices tested with a dierent specimen pool and do not indicate that a test will have dierent clinical performance
compared to other EUA authorized tests. Compared to an EUA authorized RT-PCR method, the BinaxNOW COVID-19
Ag Card detected 100% of live virus Omicron samples at a Ct-value of 28.7 (n=5). Testing was also compared to additional
EUA authorized OTC antigen tests (Assay #1 and Assay #2). Omicron dilutions at lower viral concentrations (Ct-values
greater than 28.7) were not detected by the BinaxNOW COVID-19 Ag Card in this study.
Omicron Pool 1 – Live
Omicron Clinical Samples
Average N2
Ct (n=9)
Assay #1
Percent Positive
(n=5)
Assay #2
Percent Positive
(n=5)
Binax NOW
COVID-19 Ag
Card Percent
Positive
(n=5)
Dilution 1 19.9 100 100 100
Dilution 2 21.0 100 100 100
Dilution 3 22.3 100 100 100
Dilution 4 23.4 100 100 100
Dilution 5 25.0 100 100 100
Dilution 6 26.6 100 100 100
Dilution 7 27.3 0 100 100
Dilution 8 28.7 0 0 100
Dilution 9 30.1 0 0 0
Dilution 10 31.0 0 0 0
Dilution 11 32.1 0 0 0
13 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
Cross Reactivity (Analytical Specificity) and Microbial Interference
Cross reactivity and potential interference of BinaxNOW COVID-19 Ag Card Home Test was evaluated by testing 37
commensal and pathogenic microorganisms (8 bacteria, 14 viruses, 1 yeast and pooled human nasal wash) that may be
present in the nasal cavity. Each of the organism, viruses, and yeast were tested in triplicate in the absence or presence of
heat inactivated SARS-CoV-2 virus (45 TCID/swab). No cross-reactivity or interference was seen with the following
microorganisms when tested at the concentration presented in the table below.
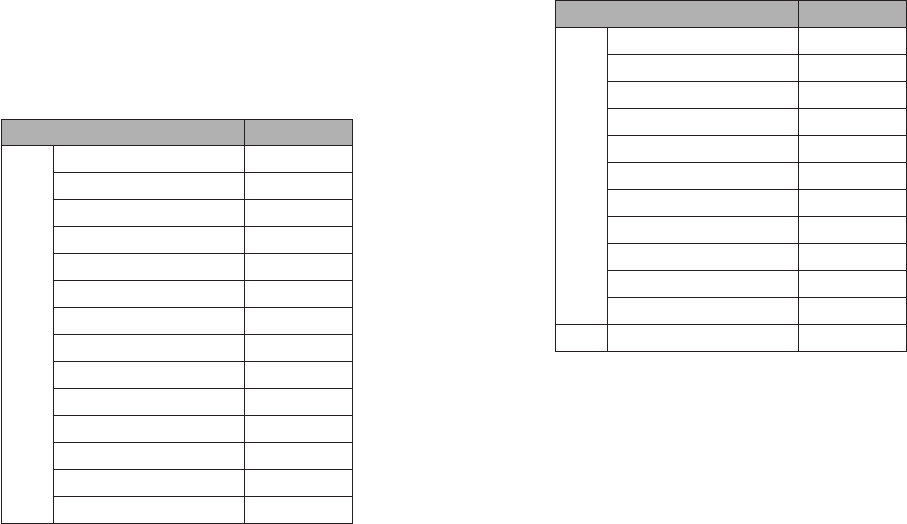
Potential Cross-Reactant Test Concentration
Virus
Adenovirus
1.0 x 10
⁵
TCID
/mL
Human metapneumovirus (hMPV) 1.0 x 10⁵ TCID/mL
Rhinovirus 1.0 x 10⁵ PFU/mL
Enterovirus/Coxsackievirus B4 1.0 x 10⁵ TCID/mL
Human coronavirus OC43 1.0 x 10⁵ TCID/mL
Human coronavirus 229E 1.0 x 10⁵ TCID/mL
Human coronavirus NL63 1.0 x 10⁵ TCID/mL
Human parainfluenza virus 1 1.0 x 10⁵ TCID/mL
Human parainfluenza virus 2 1.0 x 10⁵ TCID/mL
Human parainfluenza virus 3 1.0 x 10⁵ TCID/mL
Human parainfluenza virus 4 1.0 x 10⁵ TCID/mL
Influenza A 1.0 x 10⁵ TCID/mL
Influenza B 1.0 x 10⁵ TCID/mL
Respiratory Syncytial Virus A 1.0 x 10⁵ PFU/mL
Potential Cross-Reactant Test Concentration
Bacteria
Bordetella pertussis 1.0 x 10⁶ cells/mL
Chlamydia pneumoniae 1.0 x 10⁶ IFU/mL
Haemophilus influenzae 1.0 x 10⁶ cells/mL
Legionella pnuemophila 1.0 x 10⁶ cells/mL
Mycoplasma pneumoniae 1.0 x 10⁶ U/mL
Streptococcus pneumoniae 1.0 x 10⁶ cells/mL
Streptococcus pyogenes (group A) 1.0 x 10⁶ cells/mL
Mycobacterium tuberculosis 1.0 x 10⁶ cells/mL
Staphylococcus aureus 1.0 x 10 org/mL
Staphylococcus epidermidis 1.0 x 10 org/mL
Pooled human nasal wash N/A
Yeast Candida albicans 1.0 x 10⁶ cells/mL
To estimate the likelihood of cross-reactivity with SARS-CoV-2 virus in the presence of organisms that were not available
for wet testing, In silico analysis using the Basic Local Alignment Search Tool (BLAST) managed by the National Center for
Biotechnology Information (NCBI) was used to assess the degree of protein sequence homology.
• For P. jirovecii one area of sequence similarity shows 45% homology across 18% of the sequence, making cross-
reactivity in the BinaxNOW COVID-19 Ag Card highly unlikely.
• No protein sequence homology was found between M. tuberculosis, and thus homology-based cross-reactivity can be
ruled out.
• The comparison between SARS-CoV-2 nucleocapsid protein, MERS-CoV and human coronavirus HKU1 revealed that
cross-reactivity cannot be ruled out. Homology for KHU1 and MERS-CoV is relatively low, at 37.8% across 95% of
the sequence and 57.14% across 87% of the sequence, respectively.
High Dose Hook Eect
No high dose hook eect was observed when tested with up to a concentration of 1.6 x 10 TCID/mL of heat inactivated
SARS-CoV-2 virus with the BinaxNOW COVID-19 Ag Card Home Test.
14 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use

Endogenous Interfering Substances
The following substances, naturally present in respiratory specimens or that may be artificially introduced into the nasal
cavity or nasopharynx, were evaluated with the BinaxNOW COVID-19 Ag Card Home Test at the concentrations listed
below and were found not to aect test performance.
Substance Active Ingredient Concentration
Endogenous
Mucin 2% w/v
Whole Blood 1% v/v
OTC Nasal Drops Phenylephrine 15% v/v
OTC Nasal Gel Sodium Chloride (i.e. NeilMed) 5% v/v
OTC Nasal Spray 1 Cromolyn 15% v/v
OTC Nasal Spray 2 Oxymetazoline 15% v/v
OTC Nasal Spray 3 Fluconazole 5% w/v
Throat Lozenge Benzocaine, Menthol 0.15% w/v
OTC Homeopathic Nasal Spray 1
Galphimia glauca, Sabadilla, Lua
opperculata
20% v/v
OTC Homeopathic Nasal Spray 2 Zincum gluconium (i.e., Zicam) 5% w/v
OTC Homeopathic Nasal Spray 3 Alkalol 10% v/v
OTC Homeopathic Nasal Spray 4 Fluticasone Propionate 5% v/v
Sore Throat Phenol Spray Phenol 15% v/v
Anti-viral Drug Tamiflu (Oseltamivir Phosphate) 0.5% w/v
Antibiotic, Nasal Ointment Mupirocin 0.25% w/v
Antibacterial, Systemic Tobramycin 0.0004% w/v
Testing demonstrated false negative results at concentrations of 5 mg/mL (0.5% w/v). Standard dose of nasal ointment:
2 mg (2% w/w) of mupirocin in single-use 1-gram tubes.
Human Factors Study
Abbott conducted a human factor’s study to evaluate whether home user patients or caregivers (lay user) could perform
the test and accurately interpret test results from the BinaxNOW COVID-19 Ag Card under the supervision of a trained
proctor.
In this study, a total of 31 lay users, age 15 and older with either good or corrected vision (far/near-sighted or wear bifocals)
participated in a 45-minute session including an introduction, a product overview, and simulated use cases of BinaxNOW
COVID-19 Ag Card Home test result interpretation. Participants were asked to read and interpret a panel of 9 dierent
BinaxNOW COVID-19 Ag Card test results, including high positive, low positive, negative and invalid under the guidance
of a virtual proctor. Participants and virtual proctors were blinded to the test card results.
22/30 participants described the process of reading and interpreting the test card results as being easy. However, 8/30 of
the participants commented that it was dicult to see some of the fainter line conditions.
A total of 270 trials were recorded in this study. Participants were able to perceive and interpret the results correctly for
239 trials, or 89% of the time. Positive results with stronger intensity lines were easier to read than the positive lines with
less intensity. As the line intensity became fainter, the ability to read the result correctly ranged from 83% to 60%, with an
overall rate of 70%.
After the human factors evaluation, participants were asked for their overall impressions of the instructional materials they
were provided. Nearly all participants (29/30) thought the instructions were straightforward and easy to understand and
follow.
Based on the learnings from this study improvements were made to the Quick Reference Guide and Proctor training.
Usability Study
Abbott conducted a study to evaluate whether a home user can follow instructions from a trained proctor through a
virtual platform and successfully perform the test steps for the BinaxNOW COVID-19 Ag Card test, including nasal swab
collection at home, and correctly interpreting the results.
60 home users, including individuals (n=30) and caregivers (n=30), participated in the study. Each individual or caregiver
pair participated in a 45-minute session with a single proctor. The usability evaluation session included one simulated use
of the BinaxNOW COVID-19 Home Test Kit in which a user was already connected with a proctor, knowledge tasks, and
opportunities to provide feedback.
96.7% (58 out of 60) home users produced a valid result (all negative) and 2 participants produced an invalid result.
(The causes of the invalid tests were insucient amount of reagent added, and damage to the test strip). 58 out of 60
participants interpreted their test result correctly and 2 participants interpreted their result incorrectly (where they
perceived a faint line in the sample window (as positive) when there was none (all results were verified by the study
moderator).
The individual home use group completed 96.8% (1103/1140) of the total tasks/steps correctly. The caregiver home user
group completed 97.3% (1109/1140) of the total tasks/steps correctly. The most common use errors observed during
critical tasks included incorrectly swabbing the nostril to obtain a nasal sample and contacting the test strip with the hands
or with the surface.
15 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use

90% (56 out of 60) of the home (individual and caregiver) participants had positive impressions of the BinaxNOW
COVID-19 Ag Card Home Test Kit. The test was perceived as being easy to use. The mixed feedback from three home user
participants included that some of the labeling on the dierent components was confusing and one participant reported
that they would not be comfortable performing this test without a medical professional present.
88% (53 out of 60) participants stated the Quick Reference Guide (QRG) shown on the screen while the participant
performed simulated use of the BinaxNOW COVID-19 Ag Card Home test was clear and easy to understand. 54 out of
60 participants felt their proctor that helped guide them through the workflow was helpful and provided clear instructions.
SYMBOLS
16 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
This symbol indicates that the product has a temperature limitation.
This symbol indicates the total number of tests provided in the kit box.
This symbol indicates that the product is for single use only. It is not to be re-used.
IVD
For In Vitro Diagnostic Use.
This symbol indicates that you should consult the instructions for use.
www.globalpointofcare.eifu.abbott
This symbol indicates the product’s catalog number.
This symbol indicates the name and location of the product manufacturer.
TECHNICAL SUPPORT ADVICE LINE
Further information can be obtained from your Telehealth provider, or by contacting Technical Support on:
US
+ 1 800 257 9525 [email protected]

Abbott
BinaxNOW
COVID-19 Ag Card Home Test
PI - EN
Size:
Flat size: 8.375 in x 10.75 in
Finished: 8.375 in x 5.375 in
Printed Colors
CMYK
Incoming Inspection Colors
PMS 2995 U
Primary Blue
PMS 224 U
Magenta-Pink
PMS 303 U
Dark Blue
PN: IN195100
Rev: 6
Date of Last Revision:
6.12 2023/01/30

