
This guideline is
designed to help
Importers, Customs
Brokers, Official
Import Inspection
Establishment
Management, and the
general public:
• Understand the
import regulatory
requirements
• Take steps to
ensure products
are imported in
compliance with
applicable
regulations
USDA FSIS
FSIS Guidance for Importing Meat,
Poultry, and Egg Products into the
United States
Final – September, 2020

ii
Food Safety and Inspection Service (FSIS) Guidance for Importing Meat,
Poultry, and Egg Products into the United States
What is the purpose of this Compliance Guidance?
FSIS is providing guidance to interested parties on steps they can take to ensure meat
(including Siluriformes fish products), poultry, and egg products are imported in
compliance with the applicable statutes and regulations of the United States.
FSIS has updated its guideline based on comments received. Specifically, FSIS revised
and reorganized a section on industry supply chain best practices, clarified approaches
to levels of reinspection, added information about generic labeling approvals, food
defense, slaughter dates on import certification, and barcoding, and made minor
editorial changes to improve the guidance’s clarity.
Who is this guidance designed for?
This guidance is intended to help U.S. importers, customs brokers, official import
inspection establishments, and the general public understand the import requirements
found in 9 CFR 327 (meat), 9 CFR 381, Subpart T (poultry), 9 CFR 557 (Siluriformes
fish), 9 CFR 590.900-970 (egg products).
Although FSIS is requesting comments on this document, this document reflects FSIS’s
current position. FSIS recommends importers begin using it now.
What if I still have questions after I read this guidance?
If the desired information cannot be found within the Compliance Guidance, FSIS
recommends that users search the publicly posted Questions & Answers (Q&As) in the
askFSIS database or submit questions through askFSIS
. Documenting these questions
helps FSIS improve and refine present and future versions of the Compliance Guideline
and associated issuances.
iii
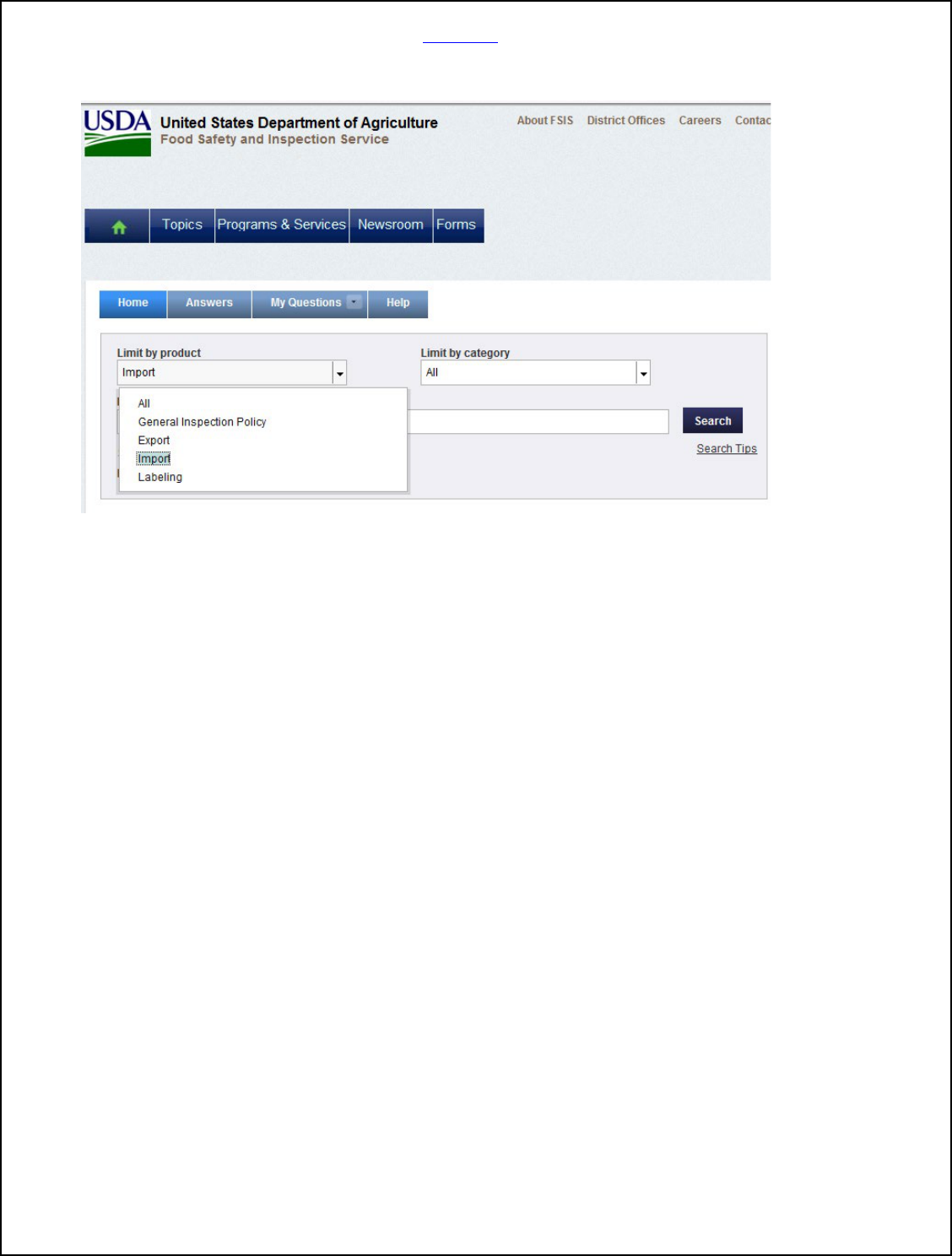
Below is the screenshot to search the askFSIS database of publicly posted Q&As:
The "Submit a Question" tab will only be activated once you've performed a search and
have accessed an answer. If you've searched the publicly posted Q&A’s and are
unable to find the answer you're looking for, you can access the "Submit a Question"
tab to submit a new question to our support staff. The system may suggest possible
answers, which you can view before finally submitting your question.
You must have a customer account in order to submit a question or access "My
Questions."
To create an account, you can either access the "Create a New Account" option or the
"Signup" option on the upper right-hand corner of the screen.

iv
Below is the screenshot to “Submit a Question,” Log in or create an askFSIS account:
If you already have a customer account, but have forgotten your username or
password, click "Forgot your username or password?" option. The next screen will
allow you to request your username or reset your password. For Username
requests, an email will be sent to the email address in your customer account. For
Password resets, your username must be correct to receive an email with a link to reset
your password. If you do not receive the email to reset your password, check to make
sure your username is correct. The username is case sensitive.
When submitting a question, use the “Submit a Question” tab, and enter the following
information in the fields provided:
Subject Field: Enter FSIS Guidance for Importing Meat, Poultry, and Egg
Products into the United States
Question Field: Enter question with as much detail as possible.
Product Field: Select Import from the drop-down menu.
Category Field: Select Basic Import Answers from the drop-down menu.
Policy Arena: Select International (Import/Export) from the drop-down menu.
When all fields are complete, press Continue.
Additional Information: FSIS Directive 5620.1 - Using askFSIS

v
FSIS Guidance for Importing Meat, Poultry, and Egg Products
into the United States
Table of Contents
Statutory Jurisdiction............................................................................................................ 1
Country Equivalence and Establishment Eligibility ............................................................. 1
Certification ........................................................................................................................... 7
Labeling and Marking ...........................................................................................................11
FSIS Sampling Process........................................................................................................14
Test and Hold on Shipments Pending FSIS Testing Results ..............................................15
U.S. Refused Entry Process .................................................................................................16
U.S. Export Products Returned to the United States ..........................................................16
Samples for Laboratory Examination, Research, Evaluative Testing, or Trade Show
Exhibition..............................................................................................................................19
Products for Pharmaceutical Use Only ...............................................................................19
Product Containing a Small Amount of Meat, Poultry or Egg Product Ingredients ...........20
Product Entering the U.S. Destined for Foreign Commissaries .........................................20
Products for Personal Consumption ...................................................................................20
Undenatured Inedible Meat and Egg Products (includes meat, meat by products, and
technical animal fat) .............................................................................................................21
Products Not Regulated by FSIS .........................................................................................22
Imported Farmed and Wild Game Meat and Other Products ..............................................22
Casings .................................................................................................................................23
Denatured Inedible Meat, Poultry, Egg Products and Rendered Fats ................................24
Shell Eggs.............................................................................................................................24
Pharmaceutical Products Derived from Livestock and Imported into the U.S ...................25
Import Inspection Resources ...............................................................................................26

1
FSIS Guidance for Importing Meat, Poultry, and Egg Products
into the United States
Statutory Jurisdiction
The U.S. Department of Agriculture’s (USDA) Food Safety and Inspection Service
(FSIS) is the public health regulatory agency responsible for ensuring that domestic and
imported meat (which includes Siluriformes fish), poultry, and egg products are safe,
wholesome, and correctly labeled and packaged. FSIS inspects imported meat, poultry,
and egg products under the authority of the Federal Meat Inspection Act
(FMIA) (21
U.S.C. 601 et seq.), the Poultry Products Inspection Act (PPIA) (21 U.S.C. 451 et seq.),
and the Egg Products Inspection Act (EPIA) (15 U.S.C. 1031 et seq).
Country Equivalence and Establishment Eligibility
Imported meat, poultry, and egg products must originate from eligible countries and
from establishments or plants that are certified by each country’s central competent
authority (CCA) to export to the United States (21 U.S.C. 620, 466, and 1046).
Countries become eligible following an equivalence determination process
completed
by FSIS in coordination with the CCA of the exporting country.
After a country is determined to have an equivalent food safety regulatory system, FSIS
relies on the country’s CCA to carry out inspection activities. Foreign establishments
desiring to export to the United States must apply to their own CCA and a responsible
official of that country’s inspection system must certify to FSIS those establishments
that meet requirements equivalent to those of the United States. In addition to
reinspecting product presented for import into the United States, FSIS conducts periodic
audits to ensure that the foreign country’s food safety regulatory system remains
equivalent to that of the United States. These audits include an assessment of selected
exporting establishments.
FSIS maintains an Import Library on its website, which provides links to country-specific
pages for equivalent countries, detailing the eligible species, process categories,
product categories, and product groups the country can export. The information
detailed on the country-specific pages aligns with the FSIS product categorization guide
and the Public Health Information System (PHIS)
(
https://www.fsis.usda.gov/wps/wcm/connect/abbf595d-7fc7-4170-b7be-
37f812882388/Product-Categorization.pdf?MOD=AJPERES). Each eligible country
page also lists any applicable USDA, Animal and Plant Health Inspection Service
(APHIS) animal disease restrictions, and includes direct, disease-specific links to the
APHIS website and regulations. The Import Library can be found at
https://www.fsis.usda.gov/wps/portal/fsis/topics/international-affairs/importing-
products/eligible-countries-products-foreign-establishments/eligible-countries-and-
products.

2
Supply Chain Roles
This guidance is not intended to capture the wide variety, nuance and complexity of
supply chain roles and practices. However, listed below are common parties in the
majority of supply chain processes involving imported meat, poultry, and egg products:
Importer: The person primarily liable for the payment of any duties on the merchandise,
or an authorized agent acting on the importer’s behalf (such as a customs broker, see
below) (19 CFR 101.1)). The importer is responsible for filing entry and clearing the
goods through U.S. Customs and Border Protection (CBP) and other applicable U.S.
government agencies, and for ensuring that all U.S. requirements (e.g.
the statutes and
regulations enforced and administered by CBP, APHIS, and FSIS) are met prior to
moving goods into commerce. The “importer” as defined in the relevant regulations may
include the “importer of record,” or “consignee.” Importers may be
any individual, firm,
or corporation, including any partnership, association, or other unincorporated business
organization.
Customs Broker: The person acting on the importer’s behalf to ensure that U.S.
statutory and regulatory requirements are met before moving goods into commerce,
depending on the arrangements and agreements made between the commercial
parties, and any official documents signed. As noted above, an “importer” includes an
authorized agent acting on the importer of record’s behalf. Like importers, customs
brokers may be individual persons, firms, or corporations. More information on customs
brokers can be found on CBP’s website at:
https://www.cbp.gov/trade/programs-
administration/customs-brokers/becoming-customs-broker
Carrier: The person or entity transporting the shipment for compensation.
Official Import Inspection Establishment: Business operating under an FSIS Grant of
Inspection that receives meat, poultry, or egg products for FSIS import inspection. As
part of business supply chain processes and contractual agreements, the management
of the official import inspection establishment often has a role as the importer’s
designee/agent when issues arise with the reinspection of imported meat, poultry, or
egg products.
Software Developer: Any CBP-approved company or individual person that develops
software to transmit CBP’s Automated Commercial Environment (ACE) entry/cargo
release to CBP.
Supply Chain Coordination:
Acting on the importer’s behalf, the customs broker generally completes the FSIS import
inspection application (paper FSIS Form 9540-1 or electronic Partner Government
Agency (PGA) Message Set), and will be contacted by FSIS (email) regarding the
following:
• Decisions associated with the reinspection of imported products;
• Decisions associated with refused entry product;
• Responsibility for recalls requested on imported products; and

3
• Responsibility for shipments that fail to present (FTP) for FSIS reinspection.
Based on the roles appropriate to each party in the supply chain, FSIS strongly
recommends that the importer, customs broker, carrier, and official import inspection
establishment management coordinate closely to ensure the following issues are
effectively managed:
• Tracking the estimated date of arrival of shipments expected to present to FSIS
at the designated official import inspection establishment and all parties are
notified when a shipment has not arrived;
• Products are properly handled, stored, and staged for reinspection;
• Product subject to test-and-hold sampling is securely stored;
• Corrective actions such as re-labeling or shipping mark issues are handled
according to FSIS requirements;
• Product disposition (U.S. Inspected and Passed, Holds Pending Laboratory
Sample Results, U.S. Refused Entry) is properly and expeditiously executed; and
• Refused entry product is properly stored and handled, and its disposition is
handled as per the importer and FSIS requirements, and the importer is notified
when the 45-day (30 days for egg products) disposition date is approaching.
Presenting and Documenting Shipments
All imported shipments of meat, poultry, and egg products must be presented to FSIS
for inspection. FSIS import inspection occurs after the product has met CBP and
APHIS requirements at the U.S. Port of Entry (POE). CBP’s release of the shipment at
the U.S. POE allows the shipment to transfer to the designated FSIS official import
inspection establishment. It does not mean that FSIS import inspection requirements
have been met.
Import Inspection Application: The importer (person primarily liable for the payment
of any duties on the merchandise, or an authorized agent acting on the importer’s
behalf, such as a customs broker (19 CFR 101.1)), must apply for FSIS inspection of
imported product (9 CFR 327.5, 381.198, 557.5, and 590.920). Importers (i.e., the
applicant) must submit either the paper FSIS Form 9540-1 Import Inspection Application
to the FSIS inspection personnel in the designated official import inspection
establishment; or the electronic application data (PGA Message Set) into the CBP’s
ACE, which transfers to FSIS’ Public Health Information System (PHIS) Import
Component. The PHIS Import Component is an electronic data analytic system that
collects, consolidates, and analyzes data in order to improve public health. PHIS
enables importers to electronically apply for FSIS import inspection prior to arrival of
shipments destined for the United States, and electronically links with ACE. More
information about the PHIS Import Component can be found at
http://www.fsis.usda.gov/wps/portal/fsis/topics/international-affairs/importing-
products/phis-import-component

4
Basic Import Process
Importers must submit the inspection application as far as possible in advance of the
anticipated arrival of each consignment, but no later than when entry is filed with CBP (9
CFR 327.5(b), 381.198(b), 557, and 590.920(b)). Importers who submit inspection
application data electronically using the PGA Message Set automatically meet the prior
notification requirement. The prior notification requirement is particularly important for
shipments that cross land borders (U.S.-Canada and U.S. -Mexico), because of the
relative speed with which those shipments enter the United States, compared to
shipments that enter the country on ocean vessels or by air. For all shipments,
including those from Canada and Mexico, it is essential that sufficient advance notice
occur to ensure data entry can be completed in advance of the shipment arriving at the
official import inspection establishment to avoid delays at the official import inspection
establishment, and early notice of any shipments that Fail to Present for import
reinspection. Import inspection applications must be complete, including the Customs
entry number. Further guidance on the PHIS Import Component can be found in the
following industry Q&A:
http://www.fsis.usda.gov/wps/portal/fsis/topics/inspection/phis/questions-and-answers-
import-component/ct_index
Official Import Inspection Establishments: FSIS inspection occurs at the official
import inspection establishment designated by the importer in the inspection application,
after meeting CBP and APHIS (animal disease) requirements at the U.S. POE. Official
import inspection establishments are privately owned facilities that operate under an
FSIS grant of inspection. An establishment’s grant of inspection (i.e. approval to host
FSIS import inspection) identifies the specific products approved for FSIS import
inspection at the establishment. A list of official import establishments can be found in
the FSIS Meat, Poultry, and Egg Product Inspection Directory at
http://www.fsis.usda.gov/wps/portal/fsis/topics/inspection/mpi-directory, with an “I” prefix
Eligible Foreign
Est.
• Eligible est. in equivalent country exports certified shipment to United
States.
U.S. POE
• U.S. importer (or agent) files entry with CBP, submits import inspection
application to FSIS, and ensures shipment clears CBP and APHIS
(animal health requirements) at U.S. POE.
Official Import
Ins pection Es t.
• After clearance at U.S. POE, U.S. importer (or agent) ensures properly
documented shipment is transported directly to the designated official
import inspection est. and presented to FSIS for reinspection.

5
in the establishment’s name/number.
Failure to Present (FTP): Any shipment of meat, poultry, or egg product that has
entered commerce without FSIS import inspection violates the FMIA; the PPIA; or the
EPIA, as well as the associated implementing regulations (9 CFR 327.6, 381.199, 557,
and 590.925). Meat, poultry, and egg products from outside the U.S. are considered
"in-commerce" when they are off-loaded at a location other than the official import
inspection establishment or the official egg processing plant designated on the import
inspection application. FSIS considers such product to be a FTP, and therefore
ineligible for FSIS inspection. When a product has been identified as a FTP, FSIS will
request, through CBP, a redelivery of the shipment and appropriate penalties.
FTP product still in the original shipping containers may either be destroyed or re-
exported. If any imported product identified as FTP has been removed from the original
cartons or further processed, FSIS will initiate a regulatory control action on the product,
including any further processed product that contains the FTP product, to ensure
appropriate disposition (i.e., destruction). FSIS will likely request that the importer recall
the FTP product.
NOTE: The importer or customs broker can update the official import inspection
establishment or the estimated date of arrival (EDA) as long as the product remains
intact and is not offloaded, so that the product can be reinspected as required. The
EDA is the date that the product is expected to arrive at the designated FSIS
Bulk Pasteurized Egg Product Shipments: Tankers and large
bulk shipments of pasteurized egg products, which cannot be
safely opened (i.e. railroad tanker) and checked organoleptically
at the official import inspection establishment, must be checked
by FSIS at the receiving egg products plant (
9 CFR 590.424(b)).
Other containers of pasteurized egg products (e.g. 5-gallon
containers) will be checked organoleptically by FSIS at the official
import establishment, where safe and appropriate.
Unpasteurized Egg Products (currently permitted from Canada only) are not
required to present for FSIS reinspection at an official import inspection
establishment (9 CFR 590.925). All unpasteurized egg products shipments
must proceed directly to an official FSIS egg product plant in the United States
and be presented for FSIS reinspection.
For unpasteurized egg products, the importer (or agent) must submit as early
as possible, but no later than when the entry is filed with CBP, an electronic or
paper import inspection application, FSIS Form 9540-1, and the official
inspection certificate to FSIS, Office of Field Operations, Recall Management
and Technical Analysis Division (email:
ImportInspection@usda.gov. The FSIS
reinspection assignment will be scheduled in PHIS and reinspection will occur
at the official FSIS egg products plant designated on the electronic or paper
import inspection application.

6
establishment for import inspection. If the shipment is canceled, the broker/applicant
should provide notification of the cancellation to the import inspection personnel to avoid
a FTP.
PGA Message Set: The Security and Accountability for Every Port Act of 2006 (SAFE
Port Act) (Public Law 109-347)
http://www.gpo.gov/fdsys/pkg/PLAW-
109publ347/html/PLAW-109publ347.htm requires all agencies involved in clearing or
licensing the importation and exportation of cargo to participate in the International
Trade Data System (ITDS). The SAFE Port Act assigns CBP the responsibilities for
building and operating ITDS as part of the ACE project, an effort to modernize and
expand U.S. government agencies’ automated systems for processing imports and
exports. The ITDS is an electronic information exchange capability or “Single Window”
through which businesses will transmit data required by U.S. government agencies for
the importation or exportation of cargo. ACE is a CBP system designed to facilitate
legitimate trade while enhancing border security. ACE streamlines and automates
collection, sharing, and processing of information submitted to CBP and U.S.
government agencies, eliminating the need to submit a paper copy, and enabling the
international trade community to more easily and efficiently comply with U.S. laws and
regulations.
FSIS participates in the Single Window initiative through the PGA Message Set. The
PGA Message Set extends the electronic data that is currently provided to CBP to
include the additional import inspection application data required by FSIS. Use of the
PGA Message Set automates the collection of information provided by the importer on
FSIS form 9540-1. These data elements are transmitted electronically from ACE to
FSIS’ PHIS when the entry is filed with CBP, eliminating the need for importers to
submit a paper copy of the FSIS form 9540-1 to FSIS. This process expedites data
entry and shipment clearance by FSIS, and automatically meets FSIS’ prior notification
requirement.
Importers must use a software developer that has been certified by CBP according to
Automated Broker Interface (ABI) requirements. ABI-certified software developers who
are interested in developing PGA message set software may find the requirements for
the development of the PGA Message Set capabilities outlined in the Customs and
Trade Automated Interface Requirements (CATAIR)
http://www.cbp.gov/trade/ace/catair
. Current ABI-certified software developers can be
found on CBP’s website at https://www.cbp.gov/document/guidance/abi-software-
vendors-list. More information about ABI can be found on CBP’s website at
https://www.cbp.gov/trade/acs/abi/contact-info.
FSIS has further defined and developed additional guidance that is intended to assist
importers, customs brokers, and software developers in understanding the required
FSIS-specific data that will need to be submitted through CBP’s ACE system in order to
complete the import application process. FSIS has posted this compliance guide on the
following webpage:
FSIS Partner Government Agency (PGA) Message Set Compliance
Guide.
FSIS continues to work with interested software developers, importers, and customs
brokers. For more information on becoming an active participant in the PGA Message

7
Set with FSIS, contact the Import/Export Policy Development Staff, telephone:
.
Certification
• Shipment Certification Requirements
The foreign government (i.e. CCA) is responsible for the certification of each
consignment (i.e. shipment of product to the United States, 9 CFR 327.4, 381.197,
557.4, and 590.915). The consignment is the product as it is represented on the
official inspection certification. Each consignment must have an electronic
inspection certification or a paper inspection certificate issued by an authorized
official of the foreign government agency responsible for the inspection and
certification of the product. PHIS provides for a government-to-government
electronic transmission of the inspection certificate data as an alternative to the
paper certificate. Foreign governments interested in pursuing electronic certification
(e-Cert) should contact the FSIS, Office of International Coordination, telephone:
(202) 708-9543, email: ImportExport@usda.gov
for further guidance and
requirements.
The certificate must include a statement that any product described on the official
certificate was produced in accordance with the regulatory requirements in 9 CFR
327.2, 381.196, 557.2 or 590.910, or their equivalent.
NOTE: The electronic certification will be transmitted directly to FSIS in PHIS before
the product’s arrival at the official import inspection establishment (9 CFR 327.4,
381.197, 557.4, and 590.915).
The paper official inspection certificate must accompany each consignment; be
submitted to import inspection personnel at the official import inspection
establishment; be in English; and bear the official seal of the foreign government
responsible for the inspection of the product, including the name, title, and signature
of the official authorized to issue inspection certificates for products imported to the
United States.
Non-CBP Locations: A paper FSIS Form 9540-1 will continue to be
required for shipments of meat, poultry, and egg products imported
into ports where the U.S. CBP ACE system is not available
(American Samoa, Guam, Commonwealth of Northern Mariana
Islands) due to a lack of CBP jurisdiction in these ports. FSIS does
not require a hard copy of the official inspection certificate when the
data from that certificate transfers to PHIS from a government-to-
government connection (eCERT).

8
Both the electronic certification and the paper official inspection certificate must
contain:
• The date;
• The foreign country of export and the producing foreign establishment
number;
• The species used to produce the product and the source country and foreign
establishment number, if the source materials originate from a country other
than the exporting country;
• The product’s description, including the process category, the product
category, and the product group (see Product Categorization below);
• The name and address of the importer or consignee;
• The name and address of the exporter or consignor;
• The number of units (pieces or containers) and the shipping or identification
mark on the units;
• The net weight of each lot; and
• Any additional information the Administrator requests to determine whether
the product is eligible to be imported into the United States.
Examples of additional Information Requirements
1. Pasteurized Egg Products: bulk packed shipments (tankers or large tote
shipments) of pasteurized egg products must have foreign government
documentation attesting to negative laboratory results for Salmonella. This
may be on the official inspection certificate or on a separate paper with the
foreign government’s letterhead.
2. Poultry Grading Certificates: USDA’s Agriculture Marketing Service
recognizes the Canadian Food Inspection Agency’s (CFIA) poultry product
grade designations provided the poultry graded for export to the U.S. satisfies
the criteria for U.S. grades. Canadian product labeled with the CFIA grades
will be accepted provided the shipment is accompanied by an official CFIA
grading certificate stating that the product meets USDA grade standards (e.g.
“Young Turkey Canada A”). This certificate is in addition to the requirement
that there be an official inspection certificate. If graded product is received
without the accompanying grading certificate, FSIS will refuse entry on the
shipment until either a grading certificate is provided or the reference to the

9
poultry grade has been obliterated or removed.
3. Production Dates: Production dates are required on the official inspection
certificate when the producing foreign establishment has had a period of
ineligibility (delisted for noncompliance), or to distinguish the lots when a
shipment includes multiple lots of like product that bear the same shipping
mark.
4. Slaughter Dates: Slaughter dates may be required on the official inspection
certificate when FSIS has first determined that a country’s system is
equivalent to the United States, or when a country’s equivalence status has
been reinstated.
5. Sealing of Specific Products (Liquid Egg Products; Carcasses from Canada, and
Undenatured Inedible Fat): Bulk shipments of liquid egg product, meat or poultry
carcasses from Canada, and undenatured inedible fat (rendered grease, tallow,
or other fat) are required to be sealed with a foreign government seal(s) and the
seal numbers included on the official inspection certificate. If the seal(s) are
missing or broken, except for instances where CBP has removed the seal, FSIS
will refuse entry of the shipment.
• Product Categorization
To help foreign exporters and governments accurately identify eligible meat,
poultry, and egg products intended for export to the United States, FSIS has
published an updated Product Categorization
guide on the Agency’s website at
http://www.fsis.usda.gov/wps/wcm/connect/abbf595d-7fc7-4170-b7be-
37f812882388/Product-Categorization.pdf?MOD=AJPERES. It is essential that
the product is properly categorized, as this impacts what types of inspection the
products will be subjected to, including product sampling.
The guide now aligns more closely with product categorization in PHIS and
includes all species that are now under FSIS jurisdiction.
The guide outlines the three levels of product description required by FSIS on
official inspection certification for each shipment of imported meat, poultry, and
egg products (9 CFR 327.4, 381.197, 557.4, and 590.915):
Process Category: For imported product, process categories are used
as high-level groupings that identify whether and how products are further
processed after the slaughter operation. Thus, “slaughter” would not be a
correct process category for imported product. For example, the process
category “Raw Product - Non-Intact” applies to establishments that use
further processing steps such as grinding, comminuting, injecting product

10
with solutions, or mechanical tenderization by needling, cubing, pounding
devices or other means of creating non-intact product.
Product Category: Product categories are a more specific classification,
linked to the appropriate species for each category. For example, a
product category for beef or veal species is “Raw ground, comminuted, or
otherwise non-intact beef.”
Product Group: Product groups further define the product to a level of
specificity that FSIS can program appropriate types of inspections (TOI) in
PHIS for examinations and laboratory sampling. “Beef Patty Product” is an
example of a product group.
Because of the length of some process categories and product categories, FSIS
accepts easily-identifiable abbreviations or acronyms for either product or process
categories on official inspection certification. However, if the official inspection
certification does not include product categorization (process category, product
category, and product group) that is consistent with the guide, replacement certification
is required to proceed with import reinspection.
Palletized, consumer packaged (including food service—hotel, restaurant, or institution
(HRI)), fully marked and labeled products may be presented with the shipping mark and
shipping container label applied to the outside of the pallet, rather than to individual tray
packs or cartons. When products are presented in this manner, only one type and size
of product may be presented as a lot, and the foreign inspection certificate identifies all
the retail package production codes present (such as date codes imprinted on the can
or package) in the shipment. When utilizing alternative packaging procedures, the
Product Lot Grouping and Certification
FSIS requires product to be presented for reinspection in lots certified on the official
inspection certificate. PHIS assigns reinspection tasks based on the lots included on the
certificate/eCert. The lots on the FSIS 9540-1 Import Inspection Application
or PGA
Message Set must align with those on the inspection certificate/eCert. The lots on the
inspection certificate/eCert cannot be changed at import reinspection.
Therefore, it is critical that the importer (or agent) correlates the lotting and certification of
the product with the foreign exporter before the foreign government certifies the
shipment. The importer (or agent) must resolve any discrepancies between the import
application and the official inspection certificate before FSIS reinspection takes place.
PHIS allows this process to take place electronically between the importer (or agent) and
the FSIS import inspector.
Lot groupings are based on like product by species, process category, product category
and product group. The FSIS Product Categorization Chart
provides product
descriptions to determine lot grouping on the official inspection certificate. When a
shipment includes multiple lots of like product with the same shipping mark, additional
information must be provided (e.g. production dates) to identify the lots.

11
importer is responsible for assuring that the entire pallet will be distributed to retail or the
end user as an intact unit.
NOTE: For Canadian shipments, different sized boxes of the same product may be
presented on the same pallet when the pallets in the lot will be split for distribution to
specific end users.
Labeling and Marking
Meat, poultry, and egg products exported to the United States must adhere to all
applicable labeling
requirements in the FMIA, PPIA, EPIA, and FSIS regulations, and
policies, or their equivalent.
Consistent with the FMIA, PPIA, and EPIA, FSIS regulations prohibit misbranded and
economically adulterated meat, poultry, and egg products, ensuring that all labels are
truthful and not misleading. FSIS reinspection includes verification of labeling
requirements. If a product is deemed to be incorrectly labeled, a wide range of penalties
can be imposed, including rescinding the use of the label, product retention or detention
(prohibiting the sale from anywhere in the chain of commerce), relabeling, or request for
product recall. Additionally, bringing misbranded product into compliance is performed
under reimbursable services, meaning the importer will be charged for these services.
FSIS’ meat and poultry regulations permit certain labels, including those that are not
likely to present significant policy issues that have health or economic significance, to
be “generically approved” without having to be submitted to the Agency’s headquarters-
based labeling staff, as long as the labels comply with FSIS regulations. Although not
submitted to FSIS, generically approved labels are approved by FSIS by being in
compliance with applicable regulations. Any entity responsible for designing or
modifying meat or poultry product labels may use generic approval of labels, including
foreign exporters and U.S. importers. In August 2017, FSIS published a
compliance
guide on generic labeling to assist industry in realizing the efficiencies of generic
labeling.
Further guidance on labeling is available at:
http://www.fsis.usda.gov/wps/portal/fsis/topics/regulatory-compliance/labeling
.

12
•
Missing or Completely Illegible Shipping Marks
Shipping marks are normally applied by the exporting country when product is
exported. Missing or illegible shipping marks may be applied (or reapplied) under
the supervision of a representative of the CCA of the exporting country (9 CFR
327.4, 381.197, 557.4, and 590.915).
NOTE: FSIS permits official import inspection establishment personnel to apply the
shipping mark to shipping units in lieu of an official representative of the foreign
inspection system having to be on-site, provided that FSIS IPP can verify the unique
identifier within a barcode on the shipping unit using the supporting documentation
from the foreign country. This process applies only to countries that are eligible for
the use of barcodes, and for shipping units when a shipping mark is missing or
completely illegible. Countries must first submit a barcoding plan to FSIS to be
approved for this process.
Approval for a Pre-Stamping Program
Official import inspection establishments may request approval for a Pre-Stamping
Program. The Federal Meat and Poultry Products Inspection regulations (9 CFR
327.10(d), 381.204(f), and 557.10) permit official import inspection establishments, with
an approved program, to place the official inspection legend on imported meat and
poultry products before the completion of an official import reinspection. However, the
regulations require that the official import inspection establishment has a controlled pre-
Seal Variations for Certain Egg Products
When pasteurized liquid egg products are shipped in bulk shipments (tanker trucks or
portable totes weighing approximately 1,000 pounds or more):
• The shipment must be presented at an official import inspection establishment;
• The transport container(s) must be sealed with the exporting government’s seals
and the seal numbers identified on the inspection certificate or other CCA
documentation containing the exporting country’s official seal numbers; and
• The testing results for Salmonella must be presented with the shipment and
identified as negative on the official inspection certificate or other CCA
documentation for the production lot of pasteurized egg products that includes the
product offered for entry; the certificate and product information must match.
Unpasteurized egg products shipped from Canada must be sealed with government
seals and the seal numbers identified on the inspection certificate or other
documentation containing Canada’s official seal numbers.

13
stamping procedure approved by the FSIS. The pre-stamping procedure must identify
how the controlled stamping of product will be accomplished. Requests to establish a
pre-stamping procedure must be submitted in writing on company letterhead to the
District Office with jurisdiction for that state
for review.
The written request must:
• State that pre-stamping is limited to only those meat and poultry lots that FSIS
import inspection personnel can fully inspect on the day on which pre-stamping
occurs;
• Identify where product that is pre-stamped will be stored in the official import
inspection establishment until reinspection has occurred;
• Include a control procedure for removing or obliterating the official inspection
legend from pre-stamped lots that fail reinspection;
• Describe how the official import inspection establishment will maintain a daily
pre-stamping log that includes the date the product was pre-stamped/re-
inspected, the country of origin, the foreign establishment number, the name of
the product, the number of units in the lot, the shipping or identification mark, and
the official inspection certificate number; and
• Provide for the retention of the daily pre-stamping log in accordance with
9 CFR 320.3, 381.177, and 550.3 for making it available to import inspection
personnel on a daily basis.
Approved requests will be signed by the FSIS District Manager and the letter returned to
the requesting official import inspection establishment management.
Sanitation Standard Operating Procedures (Sanitation SOPs) are written procedures
that official establishments and official import inspection establishments develop,
implement, and maintain to prevent direct contamination or adulteration of meat or poultry
products. All official import inspection establishments must develop and maintain
Sanitation SOPs (9 CFR 304.3(a), 381.22(a), and 416.11 through 416.17). Official import
inspection establishments must also ensure that reinspected, imported egg products do
not become contaminated or adulterated.
FSIS will perform directed procedures within the official import inspection establishment,
including Sanitation SOP verification, pre-stamping compliance, as well as unscheduled
procedures prompted by a trigger event, such as when a pre-stamping violation occurs.
FSIS will maintain establishment profiles in PHIS, which will provide the ability for
compliance and non-compliance of verification activities to be documented. Official import
inspection establishment management may find out how to access PHIS for these
purposes at How to obtain a Level 2 USDA eAuthentication account
.

14
FSIS Sampling Process
FSIS reinspection sampling is allocated by country, process category, product category,
product group and in some instances, species. Each imported product shipment is to
be identified by the foreign exporting establishment with a unique shipping mark or
identification mark that is entered into PHIS for the initial identification and subsequent
tracking of the product while in U.S. commerce distribution channels. When a lot of
imported product is selected for reinspection, several TOIs may be assigned and
performed by FSIS import inspection personnel. These include a physical examination
of the product for visible defects and an examination of container condition.
At set intervals, FSIS also collects and analyzes samples for pathogens, food chemistry,
and drug and chemical residues. There are three levels of reinspection (LOR) relative
to sampling: Normal LOR is randomly selected for lots based on the annual sampling
plan; Increased LOR is targeted based on a FSIS management decision; and
Intensified LOR is automatically generated by PHIS after a lot fails a TOI. Note that
intensified LORs correspond to the failed TOI. Normal, increased and intensified LORs
may include TOIs other than laboratory sampling. For example, FSIS performs 100
percent certification and label verification on every commercial shipment presented for
import.
If a shipment fails reinspection, the result is recorded in PHIS. Thereafter, PHIS will
automatically generate an intensified rate of reinspection. For example, a failure for
physical defects that is not of public health concern results in assignment of
reinspections a minimum of 10 lots and 10 times the weight of the failed lot applied to
the processing establishment based on the same Product Category/Process
Category/Product Group. In the case of failures considered to be of public health
concern, such as a laboratory analyses failure, or off condition, a minimum of 15 lots
and 15 times the weight of the failed lot applied to the processing establishment based
on the same Product Category/Process Category/Product Group are selected for repeat
analysis. Products that fail reinspection are refused entry into the United States. If the
importer wants to appeal a failed TOI, they are to refer to FSIS appeal regulations (9
CFR 327.24, 381.202(6)(d), or 557.24), ensure the appeal is made to the program
employee’s immediate supervisor,
and continue to go through the appropriate chain of
command within the Office of Field Operations.
Products that pass reinspection are allowed to enter U.S. commerce. Under U.S. meat,
poultry, and egg products inspection laws, reinspected and passed imported articles
are, upon entry into the United States, deemed and treated as domestic articles in
commerce.
Appeals
FSIS regulations (327.24, 381.202(6)(d), and 557.24) provide that
establishments may appeal any inspection decision. Appeals are to be
submitted to an immediate supervisor for a determination (e.g. Front Line
Supervisor). More information on the FSIS appeals process can be found
on
FSIS’ website.

15
Test and Hold on Shipments Pending FSIS Testing Results
When FSIS schedules a laboratory TOI of selected products for specific adulterants, the
selected product shipment must be held pending acceptable results.
The test and hold policy above applies to imported shipments of:
1. Non-intact raw beef product or intact raw beef product intended for non-intact use
that is tested for Escherichia coli O157:H7 (E. coli O157:H7) and other shiga-
toxin producing E. coli (STEC) that FSIS considers to be adulterants;
2. Ready-to-eat products tested for Listeria monocytogenes or Salmonella;
3. Livestock carcasses and meat products tested for residues; and
4. Egg products.
The test and hold policy above does not apply to imported shipments of:
1. Raw meat or poultry products tested for Salmonella or other pathogens that FSIS
has not designated as adulterants in those products; or
2. Poultry carcasses or raw poultry parts sampled for residues.
The importer, or the importer’s agent, is responsible for controlling the product that is on
hold. The sampled lot may move off-site, however, the held shipment must be
controlled (e.g., company seals) and not enter into commerce until acceptable results
are received and the FSIS reinspection is completed. The sampled lot may move off-
site if the laboratory TOI is assigned at the Normal LOR. When the laboratory TOI is
assigned at the Intensified LOR, the lot is ineligible to move from the official import
inspection establishment until acceptable laboratory sample results are reported.
Offsite movement when laboratory TOIs are assigned at the Increased LOR is an
evidence-based FSIS management decision applied on a case-by-case basis. The
importer is primarily responsible for ensuring that the product does not move into
commerce prior to the receipt of acceptable laboratory results and the completion of the
product reinspection by FSIS.
If the laboratory analysis results show that the product is not in compliance, shipments
on hold must be returned to the official import inspection establishment, have the marks
of inspection obliterated, and will be stamped “United States Refused Entry.” The
importer and the import establishment management can receive laboratory results
electronically directly from the FSIS laboratory by providing an e-mail address on the
FSIS import inspection application. FSIS has prepared a compliance guideline for the
test and hold requirements: FSIS Compliance Guideline for Controlling Meat and
Poultry Products Pending FSIS Test Results:
http://www.fsis.usda.gov/wps/wcm/connect/6b7b5a65-9ad3-4f89-927d-
078e564aca24/Compliance_Guide_Test_Hold_020113.pdf?MOD=AJPERES

16
U.S. Refused Entry Process
Meat, poultry, and egg products that do not comply with U.S. requirements are not
allowed by FSIS to enter U.S. commerce and are identified with a “United States
Refused Entry” stamp or tag. When FSIS refuses entry, FSIS sends a notification from
PHIS to the applicant by e-mail, utilizing the email address provided on the import
inspection application. Product that is securely held at a location other than the official
import establishment, pending lab results, must be returned to the official import
establishment if the product is Refused Entry. Within 45 days for meat and poultry and
30 days for egg products, the importer must complete disposition of the refused entry
product (9 CFR 327.13(a)(2), 381.202(a)(2), 557.13, and 590.945(a)). Disposition
options for refused entry products include: rectification (i.e. misbranded brought into
compliance within the product-specific time frames listed above); exportation (return) to
originating country or third country; landfill; rendering; incineration; denaturing the
product not for human food; or conversion of product to animal food with prior U.S. Food
and Drug Administration (FDA) approval. The importer is to notify FSIS in writing if
he/she wishes to bring refused entry product into compliance or appeal the refusal.
FSIS provides for extensions to the timeframes for product to be re-exported, which
may be requested by the importer when extreme circumstances warrant it (e.g., a
dockworkers strike or unforeseeable vessel delay). No product that has been refused
entry and exported to another country, including the country that processed the product,
may be returned to the United States under any circumstance (9 CFR 327.13, 381.202,
and 557.13.)
Partial Refused Entry
Shipments containing cartons with transportation damage, missing or completely
illegible shipping marks are to be sorted, and the non-compliant product removed from
the lot by the official import inspection establishment under FSIS supervision (9 CFR
327.13(a)(2), 381.202(a)(2), 557.13, and 590.945(a)).
U.S. Export Products Returned to the United States
The FSIS Office of Field Operations (OFO), Recall Management Technical Analysis
Division (RMTAD) must approve requests to return U.S. exported meat, poultry, and
egg products prior to the product arriving at the U.S. POE. The product may be subject
to reinspection in an official FSIS establishment prior to release into commerce (9 CFR
327.17, 381.209, 557.17, and 590.965).
Prior to submitting a request to FSIS for approval to return exported products, the
applicant must verify that APHIS will permit entry. Applicants can contact USDA,
APHIS via telephone at 301-851-3300 option 1 or via e-mail at
asknies.products@usda.gov
. Information may be found on the website at
http://www.aphis.usda.gov/import_export/animals/animal_import/animal_imports_anpro
ducts.shtml. APHIS import permits: http://www.aphis.usda.gov/permits/.
Applicants returning U.S. exported products to the U.S. must also file the proper
customs entry paperwork with the appropriate port director (
U.S. Customs and Border
Protection Requirements).

17
FSIS requires that the exporter/applicant complete the FSIS Form 9010-1
, "Application
for the Return of Exported Products to the United States" (Fillable PDF). The form
should be completed and submitted by email to USReturnedExports@usda.gov with a
copy of the export certificate (FSIS Form 9060-5 or 9135-3) and any additional
supporting documentation related to the product prior to the shipment arriving in the
U.S. The “Submit via Email” button at the bottom of the form may also be used for
submission.
When the form and appropriate documentation are received by RMTAD, the information
will be assessed to determine whether the shipment needs to be reinspected prior to its
release into commerce. RMTAD will seek information about the shipment and consider
the information obtained. This information may include, but is not limited to, the
following:
• Is the product in its original shipping conveyance?
• What is the condition of the product, its shipping cartons, and its immediate
packaging?
• How long has the product been out of the country?
• Where has the product been since it was exported from the U.S.?
• Under what conditions has the product been held?
• What has been the chain of ownership of the product?
• Was the product ever abandoned?
• Is the product labeled in compliance with U.S. regulations?
• Was the product refused entry by a foreign government, and, if so, what were the
circumstances behind the refusal? (A copy of the official inspection report may be
required.)
When the exporter/applicant provides the above information with their original
submission, it may expedite RMTAD’s ability to make its determination.
If reinspection is required, RMTAD will inform the applicant, who must coordinate and
provide an appropriate plan for examination from the designated official FSIS
establishment where the reinspection is to occur. RMTAD will coordinate with the
appropriate District Office
(DO) and provide all information obtained including the plan
for reinspection. Once the DO has concurred with the reinspection location, RMTAD
will advise the applicant that the shipment can proceed to the designated establishment
for reinspection. FSIS inspection personnel at the official establishment will reinspect
the product, including taking samples as needed, to assure it is not adulterated or
misbranded and that there are no signs of product tampering. The DO will report the
results of the reinspection to RMTAD, and RMTAD will make a final determination on
admissibility.
NOTE: The product will be retained if it is adulterated or misbranded. Product
retention, approval for release into commerce, or other results will be designated on the
application (
FSIS Form 9010-1), which will be transmitted to the applicant from RMTAD.
For more information on returning exported U.S. meat, poultry, or egg products,

18
720-9904.
Food Defense
Food defense is the protection of food products from intentional contamination or
adulteration where there is an intent to cause public health harm and/or economic
disruption. Implementing food defense measures is voluntary; however, it is
encouraged as a good business practice and to further protect public health.
In order to better prevent and prepare for an intentional attack on meat, poultry, and
processed egg products, FSIS conducts vulnerability assessments of these food
systems. Based upon its assessments, FSIS develops countermeasures or food
defense practices that establishments can implement to protect the food supply. The
Agency also develops guidance materials and food defense training programs. These
initiatives are conducted in collaboration and coordination with other Federal, State, and
local partners, including academia and industry.
The Agency currently monitors FSIS-regulated facilities to determine the extent to which
establishments are implementing food defense practices. This is done through food
defense tasks performed by FSIS in-plant inspection personnel. The information from
these tasks assists FSIS in determining what further measures may need to be taken in
the area of food defense.
A functional food defense plan can help establishments, including official import
inspection establishments, identify steps to take to minimize the risk that food products
in the establishment will be intentionally tampered with or contaminated. A functional
food defense plan is: 1) developed (documented and signed), 2) implemented (food
defense practices are implemented), 3) tested (food defense practices are monitored
and validated), and 4) reviewed and maintained (at least annually).
FSIS does not require official establishments to adopt a functional food defense plan;
however, the Agency strongly encourages establishments to do so. Although the plan
should be in place at all times, it may be particularly helpful during emergencies. During
a crisis, when stress is high and response time is at a premium, a documented set of
procedures improves an establishment’s ability to respond quickly. A functional food
defense plan helps establishments maintain a safe working environment for employees,
provide a quality product to customers, and protect the bottom line.
FSIS developed a generic food defense plan template, in addition to food defense
guidelines. To access these documents and other food defense resources, visit the
FSIS web site at www.fsis.usda.gov/fooddefense
. In addition, questions regarding food
defense can be sent to: FoodDef[email protected].

19
Exemptions to FSIS Import Inspection
Samples for Laboratory Examination, Research, Evaluative Testing, or Trade
Show Exhibition
Meat, poultry, and egg product
samples destined for laboratory examination, research,
evaluative testing, or trade show exhibition are not subject to FSIS import reinspection
requirements. Such samples are subject to USDA, APHIS requirements and animal
disease restrictions, and may require, depending on any animal health restrictions in the
country or region of origin, an APHIS permit prior to importing. Meat, poultry and egg
product samples may originate from any foreign country, provided there are no animal
health restrictions imposed by APHIS.
Weight restrictions for sample shipments: red meat or poultry shipments cannot exceed
50 lbs. (22.7 kg) for each species of product and egg product shipments cannot exceed
30 lbs. (13.6 kg) for liquid or frozen egg products, or 50 lbs. (22.7 kg) for dried egg
products.
Once in the United States, samples may be used only for laboratory examination,
research, evaluative testing, or trade show exhibition. They may not be sold,
distributed, or consumed by the public. If the products are used for consumer test
marketing or sales promotions, they will be regarded as commercial shipments and
subject to FSIS import regulations, including the requirement that they must originate in
eligible foreign countries and establishments and must be certified for export to the
United States.
NOTE:
An official foreign inspection certificate is not required for sample shipments.
However, FSIS Form 9540-5
, Notification of Intent to Import Meat, Poultry, or Egg
Products Samples for Laboratory Examination, Research, Evaluative Testing or Trade
Show Exhibition, must be completed and submitted to RMTAD via e-mail at
importinspect[email protected]ov.
Importers should contact USDA, APHIS for their requirements prior to importing
samples at 301-851-3300 option 1 or via e-mail at asknies.products@usda.gov
.
Information may be found on the website at
http://www.aphis.usda.gov/import_export/animals/animal_import/animal_imports_anpro
ducts.shtml. APHIS import permits: http://www.aphis.usda.gov/permits/
Products for Pharmaceutical Use Only
Glands and organs intended for pharmaceutical use only may be imported into the U.S.
provided the containers are properly identified as “For Pharmaceutical Use Only” and do
not contain a foreign mark of inspection (9 CFR 325.19, 318.1(g), and 316.13(f)). An
undenatured inedible permit issued by FSIS is not required. If the containers of FSIS-
amenable product contain a foreign mark of inspection, then the product is considered
as edible and must meet all FSIS import requirements, and consignments must be
accompanied by an official inspection certificate and must be presented for FSIS import
reinspection.

20
Importers should contact APHIS for their requirements prior to importing products for
pharmaceutical use at 301-851-3300 option 1 or via e-mail at
asknies.products@usda.gov
. Information may be found on the website at
http://www.aphis.usda.gov/import_export/animals/animal_import/animal_imports_anpro
ducts.shtml. APHIS import permits: http://www.aphis.usda.gov/permits/
Product Containing a Small Amount of Meat, Poultry or Egg Product Ingredients
While FSIS statutes provide authority to exempt certain foods containing relatively
small
amounts of meat and poultry, or egg product ingredients from FSIS inspection, FSIS
must ensure that these FSIS amenable ingredients are not adulterated. Accordingly,
the meat, poultry, or egg products ingredients used in FSIS-exempted products must be
from an
eligible source. Further information can be found at:
https://www.fsis.usda.gov/wps/portal/fsis/topics/international-affairs/importing-
products/imported-food-products-containing-a-small-amount-of-meat-poultry-or-
processed-egg-product-ingredients.
Importers should contact USDA, APHIS for their requirements prior to importing FSIS-
exempted products at 301-851-3300 option 1 or via e-mail at
asknies.products@usda.gov
. Information may be found on the website at
http://www.aphis.usda.gov/import_export/animals/animal_import/animal_imports_anpro
ducts.shtml. APHIS import permits: http://www.aphis.usda.gov/permits/.
Foods with small amounts of meat and poultry that are not under FSIS jurisdiction are
under FDA jurisdiction. Further information on FDA’s requirements can be found at:
https://www.fda.gov/food/food-imports-exports/importing-food-products-united-states.
Product Entering the U.S. Destined for Foreign Commissaries
Under the North Atlantic Treaty Organization (NATO) Agreement, (member countries:
http://www.nato.int/cps/en/natolive/nato_countries.htm
), importing goods for use by a
foreign military force is permitted, providing the foreign military and their families with
food and other items sold within their country. The foreign commissary may import
“provisions” (e.g., meat, poultry, or egg products) from the sending country into the
receiving country duty free. Although this product is not subject to reinspection at
official import inspection establishments, FSIS investigators visit foreign commissaries
located in the U.S. to verify that products held for sale at these commissaries meet the
following requirements: the commissary is located on a military base or is restricted to
military personnel from the foreign country in which the product was produced; the
purchasing of products from the commissary is restricted to only people with proper
credentials from the foreign military to which the store sells; purchases are in foreign
currency only; and products must be labeled “For Commissary Sale Only.”
Website: http://www.nato.int/cps/en/natolive/official_texts_17265.htm
Products for Personal Consumption
All travelers entering the United States are required to declare any products of animal
origin they may be carrying. The declaration must cover all items carried in checked

21
baggage, in carry-on luggage, or in a vehicle. U.S. CBP agriculture specialists at U.S.
POEs will examine the items and determine if they meet the entry requirements of the
United States. Additional information on
bringing agricultural products into the United
States is available from CBP. CBP Basic Importing and Exporting information:
https://www.cbp.gov/trade
FSIS inspection regulations permit the entry of small quantities of meat, poultry or egg
products for personal consumption
(9 CFR, Part 327.16, 381.207, 557.16, and
590.960). The products must not exceed 50 pounds for meat, poultry and dried egg
products, and 30 pounds for liquid or frozen egg products; and must be for personal use
only and cannot be sold or distributed in U.S. commerce. Such products are exempt
from FSIS import regulations, but they are subject to APHIS animal health
requirements.
The animal health requirements change frequently, and travelers should contact APHIS
for up-to-date information by visiting the website or calling (301) 851-3300. USDA,
APHIS information:
http://www.aphis.usda.gov/import_export/animals/animal_import/animal_imports_anpro
ducts.shtml
Undenatured Inedible Meat and Egg Products (includes meat, meat by products,
and technical animal fat)
FSIS is responsible for ensuring that domestic and imported meat and egg products
distributed in U.S. commerce as human food are wholesome, unadulterated, and
properly labeled. This authority extends to the control of
undenatured inedible meat
(meat, meat by-products, and animal fat) and egg products that have the appearance of
being fit for human consumption, but are intended for the manufacture of articles not for
human food. FSIS permits the movement of imported undenatured inedible meat and
egg products and animal fat, subject to approval of a permit issued by FSIS and prior
notice given to FSIS in advance of the arrival of each shipment moving under this permit
(9 CFR 327.20). Prior notice is made through completion of FSIS Form 9540-4, Permit
Holder Notification - Importation of Undenatured Inedible Product, available through
RMTAD. The completed form is submitted via e-mail to RMTAD at
importinspect[email protected]ov.
Applicants for a permit to move undenatured inedible meat and egg products will be
requested to provide the following information (9 CFR 325.11(e)(1)): applicant name;
applicant address; applicant telephone number; applicant facsimile number; applicant E-
mail address; description of the type of business operations; and the purpose of making
such application. It is helpful if the purpose includes information on the intended use of
such product, such as “product to be used for dog treats.”
To apply for approval to import undenatured inedible meat, meat by-products, animal fat
or egg products, applicants may contact the FSIS OFO, RMTAD at
202-720-9904, or e-mail importinspection@usda.gov
for more details.

22
NOTE: Inedible poultry must be denatured regardless of the intended use (9 CFR
381.193).
For additional information on importing denatured products such as pet food ingredients
or inedible tallow, contact the FDA Animal Food and Feeds office:
https://www.fda.gov/animal-veterinary/import-exports/importing-animal-food; and for pet
food https://www.fda.gov/animal-veterinary/animal-food-feeds/ingredients-additives.
Products Not Regulated by FSIS
Imported Farmed and Wild Game Meat and Other Products
The U.S. Fish and Wildlife Service has jurisdiction over imported wildlife products.
Wildlife is any living or dead wild animal, its parts, and products made from it. Wildlife
not only includes mammals, birds, reptiles, amphibians, and fish, but also invertebrates
such as insects, crustaceans, arthropods, mollusks and coelenterates. Wild game are
wild land mammals (including those living within an enclosed area under conditions of
freedom), which are hunted, and wild birds. For example, large native game animals in
the United States include antelope, bear, caribou, deer, elk, moose, and reindeer. Wild
game birds include wild turkeys, wild geese, wild ducks, grouse, quail, pheasant, and
other non-domesticated species of fowl. APHIS may have restrictions on imported
game depending on the source of the product.
U.S. Fish and Wildlife Service:
http://www.fws.gov/le/ImpExp/Info_Importers_Exporters.htm
USDA APHIS:
https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-and-animal-product-
import-information
All imported foods not under the exclusive jurisdiction of USDA are regulated by FDA.
The FDA, under the Public Health Security and Bioterrorism Preparedness and
Response Act of 2002, requires that it receive prior notification of food, including animal
feed that is imported or offered for import into the United States. Examples of food
subject to prior notice include: Fish and seafood, live food animals, dairy products, shell
eggs, fruits, vegetables, raw agricultural commodities for use as food or as components
of food and animal feed (including pet food), food and feed ingredients, food and feed
additives, infant formula, beverages (including alcoholic beverages and bottled water),
bakery goods, snack foods, candy, canned foods, and dietary supplements and dietary
ingredients.
For additional information on prior notice of food for importation see:
https://www.fda.gov/media/98263/download
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/what-you-
need-know-about-prior-notice-imported-food-shipments
https://www.fda.gov/food/importing-food-products-united-states/quick-start-guide-prior-
notice-food-articles-prior-notice-system-interface-pnsi

23
Meats and animal products which are subject to FDA regulations are:
• Meats such as bison, buffalo, venison, rabbit, game meat, mollusks, crustaceans,
coelenterates, amphibians, aquatic reptiles, all fish other than Siluriformes fish,
and all other foods not covered by the Federal meat, poultry, and egg products
inspection laws.
• Livestock casings
• Imported inedible meat, poultry, and egg products and rendered fats which are
denatured
• Shell eggs
• Imported pharmaceutical commodities (e.g., insulin from the pancreas of cattle
and swine) that are derived from livestock and not covered by the Federal meat,
poultry and egg products inspection laws.
Products from animals not amenable to the Federal Meat Inspection Act and Poultry
Products Inspection Act are subject to Federal regulation by the FDA as "food" under
the Federal Food, Drug, and Cosmetic Act (FD&C Act). Facilities that manufacture,
process, pack, or hold food for consumption in the U.S. are required to register with
FDA (unless an exemption applies) as a food facility under 21 CFR Part 1, Subpart H.,
and comply with all applicable FDA regulations including 21 CFR Part 117, Current
Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventative Controls
for Human Food found at
https://www.govinfo.gov/content/pkg/CFR-2019-title21-
vol2/pdf/CFR-2019-title21-vol2-part117.pdf.
For additional information on food facility registration, visit:
https://www.fda.gov/food/guidance-regulation-food-and-dietary-
supplements/registration-food-facilities-and-other-submissions
U.S. FDA Import Program:
https://www.fda.gov/ForIndustry/ImportProgram/ImportBasics/default.htm
http://www.fda.gov/ForIndustry/FDABasicsforIndustry/ucm237623.htm
https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-foreign-
supplier-verification-programs-fsvp-importers-food-humans-and-animals
Casings
Livestock casings are under the FDA jurisdiction. Imported livestock casings are also
required to comply with USDA, APHIS requirements: 9 CFR 96.3 Certificate for animal
casings:
http://www.ecfr.gov/cgi-bin/text-
idx?c=ecfr&SID=a9618bb1a0adc95e051d8399ee0d727c&rgn=div8&view=text&node=9:
1.0.1.4.38.0.73.3&idno=9

24
Denatured Inedible Meat, Poultry, Egg Products and Rendered Fats
The FDA has jurisdiction over imported inedible meat, poultry, and egg products and
rendered fats which are denatured.
For additional information on importing denatured products such as pet food ingredients
or inedible tallow, please contact the FDA Center for Veterinary Medicine, Division of
Animal Feeds Office:
http://www.fda.gov/AnimalVeterinary/Products/AnimalFoodFeeds/default.htm
Shell Eggs
Jurisdiction over imported shell eggs is shared by three federal agencies: U.S. FDA,
USDA’s APHIS, and the USDA’s Agricultural Marketing Service (AMS). FDA has
regulatory authority over imported shell eggs under the FD&C Act. Foreign producers
must comply with FDA requirements in 21 CFR Part 118 and 21 CFR Part 101. Certain
farms that produce eggs for export to the U.S. must register with FDA in accordance
with 21 CFR Part 118. To register, go to www.access.fda.gov
and select “FURLS Shell
Egg Producer Registration Module.” If the eggs are transported to another facility to be
packed or consolidated with other eggs prior to export, that facility may be required to
register with FDA as a food facility under 21 CFR Part 1, Subpart H. For that
registration, go to
www.access.fda.gov and select “FURLS Food Facility Registration
Module.” Finally, prior notice must be given to FDA before shell eggs are imported into
the U.S. (unless an exemption applies). Prior notice may be provided by anyone with
knowledge of the required information. Go to
www.access.fda.gov and select “Prior
Notice System Interface.” If the eggs are for breaking only (and not for table use), they
must comply with only the registration requirements (above) and the refrigeration
requirements in 21 CFR Part 118. These regulations require that all eggs going to an
official USDA egg products plant for breaking and pasteurization must be refrigerated at
45 degrees Fahrenheit or below ambient temperature beginning 36 hours after the time
that they are laid up until they reach the official USDA egg products plant in the
U.S. More information can be found at:
https://www.govinfo.gov/content/pkg/CFR-2019-
title21-vol2/pdf/CFR-2019-title21-vol2-part118.pdf.
FDA requires the following information on most shell egg labels:
• A statement of identity (21 CFR 101.3)
• the name and place of business of the manufacturer, packer, or distributor (21
CFR 101.5)
• net quantity of contents (21 CFR 101.7)
• nutrition labeling (21 CFR 101.9) unless exempt in accordance with 21 CFR
101.9(j)
• material fact (section 403(a) and section 201(n) of the FD&C Act and 21 CFR
1.21), when applicable which includes the safe handling instructions for shell eggs that
have not been processed to destroy all viable Salmonella (21 CFR 101.17(h))

25
More information about FDA’s food labeling requirements can be found at:
https://www.fda.gov/food/food-labeling-nutrition
. For questions, contact the FDA at
(888) 723-3366.
APHIS protects America's animal and plant resources from agricultural pests and
diseases. One way APHIS does this is by ensuring that all imported agricultural
products shipped to the United States from abroad, including shell eggs and egg
products, meet that Agency's entry requirements to exclude pests and diseases of
agriculture. Importers should contact the APHIS Import staff to determine a foreign
country’s eligibility to export to the United States at (301) 851-3300 or
AskNIES.Produc[email protected]
. Permits may be required by some countries. Additional
information can be found at:
http://www.aphis.usda.gov/import_export/animals/animal_import/downloads/importer_let
ter_shell_eggs.pdf.
Under the EPIA, AMS is responsible for inspecting imported shell eggs. AMS does this
to control the disposition of restricted eggs (eggs that are undesirable for human
consumption without processing in an official egg products plant) and to assure that the
eggs sold to consumers are properly labeled and documented, and contain no more
restricted eggs than permitted in the standards for U.S. Consumer Grade B shell eggs
(7 CFR part 57). AMS notifies FDA when applications are made to import table eggs
into the United States and before releasing any lots of table eggs for domestic
commerce. AMS also ensures imported eggs originate from foreign farms that are
registered with FDA in accordance with 21 CFR 118.11 and are refrigerated in
accordance with 21 CFR 118.4(e).
An application to import shell eggs into the U.S. must be made to AMS on Form LPS-
222 and be accompanied by a foreign health certificate. Import details for shell eggs
can be found at: AMS Website
.
Pharmaceutical Products Derived from Livestock and Imported into the U.S.
The U.S. FDA has jurisdiction over imported pharmaceutical commodities (e.g., insulin
from the pancreas of cattle and swine) that are derived from livestock and not covered
by the Federal meat, poultry and egg products inspection laws.
Q & As on the U.S. FDA Import Program for insulin from livestock for personal use:
http://www.fda.gov/Drugs/ResourcesForYou/Consumers/QuestionsAnswers/ucm17390
9.htm
FSIS Contact Information for Import Inspection
For FSIS, Office of Field Operations, RMTAD Import, please contact:
USDA, Food Safety and Inspection Service
Office of Field Operations
Recall Management Technical Analysis Division (RMTAD Import)
1400 Independence Avenue, SW
Room 0205 South Building
Washington, D.C. 20250-3700
RMTAD e-mail: ImportInspect[email protected]

26
For FSIS, Office of Field Operations, District Offices, please see the link below
and on the next page the District Office Map with locations of official import
inspection establishments:
District Offices: http://www.fsis.usda.gov/wps/portal/informational/districtoffices
For FSIS, Office of Policy and Program Development, IEPDS, please contact:
USDA, Food Safety and Inspection Service
Office of Policy and Program Development
Import/Export Policy Development Staff (IEPDS)
1400 Independence Avenue, SW
Room 2925 South Building
Washington, D.C. 20250-3700
Phone: (202) 690-4354
Fax: (202) 720-4929
Email: ImportExport@usda.gov
Import Inspection Resources
FSIS homepage: http://www.fsis.usda.gov/wps/portal/fsis/home
FSIS Meat, Poultry and Egg Product Inspection Directory, includes official import
inspection establishments:
http://www.fsis.usda.gov/wps/portal/fsis/topics/inspection/mpi-directory
FSIS website on importing meat, poultry, or egg products to the United States:
http://www.fsis.usda.gov/wps/portal/fsis/topics/international-affairs/importing-products
Eligible Countries, Products, and Foreign Establishments:
http://www.fsis.usda.gov/wps/portal/fsis/topics/international-affairs/importing-
products/eligible-countries-products-foreign-establishments
FSIS Public Health Information System (PHIS):
https://www.fsis.usda.gov/wps/portal/fsis/topics/inspection/phis
PHIS Import Component Information:
http://www.fsis.usda.gov/wps/portal/fsis/topics/international-affairs/importing-
products/phis-import-component
eAuthentication: How to obtain a USDA eAuthentication account
FSIS Partner Government Agency (PGA) Message Set Compliance Guide
http://www.fsis.usda.gov/wps/wcm/connect/98c45f3c-8a5b-47d7-9907-
2fdc7352547f/Data_Samples_Guidelines_PGA_Message_Set.pdf?MOD=AJPERES

27
FSIS Directives, 9000 series, address the import of meat, poultry, and egg products.
Directives provide communications to FSIS personnel in carrying out their functions.
http://www.fsis.usda.gov/wps/portal/fsis/topics/regulations/directives/9000-series
FSIS Form 9540-1, Import Inspection Application: FSIS Form 9540-1, Import Inspection
Application (Meat, Poultry and Egg Products), dated 2/26/2014.
FSIS Product Categorization Chart: FSIS Product Categorization Chart (5/18/2016)
http://askfsis.custhelp.com/
FSIS/USDA
www.fsis.usda.gov
2020
